Abstract
Sorivudine (1-β-d-arabinofuranosyl-E-5-[2-bromovinyl] uracil; BV-araU; SQ32,756) is an antimetabolite which is a synthetic analogue of thymidine. This drug has demonstrated antiviral activity against varicella zoster virus, herpes simplex type 1 virus, and Epstein-Barr virus. Clinical studies in Japan and subsequently worldwide showed this drug to be a potent agent for treating varicella zoster infections. Although in general well tolerated, a fatal drug interaction with fluoropyrimidine drugs was subsequently observed. While three deaths resulting from this interaction were recognized to have occurred during the initial clinical evaluation in Japan, the full impact of the interaction was not recognized in Japan until post-marketing when an additional 23 cases of severe toxicity were reported including 16 patients who subsequently died from fluoro-pyrimidine toxicity. Worldwide recognition of this potentially fatal drug-drug interaction led to subsequent disapproval in the US and elsewhere. The interaction has been shown to be due to suppression of 5-fluorouracil (5-FU) catabolism, resulting in higher levels of 5-FU than would normally be observed. The mechanism of this interaction is mediated through inhibition of the 5-FU rate-limiting catabolizing enzyme dihydropyrimidine dehydrogenase (DPD) by the BV-araU metabolite BVU. This drug-drug interaction of sorivudine and 5-FU further emphasizes the critical importance of DPD on the clinical pharmacology of 5-FU.
Keywords: sorivudine, 5-fluorouracil, drug interaction, dihydropyrimidine dehydrogenase
Introduction
Over the past few decades, there has been an awareness of the increasing frequency of drug-drug interactions [1]. Nevertheless, it is important to emphasize that physicians and pharmacists must continue to have a high degree of vigilance for the possibility of new drug-drug interactions. Patients today are typically treated with an ever increasing number of diverse agents from different therapeutic classes. Many of the interactions that occur are in fact secondary to drugs prescribed by different physicians who at times may be unaware of the drugs prescribed by other physicians. The interactions can be particularly severe when one of the two drugs has a relatively narrow therapeutic window and the second drug is able to alter the kinetics of the first drug.
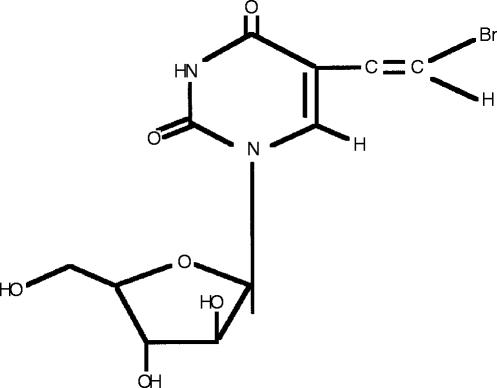
An illustrative example of a recent drug-drug interaction demonstrating several of these points is the interaction between the antiviral drug sorivudine (1-β-d-arabinofuranosyl-E-5-[2-bromovinyl] uracil; BV-araU; SQ32,756, Figure 1) and the cancer chemotherapy drug 5-fluorouracil (5-FU).
Figure 1.
Structure of sorivudine (1-β-d-arabinofuranosyl-E-5-[2-bromovinyl] uracil; BV-araU; SQ32,756).
Sorivudine
Sorivudine is a synthetic pyrimidine nucleoside antimetabolite drug. It derives its antiviral activity from selective conversion by a specific thymidine kinase present in certain DNA viruses to nucleotides which can in turn interfere with viral DNA synthesis [2]. This viral thymidine kinase is different from the thymidine kinase present in mammalian cells. Sorivudine is not a substrate for mammalian thymidine kinase, thus explaining the selectivity of this drug. Sorivudine has antiviral activity against several viruses including varicella zoster virus, herpes simplex type 1 virus, and Epstein-Barr virus [3]. Its antiviral activity is clinically significant with varicella zoster infections where it has been shown to be 3000 times more potent than the currrently indicated drug acyclovir [4]. In addition, it is attractive as a therapeutic agent in the clinic since the dosing needed is less frequent than with acyclovir [5].
Clinical interaction between sorivudine and 5-FU
It is now clear that even during the initial (pre-marketing) clinical evaluation of sorivudine in Japan, significant drug interactions occurred. Three deaths occurred during this time period with inadvertant coadministration of the 5-FU pro-drug Tegafur together with sorivudine. Following marketing in 1993 of sorivudine in Japan, another 23 cases of severe bone marrow depression were reported in patients who were started on sorivudine therapy while still receiving 5-FU or 5-FU pro-drugs. Sixteen of the 23 patients later died of 5-FU complications [6].
It is notable that no further interaction of sorivudine with 5-FU or 5-FU pro-drugs have been reported during evaluation in the US or Europe [7]. This has led some investigators to propose that the frequency of interactions in Japan may have been secondary to several unique factors in Japan including general availability of some fluoropyrimidine drugs including some without prescription, and perhaps lack of pharmacy safeguards to avoid drug interactions. Despite the lack of demonstrable drug-drug interactions in the US and the suggestions of possible therapeutic differences between Japan and elsewhere, sorivudine failed to win approval by regulatory agencies in the US and Europe.
Mechanism of interaction between sorivudine and 5-FU
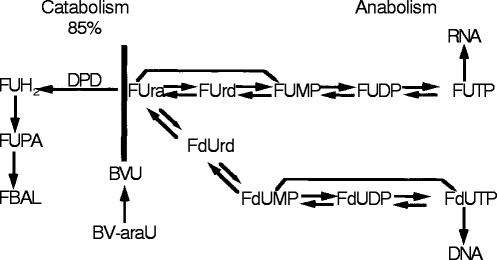
Sorivudine was hypothesized to suppress the catabolism of 5-FU resulting in higher levels of 5-FU [7]. The mechanism was thought to be mediated through conversion of sorivudine to bromovinyluracil (BVU), a known inhibitor of dihydropyrimidine dehydrogenase (dihydrouracil dehydrogenase, dihydrothymine dehydrogenase, uracil reductase, EC1.3.1.2, DPD). DPD is the initial rate-limiting enzymatic step in the catabolism of not only the naturally occurring pyrimidines uracil and thymine, but also of 5-FU [8, 9]. DPD occupies an important position in the overall metabolism of 5-FU converting over 85% of clinically administered 5-FU to 5-FUH2, an inactive metabolite, in an enzymatic step that is essentially irreversible (Figure 2). While anabolism is clearly critical in the conversion of 5-FU to the ‘active’ nucleotides FdUMP, FUTP, and FdUTP (which in turn can inhibit cell replication through inhibition of thymidylate synthase, or through incorporation into RNA or DNA respectively), catabolism controls the availability of 5-FU available for anabolism and thus occupies a critical position in the overall metabolism of 5-FU [8].
Figure 2.
Metabolic overview illustrating the critical position of DPD in the metabolism of 5-FU as well as the natural pyrimidines uracil and thymine. More than 85% of administered 5-FU is catabolized via DPD. (Abbreviations: FUH2—dihydrofluorouracil; FUPA—fluoro-ureidopropionic acid; FBAL—Fluoro-beta- alanine; FUrd—Fluorouridine; FUMP -Fluoro- uridine monophosphate; FUDP—Fluorouridine diphosphate; FUTP—Fluoro uridine triphosphate; FUdR—Fluorodeoxyuridine; FdUMP—Fluorodeoxyuridine monophosphate; FdUDP—Fluorodeoxyuridine diphosphate; FdUTP—Fluorodeoxyuridine triphosphate; BVU—bromovinyl uracil).
The actual effect of clinically relevant doses of sorivudine (40 mg orally four times a day for 10 days) on DPD has been evaluated in patients [7]. DPD was assessed directly by measuring DPD activity in peripheral blood mononuclear cells [10] as well as indirectly by measuring the plasma uracil. These levels were compared with drug (sorivudine) and metabolite (BVU) levels at the same time points. This study demonstrated that after a single dose of sorivudine, DPD was essentially completely inhibited. It remained inhibited throughout the 10 days on drug and returned to baseline levels in the majority of patients by 2 weeks, long after both sorivudine and BVU were no longer present [7]. These results suggest that patients receiving sorivudine are not only at risk to develop potentially life threatening toxicity from 5-FU or 5-FU prodrugs while receiving both drugs simultaneously but also for potentially 2 weeks after the last dose of sorivudine.
Importance of DPD for 5-FU pharmacology
It should be emphasized that DPD has a critical role in the clinical pharmacology of 5-FU that goes beyond the sorivudine interaction. This has been shown in several recent studies (Table 1) that demonstrate how DPD can influence the pharmacokinetics, bioavailability, toxicity, and antitumor effectiveness of 5-FU.
Table 1.
Importance of dihydropyrimidine dehydrogenase (DPD) in 5-fluorouracil (5-FU) pharmacology.
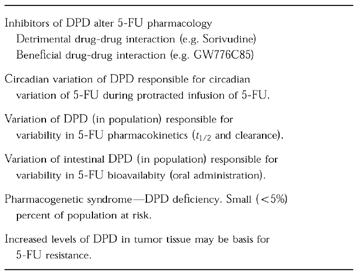
While the interaction of sorivudine with 5-FU is clearly undesireable, it should be noted that over the years there has been a concerted effort to intentionally develop inhibitors of DPD as a means of increasing the effect of 5-FU [11]. The rationale for developing these inhibitors was based on the early realization that it was essential for 5-FU to be anabolized to 5-FU nucleotides in order for antitumour activity to occur, while most of the administered 5-FU was catabolized [8]. Thus it was felt by experimental chemotherapists that it was desireable to inhibit catabolism to increase the anabolism and hence the antitumour effect. Unfortunately with many of the initial inhibitors (as with sorivudine), there was marked host toxicity. Recently there have been attempts to utilize new inhibitors of DPD to not only increase the anabolism of 5-FU and hence the antitumour activity, but also achieve desireable phamacological effects e.g. improving the bioavailability of 5-FU. One such example of a therapeutically useful inhibitor of DPD is ethynyluracil (GW 776C85) which has been shown to improve 5-FU efficacy and selected pharmacological effects (e.g. bioavailability) in both preclinical and early clinical studies [12]. Several additional DPD inhibitors are now being evaluated with 5-FU in clinical trials in an attempt to achieve similar effects.
DPD is known to follow a circadian pattern in both animals and humans [13–14]. Studies in patients receiving 5-FU infusion by automated pumps have demonstrated that the circadian variation of tissue DPD level is accompanied by an inverse circadian pattern in plasma 5-FU concentrations [15]. This has potential importance in the design of time-modified 5-FU infusions. Such regimens have been suggested to have potential benefit in the treatment of certain human cancers [16].
DPD enzyme activity in normal tissues (peripheral blood mononuclear cells and liver) has also been shown to vary from individual to individual in a Gaussian pattern with as much as a six fold variation from the lowest to the highest values [17–18]. This wide variation in DPD activity is likely responsible for the wide variation in the half-life observed in patients in population studies [19].
In addition to the variation of DPD activity in the normal population, it is clear now that an additional small percentage (<5%) of the population has DPD activity significantly below the Gaussian distribution that characterizes most of the population [20–21]. These individuals are at significant risk if they develop cancer and are given 5-FU. Thus, this is a true pharmacogenetic syndrome with symptoms not being recognized until exposure to the drug [22].
Variation in intestinal DPD activity has recently been shown to be responsible for the apparent variable bioavailabilty of orally administered 5-FU. The basis for the erratic bioavailability of 5-FU has not previously been understood, particularly since 5-FU is a small molecule with a pKa that should result in excellent absorption and bioavailability. Experimental studies in animals using DPD inhibitors have demonstrated that following inhibition of DPD the pharmacokinetic pattern resulting from oral administration of 5-FU is essentially the same as that produced by intravenous administration suggesting almost 100% bioavailability [23].
Tumours have been shown to express variable levels of DPD activity [24]. This may explain the observed varied tumour response to 5-FU. Thus tumours with high DPD levels are relatively resistant to 5-FU, while tumours with low levels of DPD are relatively sensitive to 5-FU.
References
- 1.Diasio RB. Principles of Drug Therapy. In: Bennett JC, Plum F, editors. Cecil Textbook of Medicine. 20. Philadelphia: W.B. Saunders Company; 1996. Chapter 5. [Google Scholar]
- 2.Suzatani T, Machida H, Sakuma T, Azuma M. Effects of various nucleosides on antiviral activity and metabolism of 1-β-d-arabino-furanosyl-E-5(2-bromovinyl) uracil against herpes virus types 1 and 2. Antimicrob Agents Chemother. 1988;32:1547–1551. doi: 10.1128/aac.32.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin JC, Machida H. Comparison of two bromovinyl nucleoside analogs, 1-β-d-arabinofuranosyl-E-5(2-bromovinyl) uracil and E-5 (2-bromovinyl)-2-deoxyuridine, with acyclovir in inhibition of Epstein-Barr virus replication. Antimicrob Agents Chemother. 1988;32:1068–1107. doi: 10.1128/aac.32.7.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machida H, Nishitani M. Drug susceptibilities of isolates of varicella-zoster virus in a clinical study of oral brovavir. Microbiol Immunol. 1990;34:407–411. doi: 10.1111/j.1348-0421.1990.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 5.Whitley RJ. Sorivudine: a promising drug for the treament of varicella-zoster virus infection. Neurology. 1995;45:73–75. doi: 10.1212/wnl.45.12_suppl_8.s73. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura H, Omori S, Kitada K, Mochida A. Prevention of drug interactions and its countermeasure. J Toxicol Sci. 1994;19:89–93. [PubMed] [Google Scholar]
- 7.Yan J, Tyring SK, McCrary MM, et al. The effect of sorivudine on dihydropyrimidine dehydrogenase activity in patients with acute herpes zoster. Clin Pharmacol Ther. 1997;61:563–573. doi: 10.1016/S0009-9236(97)90136-3. [DOI] [PubMed] [Google Scholar]
- 8.Daher GC, Harris BE, Diasio RB. The International Encyclopedia of Pharmacology and Therapeutics. Vol. 1. Oxford: Pergamon Press; 1994. Metabolism of pyrimidine analogues and their nucleosides, In: Metabolism and reactions of anticancer drugs. Chap. 2. [DOI] [PubMed] [Google Scholar]
- 9.Lu Z-H, Zhang R, Diasio RB. Purification and characterization of dihydropyrimidine dehydrogenase from human liver. J Biol Chem. 1992;267:17102–17109. [PubMed] [Google Scholar]
- 10.Johnson MJ, Yan J, Albin N, Diasio RB. Rapid Screening for Dihydropyrimidine Dehydrogenase (DPD) Deficiency, A Condition Associated with 5-Fluorouracil Toxicity. J Chromatography Biomed Applications. 1997;696:183–191. doi: 10.1016/s0378-4347(97)00253-3. [DOI] [PubMed] [Google Scholar]
- 11.Naguib FNM, eL Kouni MH, Cha S. Structure-activity relationship of ligands of dihydrouracil dehydrogenase from mouse liver. Biochem Pharmacol. 1989;38:1471–1480. doi: 10.1016/0006-2952(89)90187-1. [DOI] [PubMed] [Google Scholar]
- 12.Spector T, Porter DJT, Nelson DJ, et al. 5-Ethynyluracil (776C85), a modulator of the therapeutic activity of 5-fluorouracil. Drugs of the Future. 1994;19:566–571. [Google Scholar]
- 13.Harris BE, Song R, He Y, Soong S-J, Diasio RB. Circadian rhythm of rat liver dihydropyrimidine dehydrogenase: Possible relevance to fluoropyrimidine chemotherapy. Biochem Pharmacol. 1988;37:4759–4763. doi: 10.1016/0006-2952(88)90349-8. [DOI] [PubMed] [Google Scholar]
- 14.Harris BE, Song R, Soong S-J, Diasio RB. Circadian variation of 5-fluorouracil catabolism in isolated perfused rat liver. Cancer Res. 1989;49:6610–6614. [PubMed] [Google Scholar]
- 15.Harris BE, Song R, Soong SJ, Diasio RB. Relationship of dihydropyrimidine dehydrogenase activity and plasma 5-fluorouracil levels: evidence for circadian variation of 5-fluorouracil levels in cancer patients receiving protracted continuous infusion. Cancer Res. 1990;50:197–201. [PubMed] [Google Scholar]
- 16.Zhang R, Diasio RB. Pharmacologic Basis for Circadian Pharmacodynamics. In: Hrushesky WJM, editor. The Scientific Basis for Optimized Cancer Therapy. CRC Press; 1994. Chapter 4. [Google Scholar]
- 17.Lu Z, Zhang R, Diasio RB. Dihydropyrimidine dehydrogenase activity in human peripheral blood mononuclear cells and liver: population characteristics, newly identified patients, and clinical implication in 5-fluorouracil chemotherapy. Cancer Res. 1993;53:5433–5438. [PubMed] [Google Scholar]
- 18.Lu Z, Zhang R, Diasio RB. Dihydropyrimidine dehydrogenase activity in human liver: population characteristics and clinical implication in 5-FU chemotherapy. Clin Pharmacol Ther. 1995;58:512–522. doi: 10.1016/0009-9236(95)90171-X. [DOI] [PubMed] [Google Scholar]
- 19.Heggie GD, Sommadossi JP, Cross DS, Huster WJ, Diasio RB. Clinical pharmacokinetics of 5-fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;47:2203–2206. [PubMed] [Google Scholar]
- 20.Diasio RB, Beavers TL, Carpenter JT. Familial deficiency of dihydropyrimidine dehydrogenase: Biochemical basis for familial pyrimidinemia and Severe 5-fluorouracil-induced toxicity. J Clin Invest. 1988;81:47–51. doi: 10.1172/JCI113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris BE, Carpenter JT, Diasio RB. Severe 5-fluorouracil toxicity secondary to dihydropyrimidine dehydrogenase deficiency: a potentially more common pharmacogenetic syndrome. Cancer. 1991;68:499–501. doi: 10.1002/1097-0142(19910801)68:3<499::aid-cncr2820680309>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Diasio RB. Polymorphic Drug Metabolizing Enzymes. In: Schilsky RL, Milano GA, Ratain MJ, editors. Principles of antineoplastic drug development and pharmacology. New York: Marcel Dekker, Inc.; 1996. [Google Scholar]
- 23.Baccanari DP, Davis ST, Knick VC, Spector T. 5-Ethynyluracil: effects on the pharmacokinetics and antitumor activity of 5-fluorouracil. Proc Natl Acad Sci. 1993;90:11064–11068. doi: 10.1073/pnas.90.23.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W, Lu Z, He Y, Diasio RB. Dihydropyrimidine dehydrogenase activity in hepatocellular carcinoma; implication for 5-fluorouracil-based chemotherapy. Clin Cancer Res. 1997;3:395–399. [PubMed] [Google Scholar]