Abstract
Aims
The purpose of this study was to investigate the pharmacokinetics of a single oral dose of lamivudine administered to subjects with renal impairment and to determine whether lamivudine was dialysable in subjects with severe renal impairment undergoing haemodialysis.
Methods
Twenty-nine subjects were enrolled, nine with normal renal function (creatinine clearance (CLCR) 82–117 ml min−1), eight with moderately impaired renal function (CLCR 25–49 ml min−1), six with severe impairment (CLCR 13–19 ml min−1) and six with severe impairment who were also receiving haemodialysis. After an overnight fast, nondialysis subjects received a single oral dose of lamivudine. Subjects on haemodialysis were given two doses on separate occasions (intra and interdialysis). Blood was obtained before lamivudine administration and at regular intervals to 48 h post dose. Timed urine collections were performed for subjects able to produce urine. Pharmacokinetic parameters were calculated by using standard non compartmental techniques.
Results
Decreasing renal function was associated with reduced lamivudine clearance in a proportional and apparently linear relationship. Lamivudine was well dialysed with an extraction ratio in the order of 50%. However, because lamivudine has a large volume of distribution (≈100 l), a haemodialysis session of 4 h did not affect overall exposure to a clinically significant degree in most subjects.
Conclusions
Impaired renal function does require lamivudine dose modification according to the degree of impairment, but no further modification of dose is required for subjects undergoing regular haemodialysis.
Keywords: lamivudine, pharmacokinetics, haemodialysis, renal function
Introduction
Lamivudine, 2′3′-dideoxynucleoside 3′-thiacytidine (also known as 3TC), is licensed for the treatment of patients with human immunodeficiency virus (HIV). It has two chiral centres and is the (–) enantiomer of the racemic mixture GR103665X. It has comparable in vitro potency but is less toxic than other dideoxynucleosides [1] and is well tolerated in subjects with HIV. Lamivudine is also under development for the treatment of hepatitis B viral infection (HBV) and shows promising efficacy coupled with an equally good profile of safety and tolerability in these patients relative to those with HIV infection [2].
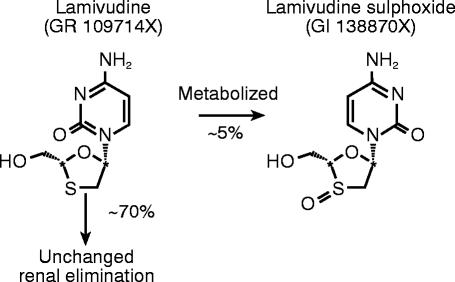
Pharmacokinetic studies in asymptomatic HIV-infected subjects and normal healthy volunteers have shown that lamivudine is rapidly absorbed following an oral dose, reaching maximal serum concentrations between 1 and 1.5 h, has good (>80%) absolute bioavailability and exhibits linear kinetics [3]. Renal clearance is the major route of lamivudine elimination with t (in the order of 5–7 h. Following oral administration ≈70% of the total dose is excreted unchanged in the urine and only 5–10% undergoes hepatic metabolism to form a trans-sulphoxide metabolite identified and characterized as GI138870X (Figure 1), which is then also renally eliminated. This metabolite is also found in dog following studies with oral lamivudine dosing and comprises up to half of the drug-related material in urine. Renal dysfunction has significant effects on the pharmacokinetics of lamivudine in HIV-infected individuals [4] such that dose modification is recommended at or below 50 ml min−1 CLCR as assessed using the Cockcroft-Gault estimation of renal function [5].
Figure 1.
Structure of lamivudine and the trans-sulphoxide metabolite.
Haemodialysis is designed to remove toxic waste products that accumulate, unable to be renally cleared and is the established treatment for subjects with endstage renal disease. Drug concentration may be significantly reduced by haemodialysis, depending on pharmacokinetic and physico-chemical properties e.g. plasma protein binding, molecular weight. Haemodialysis may require dose modification [6–8].
The present study was designed to investigate the pharmacokinetics of a single dose of lamivudine in otherwise healthy (i.e. no concurrent viral infection) renally impaired subjects to substantiate earlier findings, to assess the effect of haemodialysis on lamivudine clearance and to provide quantitative and qualitative information on the excretion of the trans-sulphoxide metabolite. Conclusions drawn from this study are essential as a guide to modification of lamivudine therapy for patients undergoing routine haemodialysis.
Methods
Subject population
Subjects were included with renal dysfunction as defined by the subjects’ records within 6 months of the start of the study and an assessment of glomerular filtration rate (GFR) as determined by serum creatinine within the 2 weeks prior to the study. Subjects were stratified according to renal function by using the method of Cockcroft-Gault [5] to estimate creatinine clearance (CLcr). All assessments of renal function were repeated for consistency on two occasions to ensure accurate stratification and correlation with lamivudine pharmacokinetics.
Twenty-nine subjects were enrolled as follows: Nine with normal renal function (creatinine clearance (CLCR) 82–117 ml min−1), Group 1; eight with moderately impaired renal function (CLCR 25–49 ml min−1), Group 2; six with severe impairment (CLCR 13–19 ml min−1. A further group of six patients with severe impairment but undergoing routine haemodialysis were also recruited (Group 3). Assessment of CLCR is flawed in such a group as serum creatinine elimination will be predominantly extra-renal where little or no urine is produced. Haemodialysis subjects were studied both intra and interdialysis i.e. on their day of haemodialysis and between haemodialysis visits.
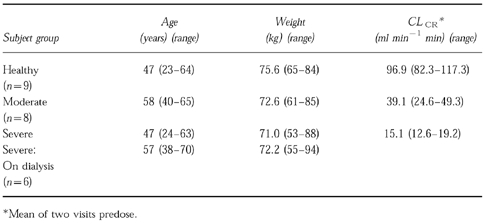
The demographics are summarized in Table 1. Other than one subject (aged 70 years) in the dialysis group, all subjects were between 18 and 65 years. Average body weight was less than 100 kg, and all subjects were able to provide oral and written informed consent. The protocol was approved by the Universitair Ziekenhuis Antwerpen Ethical Review Committee.
Table 1.
Subjects characteristics.
Study design
This was an open study and was performed at the Department of Nephrology, University Hospital and St-Jozefziekenhuis, Antwerp, Belgium. All subjects underwent an overnight fast from midnight the previous day and were not allowed food until 4 h postdose. Each subject in group 1 and those with normal renal function received a single oral 300 mg dose of lamivudine administered as 3×100 mg hard gelatin capsules. Following preliminary pharmacokinetic analysis, the dose was reduced for subsequent groups, through concern (at the time) of excessive exposure in patients with severe renal impairment. Subjects in group 2 received a single oral 100 mg dose of lamivudine. Those in group 3 received a single oral dose of 100 mg on two occasions separated by at least 1 week. These two occasions were referred to as intradialysis, where the subject received their dose on the day of haemodialysis approximately 2 h prior to haemodialysis and interdialysis, where subjects received lamivudine 1 or 2 days after haemodialysis. Lamivudine capsules were taken with 150 ml water.
Haemodialysis was performed for approximately 4 h using an F7 polysulphone dialyser (surface area 1.5 m2) (Fresenius Bad Homberg, Germany) together with Gambro AK10 dialysate monitor (Lund, Sweden) at a constant dialysate flow of 500 ml min−1. Blood flow was held constant at 200 ml min−1.
Blood for pharmacokinetic analysis was obtained in all subjects predosing and 30 min, 1, 1.5, 2, 4, 6, 8, 12, 15, 24, and 48 h postdose. Timed collections were performed for subjects able to produce urine at 0–4, 4–8, 8–12, 12–15, 15–24, 24–30, 30–36, and 36–48 h postdose. Whilst being studied interdialysis the blood sampling schedule was identical to that of the nondialysis subjects and samples were also taken from the inlet and outlet ports of the dialyser at hourly time points to the end of haemodialysis (4 h later).
Blood, processed to produce serum, and urine samples taken through the study were stored frozen at −20° C until assay. Serum samples were analysed for lamivudine and urine samples for lamivudine and the trans-sulphoxide metabolite, by validated h.p.l.c. methods using u.v. detection [9].
Safety
Safety was evaluated by monitoring adverse events and vital signs, physical examinations, clinical laboratory tests and electrocardiograms. Each subject was questioned periodically throughout the study regarding adverse effects.
Pharmacokinetic analysis
The maximum drug concentration in serum (Cmax) and the time to Cmax (tmax) were obtained directly from the concentration-time data. The terminal rate constant for lamivudine (λz) and corresponding terminal serum half-life (t1/2) were calculated following inspection of the data.
The terminal rate constant for all subjects was determined by linear least squares regression (using PCNONLIN v4.2) using logarithmically transformed points in the terminal phase. The number of points included in the terminal phase was confirmed by visual inspection. Non-compartmental pharmacokinetic analysis was used to calculate parameter estimates. The amount of lamivudine (Ae) and metabolite (corrected for lamivudine molar equivalents) recovered in the urine was used to calculate renal clearance (CLR) and excretion ratios (for lamivudine and metabolite). CLR was calculated as follows: The amount excreted to time t (where t is the last time of complete collection) was divided by the area under the curve of serum lamivudine concentration over the same time (AUCt). AUC was assessed vs time (AUCt) and extrapolated to infinite time (AUC∝), apparent oral clearance (CL/F) and the dialysis clearance of lamivudine (CLD) were also determined.
Dialysis clearance CLD was calculated as:
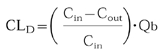 |
where Cin is the concentration of lamivudine collected prefilter and Cout is that collected postfilter with Qb being the blood flow into the dialyser (200 ml min−1). Because lamivudine has been demonstrated (in vitro) to have equal partition between plasma (or serum) and red blood cells, no correction for haematocrit and partition coefficient was made. Urea CLD was assessed as a marker of dialyser efficiency (normal extraction ratio about 60%).
Statistical analysis
Pharmacokinetic parameter results are presented as the geometric mean and 95% confidence intervals for each group. Mean ratios of the parameter estimates together with the associated 95% confidence intervals (where significance was declared if P<0.05) were obtained for healthy subjects/moderately impaired subjects, healthy subjects/severely impaired subjects and interdialysis subjects/intradialysis subjects and were analysed using analysis of variance.
tmax was compared between groups using the Wilcoxon Rank Sum test together with an estimate of the median difference for each of the comparisons of moderate-healthy and severe-healthy. All analyses were performed using SAS version 6.08.
Results
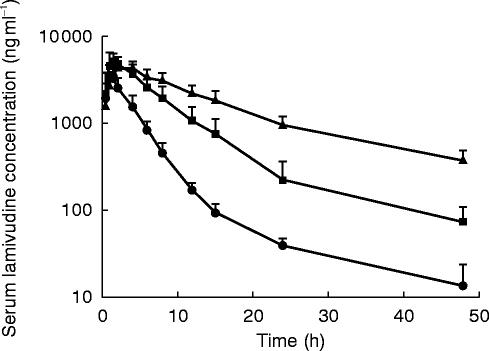
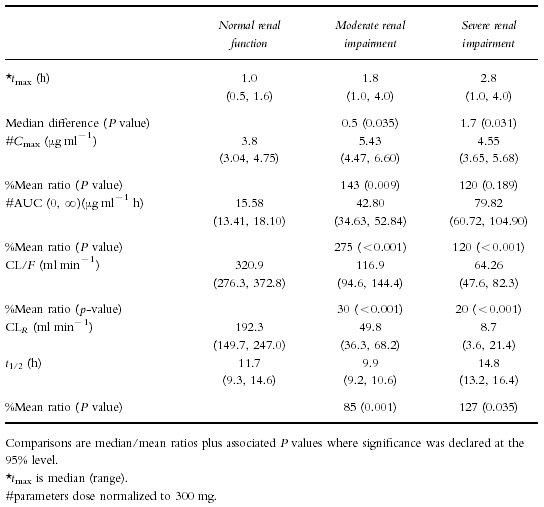
Demographic details of all subjects participating in this study are shown in Table 1. Figure 2 shows the median concentration-time profiles for healthy subjects and subjects with moderate to severe renal impairment. For visual comparison the serum data from severely impaired subjects has been dose normalized to 300 mg. In subjects with normal renal function, lamivudine concentrations rose quickly, reached a Cmax of 3.80 μg ml−1 at about 1 h and decayed in a biexponential fashion. In subjects with moderately impaired renal function, lamivudine concentrations also rose quickly, reaching a higher Cmax of 5.43 μg ml−1 at about 2 h. In subjects with severe renal impairment, lamivudine concentrations reached a (dose normalized) Cmax of 4.55 μg ml−1 at about 3 h. The severely impaired subject profiles showed noticeably longer time to peak concentrations, higher exposure (AUC) and lower clearance (CL/F) with generally longer t1/2 values than in healthy subjects.
Figure 2.
Dose-normalized (to 300 mg) mean (+s.d.) lamivudine concentrations following oral administration to healthy subjects and renally impaired subjects.
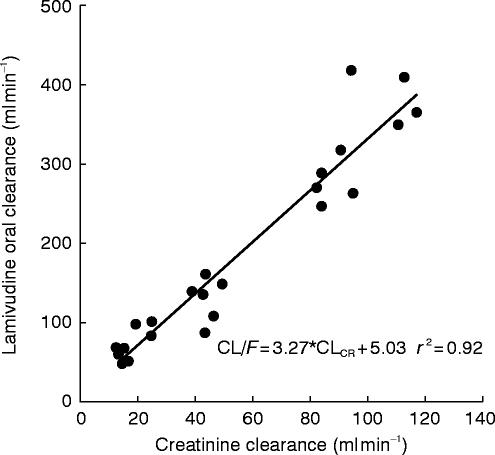
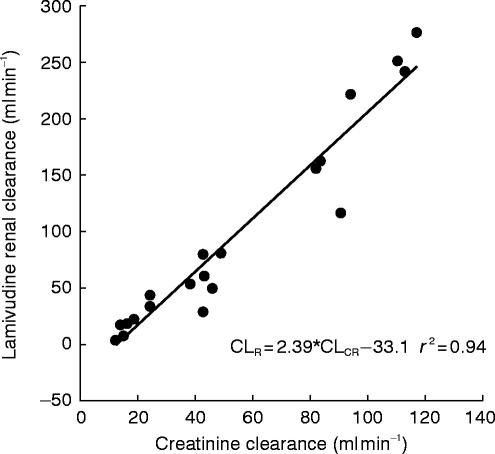
Figures 3 and 4 illustrate the proportional relationship between CL/F or CLR and CLCR which appear to be essentially linear (r2=0.92 and 0.94, respectively).
Figure 3.
Lamivudine oral clearance vs creatinine clearance in healthy subjects and renally impaired subjects.
Figure 4.
Lamivudine renal clearance vs creatinine clearance in healthy subjects and renally impaired subjects.
Haemodialysis
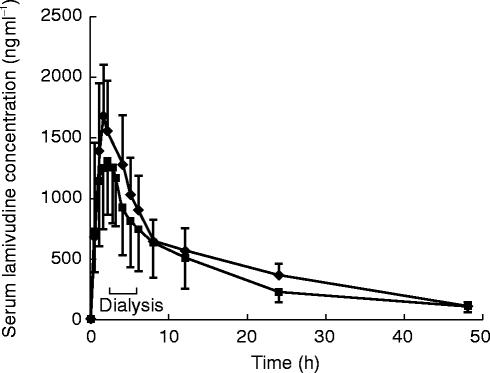
The dose-normalized median serum lamivudine concentration data from haemodialysis subjects studied intra and interdialysis are shown in Figure 4. The haemodialysis extraction ratio averaged 52.8% (range 57–68%) which was similar to urea extraction ratios (50–70%). Lamivudine mean haemodialysis clearance (CLD) was 106 ml min−1 (range 92–121 ml min−1).
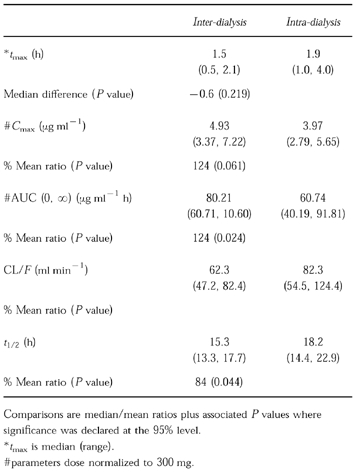
When lamivudine (as a 100 mg dose) was administered interdialysis, lamivudine concentrations rose to a Cmax of 4.93 μg ml−1 by 1.5 h (Table 3). When studied intradialysis, values of 3.97 μg ml−1 and 1.9 h were obtained for Cmax and tmax, respectively. Haemodialysis resulted in a small apparent increase in t1/2 (see discussion) and an increase in geometric mean CL/F from 62 (interdialysis) to 82 ml min−1 (intradialysis) in impaired subjects undergoing haemodialysis. However, three of the six haemodialysis subjects had intradialysis serum concentration profiles that were similar to those seen interdialysis. Haemodialysis therefore conferred a modest (24%) average decrease in AUC (o, ∝) i.e. AUC (AUC over a dosing interval at steady-state) when administered for a 4 h period following lamivudine oral dosing.
Table 3.
Lamivudine parameters (geometric mean and 95% confidence intervals) in dialysis subjects studied interdialysis and intra dialysis.
Metabolism
In progressive renal impairment the primary, renal elimination pathway of lamivudine diminishes and the secondary route (s) of elimination (metabolic) account for the majority of lamivudine clearance. Even in severely impaired subjects with CLCR of <20 ml min−1 however, lamivudine clearance is still around 50 ml min−1 (Figure 3).
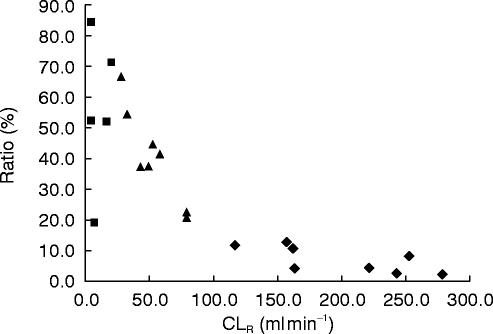
Increasing amounts of trans-sulphoxide are formed as renal function declines (Figure 6), mirroring the reduced renal elimination of parent compound. The percentage ratio of metabolite to lamivudine ranged from around 10% in healthy subjects to 50% in severely impaired subjects. Urinary recovery of drug-related material was on average 64.7% for healthy subjects (range 38.0–78.3%) and 60.1% (range 50.2–71.3%) for patients with moderate impairment. In the severely impaired subject group overall drug-related material recoveries were lower, on average 24.4% (17.9–33.9%) so that quantitative assessment of lamivudine and metabolite levels is probably not valid. By calculating the amount of lamivudine AUC extrapolated beyond 48 h to infinity and multiplying this with CLR, the median estimate of amount of lamivudine to be excreted beyond the 48 h collection in severely impaired patients was 4.1 mg (range 1.1–11.7 mg) or 1.4% of the administered dose. There was little or no urine produced by haemodialysis subjects hence no calculation of recovery.
Figure 6.
Ratio of amount of metabolite (Ae) to lamivudine excreted in urine vs renal clearance in healthy subjects (♦), moderate renally impaired subjects (▴) and severely impaired subjects (▴).
Safety profile
There were no clinically concerning changes in any safety assessment that were attributable to lamivudine. One haemodialysis subject experienced the simultaneous occurrence of a coronary thrombosis and fistula thrombosis 5 weeks after receiving lamivudine. In the investigator's opinion this was directly related to their underlying condition and unrelated to the study drug.
Discussion
Renal dysfunction was found to have marked effects on the pharmacokinetics of lamivudine, in good agreement with data obtained in HIV-infected subjects [4].
The increase in AUC was caused by the decrease in renal function, as demonstrated by the proportional and linear correlation between clearance, renal clearance and creatinine clearance. Lower overall values for lamivudine AUC and Cmax in the subjects with severe renal impairment were found in this study in comparison to that reported by Heald et al. [4] and probably reflect differences in pharmacokinetic parameter estimates in different patient populations.
There is a good linear correlation for the regression fit of CLCRvs CL/F or CLR plots even where assessment of creatinine clearance, as applied by Cockcroft & Gault at the lowest levels of renal function can be inaccurate [5, 6]. It is this inaccuracy which is the likely reason for the apparent negative intercept. Renal clearance (mean 192 ml min−1) greatly exceeded GFR (around 100 ml min−1) which confirms earlier work and implies an active secretory process is significantly responsible for overall lamivudine clearance.
Cmax values were increased in subjects with moderate and severe renal impairment over healthy controls, although only the moderate/healthy comparison was statistically significant. Absorption rate seemed similar between the groups and any potential alterations in protein binding are not a likely explanation for this as lamivudine is not extensively protein bound (<36%). Lamivudine, as stated earlier is a widely distributed drug (Vd>100 l) so changes in apparent volume of distribution coupled with greatly reduced elimination are likely explanations for the changes seen in Cmax.
Although there looks to be a lack of definition in the terminal phase of the mean plot (Figure 2) three to four points were generally used in individual profiles to establish values for (and thus half-life (t1/2). Healthy subjects demonstrate a longer terminal phase i.e. 11 h when sampling is continued to 48 h postdose than is seen with shorter sampling periods, to 8 or 12 h postdose, where t1/2 values were 2.5–3 h [1, 3]. Half-life was generally increased in renally impaired subjects relative to healthy controls although the magnitude of these increases was less than would be expected from the change observed in oral drug clearance. The calculation of these t1/2 estimates is confounded by the fact that with severely impaired subjects, the decay appears more monoexponential, implying the sampling is still mostly contained within the alpha phase. In effect, comparisons between healthy and severely impaired subject t1/2 data are therefore not valid.
Assuming a bioavailability (F) of 0.85 [11], over 90% of the orally administered dose of lamivudine was accounted for in healthy subjects. In general, the majority of administered lamivudine was excreted predominately in the first 12 h. However, as renal function declined, the overall elimination profile of lamivudine changed with a delay in excretion of the metabolite. This resulted in significant amounts of drug-related material still being present in the final (to 48 h) urine collections and consequent underestimate of total balance of recovery in subjects with severe renal impairment. Calculation of the amount of lamivudine to be excreted (<2% of the dose) beyond 48 h suggests the majority of unrecovered material would be sulfoxide metabolite still circulating which would eventually be excreted beyond 48 h. An additional hypothesis is that additional metabolite (s) may be formed, possibly uracil, a minor metabolite seen in dog folowing lamivudine administration.
The pharmacokinetics of the sulfoxide metabolite in subjects with renal dysfunction have not been established. However, significantly higher systemic exposure to lamivudine and therefore the metabolite in toxicological studies in rat and dog has yielded no effects of clinical concern and the metabolite has been shown to be pharmacologically inactive in in-vitro antiviral screens.
Haemodialysis subjects
Lamivudine, by virtue of its low molecular weight [229] and relative lack of protein binding had an average haemodialysis extraction of 53% (range 42.7–60.6%) and average haemodialysis clearance of 106 ml min−1 (range 92–121 ml min−1). This value for extraction ratio was similar to that obtained for urea which is used as a marker of haemodialysis efficiency. This indicates some similarity between the two compounds in that are both renally excreted, have low molecular weight, low protein binding and are thus efficiently dialysed.
Patients typically lost 3–4 kg during haemodialysis whilst on this study. Assuming an ultrafiltration rate of about 1 lh−1 (volume lost/time on dialysis) ultrafiltration clearance would approximate 17 ml min−1, with negligible protein binding. Thus, since dialysis clearance is in the order of 100 ml min−1 for a dialyser with a 200 ml min−1 flow rate, ultrafiltration will contribute to overall drug clearance during a period of haemodialysis. However, the impact that haemodialysis has on geometric mean oral lamivudine clearance is a modest 27% increase in CL/F with a concomitant 24% decrease in mean AUC (0, ∞). A pronounced delay to Cmax and seemingly lower overall absorption was seen in one subject possibly as a result of changes in gastro-intestinal tract function induced by predialysis fluid retention. The small, apparent increase in half-life is likely to be the result of variability in the estimates and not a genuine effect of a short period of haemodialysis. Sampling was continued to 48 h postdose, just prior to the subjects next haemodialysis session i.e. just over two half-lives (where significant renal impairment results in a prolonged lamivudine half-life). This is unlikely to yield sufficient data points for the calculation of a meaningful half-life parameter estimate.
Haemodialysis was seen to have a small but variable effect on overall systemic lamivudine exposure, probably dependent on time of lamivudine administration relative to the start of haemodialysis in these subjects. Haemodialysis primarily extracts drug from the central compartment. Lamivudine has a relatively high volume of distribution (≥100 l), consequently even though it is extracted by haemodialysis, only a small fraction of drug is removed during a single (4 h) session. This is further corroborated in some individual concentration time profiles where there is a transient increase in concentration post haemodialysis in some subjects. This may be explained as an apparent re-distribution phase where drug passes back into the central compartment from tissue compartments once haemodialysis is stopped [10].
For subjects receiving haemodialysis, dialyser flow rate and the amount of time on haemodialysis will be the determining factors for overall drug exposure. Assuming flow rates are not significantly altered, intermittent (4 h two to three times weekly, as studied here) is not considered to have a clinically significant effect on lamivudine exposure. Prolonged or continuous haemodialysis would have a greater effect on lamivudine exposure, possibly necessitating increasing lamivudine dosage from that determined by CLCR assessment alone.
In conclusion this study supports those of a previous study in HIV asymptomatic renally impaired subjects whereby reduction in renal function was directly correlated with lamivudine clearance and necessitates dosing adjustment in these subjects.
The amount of trans-sulphoxide metabolite excreted is increased with decreasing renal function, as a result of diminished renal elimination of lamivudine. This is not deemed clinically relevant as the metabolite is not associated with toxicity and is pharmacologically inactive.
Intermittent haemodialysis does not reduce lamivudine exposure to a clinically significant degree, especially with a conventional haemodialysis routine for these subjects i.e. 2/3 days a week. Therefore once the dose of lamivudine has been adjusted according to degree of renal impairment (assessed as CLCR), no further modification of dose is required for subjects undergoing routine, transient (<4 h) haemodialysis.
Figure 5.
Mean (+s.d.) serum lamivudine concentration-time plot for dialysis subjects studied inter and intradialysis.
Table 2.
Lamivudine pharmacokinetic parameters (geometric mean and 95% confidence intervals, CI) in subjects with renal impairment compared with healthy subjects.
Acknowledgments
The authors wish to thank the subjects who took part in this study, the staff at the University hospital, Antwerp and employees of GlaxoWellcome who assisted in preparation of this manuscript, in particular Miss Julie Garner and Dr Frank Hoke.
References
- 1.Pluda JM, Cooley TP, Montaner JSG, et al. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in subjects with advanced human immuno defficiency virus infection. The J Infectious Dis. 1995;171:1438–1447. doi: 10.1093/infdis/171.6.1438. [DOI] [PubMed] [Google Scholar]
- 2.Doong S-L, Tsai C-H, Shinazi RF, et al. Inhibition of the replication of hepatitis B virus in vitro by 2′3′-dideoxy-3′ thiacytidine and related analogues. Proc Nat Acad Sci. 1991;88:8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Leeuwen R, Lange JMA, Hussey EK, et al. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in subjects with HIV infection: a phase I study. AIDS. 1992;6:1417–1415. doi: 10.1097/00002030-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Heald A, Hsyu P, Yuen G, et al. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected subjects with renal dysfunction. Antimicrob Agents Chemother. 1996;40(6):1514–1519. doi: 10.1128/aac.40.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 6.Rowland M, Tozer . Clinical Pharmacokinetics: Concepts and Applications. 2. Lea and Febiger publishing; 1989. pp. 420–437. Chapter 24 ‘Dialysis’. [Google Scholar]
- 7.Reetze-Bonorden P, Bohler J, Keller E. Drug dosage in subjects during continuous renal replacement therapy. Pharmacokinetic and therapeutic considerations. Clin Pharmacokinet. 1993;24(5):362–379. doi: 10.2165/00003088-199324050-00002. [DOI] [PubMed] [Google Scholar]
- 8.Cunha BA, Friedman PE. Antibiotic dosing in subjects with renal insufficiency or receiving dialysis. Heart Lung. 1988;17(6 Part; 1):612–616. [PubMed] [Google Scholar]
- 9.Harker AJ, Evans GL, Hawley AE, Morris DM. High-performance liquid chromatographic assay for 2′-deoxy-3′-thiacytidine in human serum. J Chromatogr B Biomed Appl. 1994;657:227–232. doi: 10.1016/0378-4347(94)80092-8. [DOI] [PubMed] [Google Scholar]
- 10.Verpooten GA, Verbist L, Buntinx AP, Entwistle LA, Jones KH, De Broe ME. The pharmacokinetics of imipenem (thienamycin-formamidine) and the renal dehydropeptidase inhibitor cilastatin sodium in normal subjects and subjects with renal failure. Br J Clin Pharmacol. 1984;18:183–193. doi: 10.1111/j.1365-2125.1984.tb02451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuen GJ, Morris DM, Mydlow PK, Haidar S, Hall ST, Hussey EK. Pharmacokinetics, absolute bioavailability, and absorption characteristics of lamivudine. J Clin Pharmacol. 1995;35:1174–1180. doi: 10.1002/j.1552-4604.1995.tb04043.x. [DOI] [PubMed] [Google Scholar]