Abstract
Aims
To gain information on serious adverse events in a large number of patients exposed to perindopril.
Methods
Four thousand seven hundred and eighty-eight general practitioners throughout France collaborated in the recruitment of 47 351 patients for a 12 month postmarketing study. Data collection was undertaken by company representatives under the supervision of nine regional medical officers. Computerised data entry was performed by six pharmaceutical officers. Serious adverse events were later individually reviewed medically.
Results
Withdrawals due to adverse events occurred in 6.1% of female and 3.2% of males patients. The ascertainment of adverse events in this study approved satisfactory, as shown by the reported incidence of cough, which was 11.3% in women and 7.8% in men, this being compatible with the best estimates of the true incidence of cough during ACE-inhibitor therapy. Serious adverse reactions—anaphylaxis and blood dyscrasias—were rare.
Conclusions
This study successfully followed a large cohort of patients treated with perindopril and failed to demonstrate any unexpected hazards.
Keywords: PMS, perindopril, hypertension, ADR monitoring
Introduction
Perindopril (Coversyl®, Servier) is an angiotensin converting-enzyme inhibitor (ACE inhibitor) which was first licensed in France in 1988 for the treatment of hypertension, the indication for heart failure being added in 1992. In view of the ongoing discussions by the Committee for Proprietary Medicinal Products concerning guidelines for the conduct of postmarketing studies, it was decided to undertake a major postmarketing study of perindopril.
Servier has in France a network of regional medical officers with responsibility for supervision of ‘attachés de recherche clinique’, that is representatives trained in the conducting of postmarketing studies, and was able to utilize this organization for recruitment and monitoring of the study. The study ended in October 1990. A preliminary report of this surveillance study was published previously [1]. We now report the final results of this project which includes medical review of serious adverse events.
Methods
The study was conducted by the Servier Medical Division in France. Four thousand seven hundred and eighty-eight French general practitioners recruited patients with a target number of 10 per general practitioner. The targeted patient population was newly diagnosed hypertensives but patients included in the study could have been on previous drug therapy which was considered inappropriate due to lack of efficacy or side-effects. In order to guard against inappropriate recruitment no general practitioner could enter more than 10 patients. The only restrictions on entry were those related to the existing indications, contra-indications and precautions on use then applying to the product in France. At the time perindopril was not indicated for the treatment of cardiac failure. Patients included were over 18 years of age with nonaccelerated hypertension and diastolic blood pressure within a range of 95–114 mmHg on at least two occasions, at three different visits. There was no upper age limit. Pregnant and breast-feeding women and patients with known secondary hypertension were excluded, as were those with a history of cerebrovascular accident, myocardial infarction, and unstable angina within the 3 months preceding the study. Patients with known concomitant hepatic or renal disease (with a suggested guideline of a creatinine level over 180 (μmol l−1), or with serious illness or incapacity considered sufficient to preclude successful participation in the study, were also excluded. Patients satisfying these criteria and thought to be capable of participating were then informed of the nature of the study, and informed consent was obtained in all cases before entry into the study.
On entry into the study, patients were categorized according to whether or not the following risk factors were present: smoking, hypercholesterolaemia (as defined by a cholesterol level of more than 6.76 mmol l−1), or diabetes and obesity. The presence of associated cardiac,cerebrovascular, or renal problems was documented, along with other associated disease. Previous therapy for hypertension and other problems was noted. Patients underwent a general medical examination with blood pressure measured in both the erect and supine positions, and laboratory tests were performed in order to determine plasma urea, creatinine, electrolytes, cholesterol and apolipoprotein A1 and B. In accordance with the product monograph at the time it was advised that patients with known prior elevations of serum creatinine (greater than 180 (μmol l−1) were excluded, while those whose creatinine levels when checked on entry, were found to be greater than 180 (μmol l−1 were not excluded.
Patients already taking antihypertensive medication considered ineffective were taken off the treatment before starting perindopril. β-adrenoceptor blocking agents and centrally acting drugs were withdrawn gradually and perindopril substituted, it was recommended that diuretics were stopped 3 days before perindopril was started. Perindopril was started at a dose of 4 mg daily, this starting dose being reduced to 2 mg for patients over 70 years. At each subsequent revisit at 1, 3 and 6 months, the dose of perindopril could be doubled, or a diuretic added, if the supine diastolic blood pressure was greater than 95 mmHg. Continuance in the study was not dependent on adherence to the treatment template.
At each follow-up visit, erect and supine blood pressure and pulse rate were measured. Patients spontaneous reports of any problem whether related to therapy or not were recorded. The reporting of all serious adverse events by the general practitioner was mandatory. All serious events requiring hospital admission along with the reason for admission and all deaths were recorded. In addition, urea and electrolyte levels were monitored at every visit, as were the blood lipids at 1, 6 and 12 months. Medical examinations were performed at 6 and 12 months.
The study was monitored in the field by Servier's team of representatives who visited regularly during the study. These visits took place every 6–8 weeks after inclusion of patients in the study. The operation was supervised by company medical officers in charge of drug monitoring in nine regions in France. Completed case report forms were returned to Servier's Medical Department in Paris and validation and computer entry of anonymously coded data was performed by a team of six pharmacists. Confidentiality was maintained according to the Commission Informatique et Liberté French regulations. In accordance with generally accepted ethical considerations, in order to exclude any undue influence of the study to continue therapy against the best interests of the patient, payment was not conditional upon any milestone of therapy but was made in full 1 year after entry of the patient irrespective of the duration of follow-up.
A total of 2596 patients left the study for reasons of drug-related side-effects or intercurrent illness. There were 190 deaths, and 255 patients required hospital admission. In these cases, after examination of the case report forms, the reporting doctors were contacted by telephone by the validation pharmacists in order to confirm the circumstances surrounding the clinical event. At the conclusion of the study all reports on these patients leaving the study were reviewed by Servier's Drug Monitoring Department of Servier under the supervision of one of the authors (FW). A new database was set up for this subgroup of patients to perform a more detailed analysis.
Results
A total of 47 351 patients were recruited into the study by 4788 general practitioners. Valid entry data with adequate reporting of demographic data were available for the 47 253 subjects representing the study population. This population comprised 25 169 (53%) women and 22 084 (47%) men. The mean age at entry was 60.9 years, this being 58.9±11.9 for men and 62.7±11.1 years for women.
Mean systolic blood pressure on entry was 173 mmHg (±16.3), and mean diastolic pressure was 100 mmHg (±8.0). The mean weight of the men was 77.7±11.9 kg and that of the women 67.1±12.7 kg. The severity of the hypertension is given in Table 1 with blood pressure categorized according to the highest of three readings of supine diastolic pressure (DBP) as follows: mild 94–104 mmHg; moderate, 105–114 mmHg; and severe ≥115 mmHg.
Table 1.
Risk factors for cardiovascular disease according to age and sex.
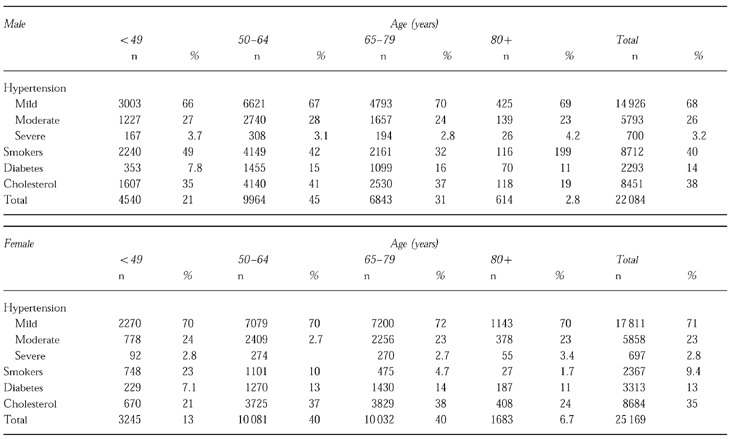
Risk factors for cardiovascular disease other than hypertension are also listed in Table 1. The most common was hypercholesterolaemia (blood cholesterol levels over 6.76 mmole l−1) affecting 38% of men and 35% of women. Smokers made up 40% of the men and 9% of the women recruited to the study.
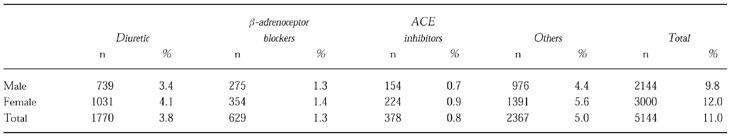
Previous treatment for hypertension had been recorded for 10% of the men and 12% of the women. The distribution of previous treatment is shown in Table 2. The most frequently previously prescribed drugs were diuretics and β-adrenoceptor blockers, followed by calcium channel blockers. Diuretic therapy had been used more frequently in older patients but there were no other age-sex associations with previous therapy. Three hundred and seventy-eight patients in the study had previously been treated with ACE inhibitors.
Table 2.
Previous antihypertensive treatment.
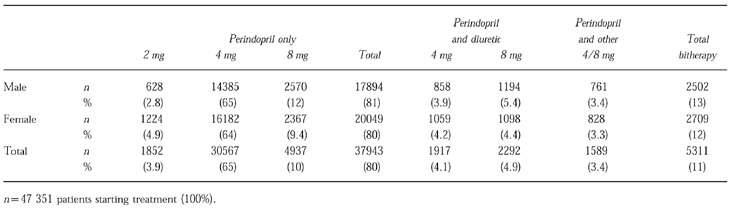
Antihypertensive treatments received after control of hypertension at 6 months in those remaining in the study at 1 year are shown in Table 3. After initial adjustments to treatment, both men (65%), and women (64%), were most commonly receiving 4 mg perindopril alone. Dual therapy was required in 13% of men and 12% of women. Data on therapy were incomplete or missing in 100 patients. Data were not available in 8% due to discontinuation in the study for any reason.
Table 3.
Treatment received during the final 6 months of the study.
A total of 43 245 patients completed the study and had assessable case records after 12 months, this being 92% of the original cohort. Of these cases at 1 year, 22 811 (53%) were women and 20 434 men (47%) were men; thus, of the original cohort, 91% of women and 93% of men could be evaluated at 1 year. The reasons for failure to complete the study are given in Table 4. In addition to the 3683 patients mentioned in Table 4, there were a further 325 patients in whom the computer data at 1 year were incomplete. However, serious adverse events were registered on these patients and were included in the analysis performed by FW.
Table 4.
Patient outcome according to sex.
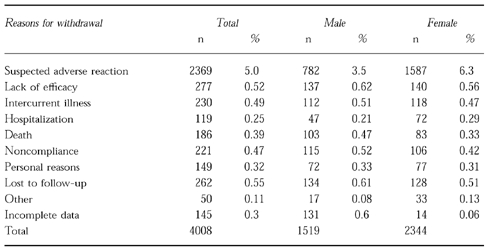
The commonest cause of failure to complete 1 year monitoring of treatment, was due to suspected adverse reaction to the drug and accounted for 2369 withdrawals. There were 1587 adverse reaction-related withdrawals among women in the study, an incidence over the year of 6.3%; and 782 (3.5%) among men. Intercurrent illness or hospitalization accounted for the withdrawal of 159 men and 200 women. There were 190 deaths (0.4%) during the course of the study. The remainder of those failing to complete 1 year's treatment monitoring were related to failure of follow-up for personal or administrative reasons.
Effects of treatment
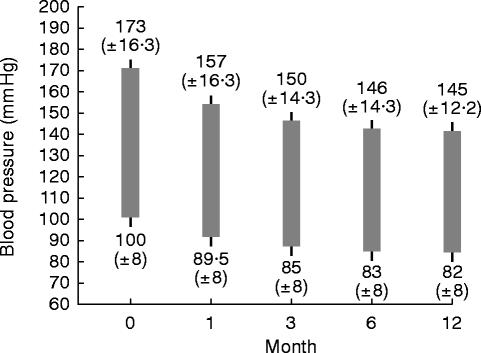
Figure 1 shows the fall in blood pressure over the 1 year period of treatment. The mean blood pressure fell from 173/100 s.d.±16.3/8 in 47 253 patients starting the study to 145/82 s.d.±12.2/8 in 46 799 patients who successfully completed 1 year's treatment (Figure 1). Lack of efficacy of treatment led to the withdrawal of 277 patients; lack of efficacy was equally common in men and women being 0.6% and 0.6%, respectively. It should be noted that 13% of patients were taking other antihypertensive medication in addition to perindopril.
Figure 1.
Mean blood pressure decrease over a 1 year period in 47 253 mild to moderately hypertensive patients treated with perindopril; at 12 months 43 245 were assessed of whom 13% required one other anti-hypertensive drug.
Suspected adverse reactions
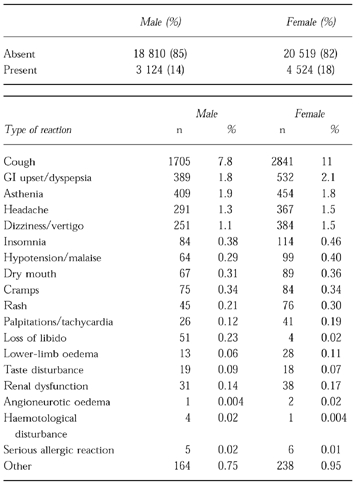
The overall recorded rate of suspected adverse reactions was 14.2% in men and 17.8% in women, as shown in Table 5. The incidence of cough was 8% in men and 11% in women. The other commonly described problems were dyspepsia, asthenia, headache, and dizziness or vertigo. Others were reported at an incidence below 1%. There were three cases of angioneurotic oedema, one in men and two in women. Other serious allergic reactions were reported in five men and six women.
Table 5.
Overall incidence of spontaneously reported clinical events.
Events leading to perindopril withdrawal
Suspected adverse reactions leading to the withdrawal of perindopril are given according to age and sex in Table 6a and 6b. There does not seem to be any trend with increasing age, with the obvious exception of reports of renal insufficiency, which in men and women over 80 years, was the second cause of drug toxicity-related withdrawal in this study.
Table 6a.
Selected suspected adverse drug reactions leading to withdrawal.
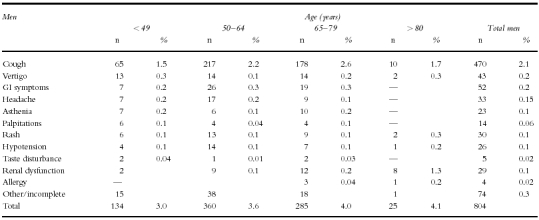
Table 6b.
Selected suspected adverse drug reactions leading to withdrawal.
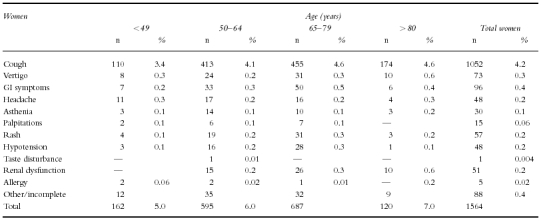
Cough
was responsible for the withdrawal of 1522 patients (3.2%) from the study, being a commoner cause of withdrawal in women, 1052 (4.2%) compared with men 470 (2.1%).
Hypotension
was given as the cause of drug intolerance in 74 cases. Women were affected somewhat more often than men, with 48 withdrawals (1.9 per thousand) compared with 26 withdrawals (1.2 per thousand) in men. There was no evidence of any increase in hypotension with age. A further 43 men and 73 women withdrew because of vertigo which may have been associated with hypotension. Since hypotension may be silent until manifesting itself by serious circulatory disorders, including cerebrovascular accident and cardiac ischaemia, all hospital admissions for cardiovascular disease occurring during the first month of perindopril treatment were reviewed. There were 18 cases, and in none of these was there any record of hypotension or diastolic blood pressure below 80 mmHg. There were 26 fatal and 24 nonfatal strokes. Hypotension was mentioned in relation to one nonfatal stroke. Of all these cases only one reported as hypotension occurred on the first day of dosing (and one on the second day) and four cases of dizziness occurred on the first day (and a further four on the second day).
Skin rashes
Eighty-seven patients withdrew because of skin rashes. Thirty men (0.14%) and 57 (0.23%) women were affected. The incidence tended to increase with age. Fifty of these reactions occurred in the first month of treatment and only three after 3 months. Only three of these were identified as serious urticarial reactions (these are included in the allergic responses described hereafter).
Renal function disturbances
A total of 80 cases were withdrawn because of renal function disturbances: 29 in men and 51 in women were affected. As can be seen in Tables 6a and 6b the incidence rises with age. None occurs below the age of 50 years and the incidence is highest in males over 80 years (1.3%). The most commonly reported changes were a rise in blood urea or creatinine not considered as being serious and reverting on discontinuation of the drug. Fourteen potentially serious cases were seen. Of these, three were found to be relatively mild elevations in creatinine (with a plasma creatinine level under 250 mmol l−1), and were rated ‘potentially serious’ because of hospital referrals. All of these cases resolved on discontinuation of treatment. Three patients had other factors which appeared to be the cause of the renal deterioration, these conditions being abdominal tumour, arteritis, and dehydration due to vertigo and vomiting. Three patients had documented chronic renal disease and were referred for haemodialysis. Three were found to have renal artery stenosis, the elevation in creatinine being found after 3–6 months of treatment. Two of these patients were successfully operated on. One patient suffered a decline in renal function which developed after the addition of a nonsteroidal anti-inflammatory drug and a potassium-sparing diuretic. One elderly male patient developed renal failure after 1 year's treatment and also suffered from diabetes and emphysema. There were no deaths from renal failure during the study.
Allergic reactions
Allergic reactions and angioneurotic oedema were reported in a total of nine patients, this being an overall incidence of 2 per 10 000. Angioneurotic oedema or severe allergic reactions were the cause of perindopril withdrawal in two men and one woman, this being an overall incidence of 0.006%. Of seven allergic reactions reported as serious the time from first exposure to allergic reaction was from 2 days to 6 months, with a median period of 2 weeks. The reaction at 6 months occurred 7 weeks after a dose increase. Two of these cases had previously experienced severe allergic reactions to other ACE inhibitors and, in these cases, the reactions occurred after 5 and 6 weeks on perindopril.
Haematological adverse reactions
Haematological adverse reactions were reported in only five patients, with four cases being serious enough to lead to drug withdrawal. Among these four cases, three had thrombocytopaenia and one had moderate pancytopaenia associated with arteritis, present before perindopril therapy was initiated. None resulted in a fatality.
Deaths
There were 186 fatalities reported during the study (0.4%), with 103 (0.5%) in men and 83 (0.3%) in women. A further four cases were found on medical review of the dropouts. Ninety-seven deaths were related to cardiovascular disease, 42 to neoplasia, 17 to trauma and 34 to a variety of other causes.
There were 27 deaths ascribed to myocardial infarction. Of these, 19 were in men (average age 67.7 years, range 52–89 years) and 8 were in women (average age 74.8 years, range 58–85 years). There were 26 stroke-related deaths: 14 in women and 12 in men. Men were aged 57–86 years, mean 74.8 years, and women were aged 66–86 years, mean 77.8 years. There were 16 deaths under the age of 60 years, five men and one woman dying of myocardial infarction, four men and three women dying of cancer, with a further seven early male deaths, five by accident and one further death from lymphoma and leukaemia (both predating therapy with perindopril).
Discussion
This study involving 4788 general practitioners in France was successful in following 43 245 patients, of 47 253 entered, who received treatment for a full year. While direct comparisons with previous studies on ACE inhibitors [2, 3] are difficult this probably represents the largest experience of an ACE-inhibitor in terms of patient-years follow-up. The study therefore provides information on serious adverse events in a large number of patients exposed to perindopril as well as assessment of potential laboratory abnormalities occurring during a full year on treatment.
As with previous large postmarketing surveillance studies on antihypertensive drugs, this study is an observational cohort study designed to detect unsuspected adverse reactions. Data on efficacy are incidental. However, in the overall context of the risk-benefit of therapy, it is important to select a dosing schedule that is both appropriate and efficacious. In this cohort, adequate control of blood pressure was achieved and mean blood pressure was reduced from 173/100 (s.d.±16.3/8) in 47 253 patients starting the study, to 145/82 (s.d.±12.2/8) in 46 799 patients who successfully completed 1 year treatment. The majority (80% of those starting) received perindopril alone. The high level of success found in the follow-up and treatment of this cohort may be explained by a number of factors; patients included were largely newly diagnosed mild to moderate hypertensives. Perindopril was not indicated for the treatment of cardiac failure at this time which may have favoured the selection of a relatively responsive population. Doctors were encouraged to incrementally increase the dose of perindopril and add diuretic therapy when required favouring continuance on perindopril.
Withdrawals due to suspected adverse reactions were reported in 2369 patients, an incidence of 5%, which is rather lower than that found in a previous study on captopril in which a withdrawal rate of 8.9% was found [2]. As found in the captopril study, withdrawals were more frequent in women than in men, but more markedly so, being 6.3%vs 3.5% in the perindopril study and 9.0%vs 7.0% in the captopril study, withdrawals being commoner in the elderly in both studies. These differences may be due to differences in the patient populations. Although both drugs were given for the treatment of hypertension, perindopril was indicated as a single agent, whereas captopril was licensed as a second-line add-on treatment at the time of the study, and more patients had had previous antihypertensive treatment in the captopril group: 38%vs 11%. However, the mean ages of the populations, 60.4 years in the captopril group and 60.9 years in the perindopril study, were similar, as was the blood pressure on entry: 180/105 mmHg and 173/100 mmHg, respectively.
It has been noted that the reported incidence of cough in association with treatment with ACE inhibitors is strongly related to ascertainment and trial design [4]. Postmarketing studies have tended to give low estimates of incidence of 0.2–1.1% [5–7]. Prescription-event monitoring found the reporting rate for dry cough for enalapril over a period of 12 months to be 2.9%. Studies specifically addressing the relationship between ACE inhibitors and cough suggest a true incidence of at least 10% [4, 8]. This study found an overall incidence of 11% in women and 8% in men (Table 5). The comparatively high reporting of this problem obtained in this postmarketing study tends to supports the relative bias favouring reporting of such well known associations as this. Reporting of adverse events is subject to bias from a variety of sources, particularly the existing recognition of a suspected adverse event at the time of the study. Minor events may or may not be recorded by individual practitioners and reporting is biased towards adverse reactions known or suspected of being drug-related. In this context the spontaneously reported data in Table 5 is biased towards the pattern of known or suspected adverse events related to ACE-inhibitors reflecting bias in reporting by the patient and recording by the doctor as previously noted by others [2]. Serious events, particularly life-threatening and fatal events, are subject to less bias in ascertainment and are discussed below.
It is noticeable that the reported incidence of idiosyncratic allergic reactions is low in this study. Eighty-seven patients were withdrawn because of rash (0.2%) and only six because of abnormality of taste. There were nine withdrawals due to apparent serious allergic reactions, and of these, only three were clearly angioneurotic oedema. Two of these cases occurred within a week, and the other occurred after 6 weeks’ therapy. Interestingly, the late case involved a patient who had previously developed a rash when taking enalapril, suggesting possible cross-sensitivity. This incidence of reported angioneurotic oedema is low compared with other ACE inhibitors: enalapril had a reported incidence of 0.1% [9] and captopril of 16 out of 67 698 patients exposed [2]. Neutropaenia has been reported to occur during treatment with captopril, particularly in patients with collagen-vascular disease [10]. While there was no exposure of this high-risk group in this study, four cases of blood dyscrasia were found: three cases of thrombocytopaenia and one with thrombocytopaenia and leucopaenia. There were no fatalities and the relationship to drug treatment was uncertain.
Secondary pharmacological problems such as hypotension and renal dysfunction are likely to be related to the patient population as, for example, high doses of loop diuretics in elderly patients with heart failure appear to predispose to renal dysfunction with ACE inhibitors [11]. However, the rate of withdrawal due to renal dysfunction was age-associated, rising to an incidence of 1.3% in males over 80 years of age. Even in an apparently fit hypertensive population, the risk of renal dysfunction in the elderly is appreciable.
Waller et al. from the UK Medicines Control Agency and members of the Committee on Safety of Medicines reviewed 31 postmarketing surveillance studies [12]. They concluded that the value of such studies is likely to be increased by increasing the sample size, by eliminating selection bias on recruitment, and the use of a comparator group.
Taking the last point first, this study lacked a comparator group. Examples of postmarketing studies which have included such a group are rare and may have the characteristics of large-scale open studies rather than postmarketing studies, with a lower number of patients recruited [13]. In a recent study which did manage to incorporate a control group of standard compared with innovatory treatment of asthma in large numbers of patients [14], it was notable that both drugs were made by the same manufacturer, enabling many of the logistical problems associated with this type of study to be overcome. Furthermore, that study involved a treatment period of only 16 weeks, appropriate for an antiasthmatic drug, whereas antihypertensive drug treatment is more appropriately followed over a longer period.
Recruitment bias may be impossible to banish completely from studies of relatively recently marketed drugs, and indications may be limited initially, thereby inevitably circumscribing the study's recruitment. Nevertheless, within the constraints of a population of patients with esssential hypertension, exclusions from this study concerned only recent unstable cardiovascular conditions, significant hepatic and renal disease and pregnant or lactating women. While this does introduce bias into the selection of the study population to some degree, it is difficult to widen the criteria significantly without raising ethical questions when studying a newly marketed drug. The lack of gratuitous exclusions, that is not excluding patients other than those outside in the licensed description of the product, is important. The absence of exclusion because of age made it possible to demonstrate significantly increased risks for certain problems in this group, particularly the relative frequency of renal dysfunction in the over 80 year olds.
This study was successful not only in recruiting, but also in following up of a large cohort of patients for 1 year. The study recruited rapidly and so had the (unproven) potential to act as a rapid alert. While confirming the feasibility of large-scale studies the experience of this study also confirms the difficulties noted by others [2] in the management of such large databases. The need to effectively manage large amounts of data should not distract attention from the paramount importance of small numbers of serious adverse events, and the value of the medical surveillance of each of these individual reports, as undertaken here, is emphasized.
This study on perindopril successfully followed a large cohort of patients and failed to demonstrate any unexpected hazards. The relatively high incidence of cough is likely to reflect a more successful ascertainment than earlier postmarketing studies of ACE inhibitors in hypertension. It is therefore unlikely that poor ascertainment in this study is the cause of the low reported rates of anaphylaxis and other idiosyncratic adverse reactions with this drug. While the absence of a comparator does not permit direct comparison with other agents, the low reported incidence of serious adverse reactions is itself reassuring.
References
- 1.Poggi L, Renucci JF, Denolle T. Treatment of essential hypertension in general practice: an open-label study of 47, 351 French hypertensive patients treated for one year with perindopril. Can J Cardiol. 1994;10(Suppl):21–24. [PubMed] [Google Scholar]
- 2.Chalmers D, Whitehead AD, Lawson DH. Postmarketing surveillance of captopril for hypertension. Br J Clin Pharmacol. 1992;34:215–223. doi: 10.1111/j.1365-2125.1992.tb04127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inman WHW, Rawson NHB, Wilton LV, Pearce GL, Speirs CJ. Postmarketing surveillance of enalapril. I. Results of prescription-event monitoring. Br Med J. 1988;297:826–829. doi: 10.1136/bmj.297.6652.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fletcher AE, Palmer AJ, Bulpitt CJ. Cough with angiotensin-converting enzyme inhibitors: how much of a problem? J Hypertens. 1994;12(suppl 2):43–47. [PubMed] [Google Scholar]
- 5.Coulter DM, Edwards IR. Cough associated with captopril and enalapril. Br Med J. 1987;294:1521–1523. doi: 10.1136/bmj.294.6586.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalmers D, Dombey SL, Lawson DH. Postmarketing surveillance of captopril (for hypertension): a preliminary report. Br J Clin Pharmacol. 1987;24:343–349. doi: 10.1111/j.1365-2125.1987.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoenberger JA, Testa M, Ross AD, Brennan WK, Bannon JA. Efficacy, safety, and quality-of-life assessment of captopril antihypertensive therapy in clinical practice. Arch Intern Med. 1990;150:301–306. [PubMed] [Google Scholar]
- 8.Yeo WW, Ramsay LE. Persistent dry cough with enalapril: incidence depends on method used. J Hum Hypertens. 1990;4:517–520. [PubMed] [Google Scholar]
- 9.Slater EE, Merrill DD, Guess HA, et al. Clinical profile of angioedema associated with angiotensin-converting enzyme inhibition. J Am Med Assoc. 1988;260:967–970. [PubMed] [Google Scholar]
- 10.Cooper RA. Captopril-associated neutropenia, who is at risk? Arch Intern Med. 1983;143:659–660. [PubMed] [Google Scholar]
- 11.Speirs CJ, Dollery CT, Inman WHW, Rawson NSB, Wilton LV. Postmarketing surveillance of enalapril. II. Investigation of the potential role of enalapril in deaths with renal failure. Br Med J. 1988;297:830–832. doi: 10.1136/bmj.297.6652.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waller PC, Wood SM, Langman MJS, Breckenridge AM, Rawlins MD. Review of company postmarketing surveilllance studies. Br Med J. 1992;304:1470–1472. doi: 10.1136/bmj.304.6840.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fallowfield JM, Blenkinsopp J, Faza A, Fowkes AG, Higgins TJ, Bridgman KM. Post-marketing surveillance of lisinopril in general practice in the UK. Br J Clin Pract. 1993;47:296–304. [PubMed] [Google Scholar]
- 14.Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatement. Br Med J. 1993;306:1034–1037. doi: 10.1136/bmj.306.6884.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]