Abstract
Aims
The aim of the study was to investigate the pharmacokinetics of recombinant human interleukin-2 (rhIL-2) in patients with metastatic renal cell carcinoma following different subcutaneous (s.c.) administration regimens.
Methods
RhIL-2 was administered subcutaneously to 10 patients according to two different dosing regimens: group A received 20×106 IU m−2 once daily and group B 10×106 IU m−2 twice daily (every 12 h). Additionally, in all patients the influence of soluble interleukin-2 receptor (sIL-2R) on the pharmacokinetics of rhIL-2 was investigated.
Results
The mean area under the serum concentration-time curve to 24 h (AUC(0,24 h)) was 627 IU ml−1 h in treatment group A and 1130 IU ml−1 h (P=0.029) in treatment group B. In both study groups Cmax and AUC(0,12 h) were not significantly different. Seventy-two hours after the beginning of s.c. rhIL-2 therapy the sIL-2R increased significantly (P=0.016), and sIL-2R levels over 1200 pmol l−1 seemed to reduce the AUC.
Conclusions
In patients with metastatic renal cell cancer administration of 20×106 IU m−2 of rhIL-2 s.c. in two daily doses (10×106 IU m−2 every 12 h) provides better bioavailability and is preferable to the single dose administration.
Keywords: interleukin-2, pharmacokinetics, soluble interleukin-2 receptor, subcutaneous
Introduction
The use of recombinant human interleukin-2 (rhIL-2) has been recommended as the best current therapy for advanced renal cell carcinoma [1, 2]. RhIL-2 was found to exert its antitumour activity via indirect effects on the immune system, including the activation and expansion of cytotoxic T-lymphocytes and natural killer cells, and the secretion of secondary cytokines such as interferon-γ and TNF-α [3, 4]. The immune modulatory capacity has been described for the i.v. administration of high dose rhIL-2, which was associated with severe adverse effects, including capillary leak-related weight gain, hypotension, malaise, fever, and chills. Subcutaneous (s.c.) rhIL-2 at doses far below the maximum tolerable dose is therapeutically effective while treatment-related toxicity is reduced [5–7]. The pharmacokinetics of intravenously and intramuscularly administered rhIL-2 are well known [8–10], but only limited data on the pharmacokinetics after subcutaneous administration are available and information on the comparison of various dose regimens of s.c. rhIL-2 is lacking.
During systemic administration of rhIL-2 in humans, elevated soluble IL-2 receptor levels have been found [11]. The soluble IL-2 receptor (sIL-2R) differs from its membrane-bound counterpart with respect to size, binding capacity and ligand specificity. The soluble form of the human IL-2 receptor is a glycosylated protein with a molecular weight between 35 and 50 kDa [12]. It binds to IL-2 with low affinity (Kd: 10 nmol l−1) which is comparable with the affinity to the membrane bound α-chain of the IL-2 receptor [13]. The α-chain is expressed on activated T cells which combines with the β-and γ-chains to constitute a high-affinity IL-2R with a 1000-fold higher affinity to IL-2 than presented by the sIL-2R [14–17]. It has been hypothesized that the competition between the membrane-bound and the soluble receptor for IL-2 causes inhibition of IL-2 dependent mechanisms. However, so far, no data are available on the influence of sIL-2R on IL-2 pharmacokinetics.
The present study attempts to provide a more detailed analysis of the pharmacokinetics of subcutaneous rhIL-2 in patients with metastatic renal cell carcinoma comparing different dosing regimens. In addition, pharmacokinetic data of s.c. rhIL-2 were analysed in relation to different concentrations of the sIL-2 receptors.
Methods
Patients and treatment
The study cohort consisted of 10 patients (mean age 56±9 years) treated at our institution with histologically confirmed metastatic renal cell cancer in a clinically progressive stage; all patients had a Karnofsky performance status >70%. No chemotherapy or immune modulatory therapy was performed for at least 4 weeks prior to this protocol. After obtaining written informed consent patients were treated with s.c. rhIL-2 (Chiron, Emeryville, USA). Two different priming doses of rhIL-2 (1 IU=2.916 pg) were administered in the first week of therapy on 3 consecutive days: seven patients (mean age 58±8 years; mean body weight 71±11 kg) received 20×106 IU m−2 rhIL-2 s.c. once daily (group A) and three patients (mean age 50±10 years; mean body weight 69±12 kg) received 10×106 IU m−2 rhIL-2 s.c. twice daily at a 12 h interval (group B). This study was approved by the institutional ethical committee of the Medizinische Hochschule Hannover.
Sampling
Patients’ sera were obtained and stored at –20° C until analysis. The samples for rhIL-2 determination were collected immediately before (0 time) and 0.5, 1, 1.5, 2, 4, 6, 8, 10, 12, 14, 16, 20, 22 and 24 h after rhIL-2 s.c. injection. Additional sera were prepared for the determination of the soluble interleukin-2 receptor concentration prior to rhIL-2 injection and at least every 4 h during 24 h in all 10 patients. Concentrations of rhIL-2 and sIL-2R were determined in two patients of each treatment group during 3 days of treatment and in one patient of each treatment group during 2 days of treatment.
Interleukin-2 assay
To assay the serum concentration of rhIL-2 a commercial standard cytokine ELISA kit (Medgenix, Ratingen, Germany) was used and performed according to the manufacturer's guidelines. Standard samples, which were contained in the ELISA kit, were used. The lower limit of quantification was 0.5 IU ml−1. A three-run validation was performed to verify the precision and accuracy of the assay. The precision was expressed as the coefficient of variation (CV) of the measured concentrations. The within-run precision (n=5) for the assay at nominal concentrations of 10, 35 and 70 IU ml−1 was 3.6%, 5.7%, and 3.9%, respectively. The between-run precision of the assay at these concentrations was 6.1%, 7.5%, and 7.7%, respectively.
ELISA for soluble IL -2R (sIL -2R)
Soluble IL-2R levels were determined using a standard two-step sandwich assay (Immunotech, Marseille, France), as described previously in detail [18]. The amount of sIL-2R per sample was calculated by plotting the absorbance values against a sIL-2R standard curve. Normal donor values ranged from 25 to 115 pmol l−1 (1 pmol l−1=42 pg ml−1). The lower limit of quantification was 5 pmol l−1. The within-run precision was assessed by performing an analysis at three defined concentrations (25, 400, and 800 pmol l−1; n=5). The coefficient of variation was 6.6%, 4.9%, and 5.3%, respectively. The between-run precision of the assay at the above mentioned concentrations was 11.4%, 7.9%, and 9.5%, respectively.
Pharmacokinetic analysis
Compartment independent pharmacokinetic parameters of rhIL-2 were evaluated using the TopFit version 2.0 software [19]. The maximal concentration (Cmax), the corresponding time (tmax) and area under the curve (AUC(0,24 h)) were determined. The elimination half-time (t1/2,z) was calculated from the log-linear terminal slope (4–24 h in patients who received 20×106 IU m−2; and 4–12 h in patients who received 10×106 IU m−2 twice daily). For statistical analysis the Wilcoxon's rank sum test was used to compare both treatment regimens. Data are given as means±s.d.
Results
Subcutaneous administration of 20×106 IU m−2 rhIL -2 once daily
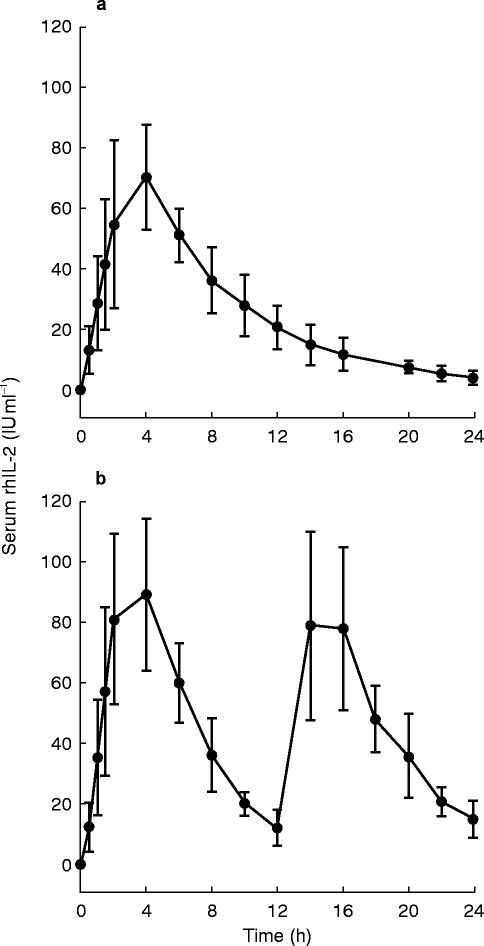
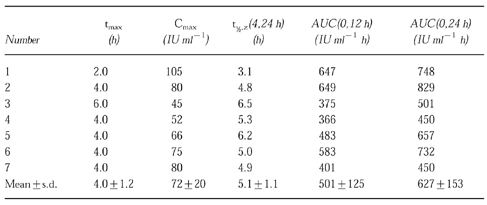
Seven patients of study group A were treated with s.c. administration of 20×106 IU m−2 once daily. The pharmacokinetic profile of the mean serum rhIL-2 concentration is presented in Figure 1(a). The pharmacokinetic parameters including tmax, Cmax, t1/2,z and AUC are summarized in Table 1. In group A the mean AUC(0,24 h)-value was 627+153 IU ml−1 h and the apparent harmonic mean t1/2-value 5.1+1.1 h. Mean Cmax was 72+20 IU ml−1 which was reached at a time of 4.0+1.2 h. The mean AUC value (AUC(0,12 h)) was 501+125 IU h ml−1 (Table 1).
Figure 1.
Pharmacokinetics of rhIL-2 in patients with metastatic renal cell cancer treated subcutaneously. Serum levels of rhIL-2 were measured with ELISA and are expressed as mean±s.d. (a) Mean levels in seven patients receiving 20×106 IU m−2 s.c. as single injection. (b) Mean levels in three patients receiving 10×106 IU m−2 s.c. twice every 12 h.
Table 1.
Pharmacokinetic parameters of a single injection of rhIL-2 at 20×106 IU m−2 s.c. in seven patients with metastatic renal cell cancer.
Subcutaneous administration of 10×106 IU m−2 rhIL -2 every 12 h (twice daily)
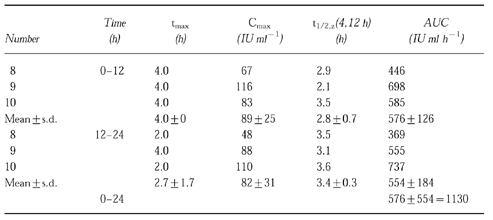
Patients of the treatment group B received 10×106 IU m−2 of rhIL-2 s.c. every 12 h (three patients). In group B Cmax of rhIL-2 in the first 12 h period amounted to 89±25 IU ml−1 at a tmax of 4.0±0 h. During the second 12-h period after the administration of 10×106 IU m−2 rhIL-2 the mean Cmax was 82±31 IU ml−1 at a tmax of 2.7±1.7 h (shown in Table 2). The mean AUC(0,12 h) of the first 12 h was 576±126 IU ml−1 h, that of the second 12 h period (AUC(12,24 h)) was 554±184 IU ml−1 h. Both mean AUC values (AUC(0,24 h)) added up to 1130 IU ml−1 h. The pharmacokinetic profile of the mean rhIL-2 concentrations is shown in Figure 1b.
Table 2.
Pharmacokinetic parameters of two consecutive injections of rhIL-2 at 10×106 IU m−2 s.c. every 12 h in patients with metastatic renal cell cancer.
Comparison of the pharmacokinetic data of both study groups
We investigated the influence of the dosage of rhIL-2 on the Cmax, tmax and AUC values after administration of single dose of 10×106 IU m−2 or 20×106 IU m−2 rhIL-2 s.c. 4.0 h after rhIL-2 administration tmax was reached in both study groups. The mean Cmax values of group A and group B (72 IU ml−1vs 89 IU ml−1) were not significantly (P=0.209) different. We also compared the mean AUC(0,12 h) values of both study groups (501 IU ml−1 h vs 576 IU ml−1 h) and found no significant difference (P=0.305). On the contrary, the AUC(0,24 h) following a twice daily administration of 10×106 IU m−2 rIL-2 was significantly (P=0.029) higher (nearly twice as high) than the AUC(0,24 h) after administration of (20×106 IU m−2) in one daily cumulative dose.
Soluble IL -2 receptor (sIL -2R)
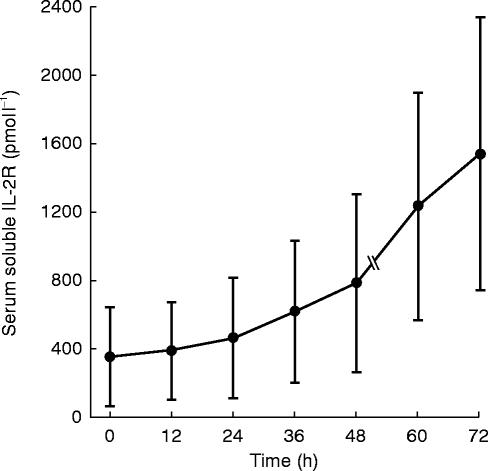
The serum concentrations of the sIL-2R of all 10 patients are shown in Figure 2. Prior to immunotherapy three patients exhibited normal sIL-2R levels (25–115 pmol l−1), while seven patients had elevated levels, which ranged from 182 to 828 pmol l−1. There was no significant difference between both study groups prior to the treatment (P=0.425), 24 h after the first injection of s.c. rhIL-2 (P=0.425) and 48 h after start of therapy (P=0.275). During rhIL-2 therapy the concentrations of the sIL-2R increased during the first 48 h continuously, but not significantly, in treatment group A from 286 pmol l−1 up to 614 pmol l−1 (P=0.237) and in treatment group B from 325 pmol l−1 up to 955 pmol l−1 (P=0.109). Three days after s.c. administration of rhIL-2 the sIL-2R seemed to increase distinctly (group A: from 286 pmol l−1 up to 1168 pmol l−1 and group B: from 325 pmol l−1 up to 1934 pmol l−1). The sIL-2R was measured only in two patients of each treatment group up to 3 days after the beginning of rhIL-2 therapy, therefore we could not calculate significance using the Wilcoxon test. When summarizing both study groups we could show that the sIL-2R increased significantly (P=0.016) 72 h after the beginning of rhIL-2 treatment.
Figure 2.
Serum concentrations (mean±s.d.) of soluble IL-2 receptor measured by ELISA during rhIL-2 therapy (n=6 until 48 h, n=4 until 72 h).
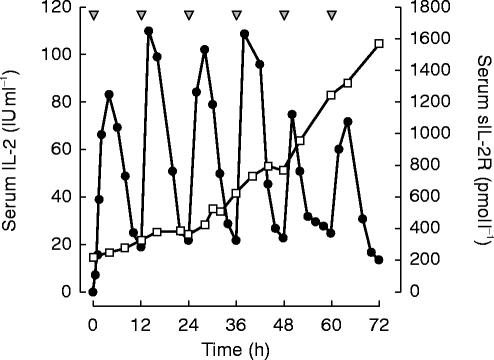
We also evaluated the influence of the increase of sIL-2R concentrations on the values of AUC(0,24 h). During the first 48 h after rhIL-2 administration, the increase of the sIL-2R had no apparent influence on the AUC values. After more than 48 h, there was a trend towards a decrease in AUC in those patients with sIL-2R concentrations over 1200 pmol l−1 (n=3), although this failed to reach statistical significance. The serum levels of sIL-2R and rhIL-2 of one typical patient who received 10×106 IU m−2 rhIL-2 s.c. are shown in Figure 3.
Figure 3.
Serum levels of rhIL-2 (•) and soluble IL-2 (□) receptor after subcutaneous application (10×106 IU m−2 s.c. every 12 h for 3 days, ▾) in one typical patient.
Discussion
In this study we have determined the pharmacokinetics of two different dose regimens of subcutaneous rhIL-2 administration in patients with metastatic renal cell cancer. Since ELISA assays have been employed, the current results do not necessarily correspond to the availability of biologically effective IL-2. We have shown for the first time that administration of 10×106 IU m−2 rhIL-2 s.c. twice daily (every 12 h) results in a significantly higher (P=0.029) total AUC(0,24 h) than s.c. administration of 20×106 IU m−2 once daily. Although pharmacokinetic parameters are best determined from i.v. bolus and infusion data there are several reports of IL-2 pharmacokinetics following i.m., i.p. and s.c. administration [8–10]. Until now no previous study described the pharmacokinetic data of different dose regimens of subcutaneous rhIL-2 administration. Only Ettinghausen et al. [20] demonstrated that intraperitoneal (i.p.) injection of rhIL-2 three times a day was more effective than the cumulative IL-2 dose administered daily i.p. or i.v.
Furthermore, we could show, that there was no significant difference between the Cmax (P=0.208) and AUC(0,12 h) (P=0.305) values after administration of a single dose of 10×106 or 20×106 IU m−2 rhIL-2 s.c. Gustavson et al. [10] reported that serum concentrations of rhIL-2 following i.v. administration (0.1–30×106 U) increased in an apparently dose-proportional manner. However, when administered s.c. (0.1–3.0×106 U), the increase in serum concentration was less than expected, which may have been due to a dose-dependent reduction in bioavailability for s.c. administered rhIL-2 [10]. We suggest that this effect is caused by an incomplete release of rhIL-2 from the subcutaneous injection site, hence, administration of doses higher than 10×106 IU m−2 rhIL-2 did not lead to significantly increased bioavailability. This observation is consistent with the results of other studies [21, 22] whereby recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) was administered s.c. in different doses. Stute et al. [21] described that Cmax and the AUC did not increase proportionally to the dose of s.c. rhGM-CSF; to explain this phenomenon a reduced absorption from the injection site was also hypothesized [21].
Our pharmacokinetic parameters t1/2=2.8–5.1 h and tmax=4.0 h are compatible with previous pharmacokinetic studies [9, 10]. Konrad et al. [9] used Cetus units (1 Cetus unit=6 IU); they reported after i.v. bolus administration of a median dose of 20×106 IU m−2 rhIL-2 a nearly two-fold higher AUC (2465 IU ml−1 h) when compared with the present AUC after s.c. rhIL-2 administration of 20×106 IU m−2 in two daily doses (10×106 IU m−2 every 12 h) in our study (AUC(0,24 h)=1130 IU h ml−1). Comparing s.c. with i.v. bolus administration, the peak levels after s.c. administration were more than 10–100 times lower than immediately after i.v. bolus rhIL-2, but were approximately constant for several hours before gradually decreasing. Therefore, the reported toxicity of i.v. rhIL-2 was much higher in comparison to the s.c. application route [9].
We found elevated sIL-2R levels in seven of 10 patients with renal cell carcinoma prior to rhIL-2 therapy. Elevated sIL-2R levels have been described earlier in patients with advanced renal cell carcinoma [23]. Furthermore, sIL-2R levels are increased in several diseases, mostly in those of infections [24, 25] or neoplastic character, like malignant melanoma, multiple myeloma, chronic myelogenous leukaemia [23, 26, 27].
After 3 days of s.c. rhIL-2 therapy the sIL-2R concentrations increased significantly (P=0.016). During the first 48 h after the start of rhIL-2 treatment the sIL-2R seemed to have no influence on the AUC values, because of the low sIL-2R concentrations. But in parallel to the increase of the sIL-2R concentrations over 1200 pmol l−1 a tendency toward reduced AUC amounts could be observed. A potential immune modulatory role of the sIL-2R has been discussed earlier [28], but not without controversy [29, 30]. The main objection against such a role was the low affinity (Kd: 10 nmol l−1) of the sIL-2R for IL-2, which is 1000-fold lower than the binding affinity of the membrane-bound heterotrimeric receptor complex (Kd: 0.01 nmol l−1) [16–18, 30–32]. Nevertheless, inhibition of IL-2 driven effects like proliferation of IL-2 dependent mouse CTLL cell line or inhibition of induction of cell-mediated cytotoxicity has been demonstrated by several investigators [30, 33–35].
In addition to the in vitro studies, in several clinical studies the putative physiological significance of sIL-2R has been investigated. During systemic administration of rIL-2 in humans, elevated sIL-2R levels have been observed earlier [15]. We described previously that during long-term s.c. rIL-2 treatment both soluble and cell surface IL-2R (CD25) exhibit a significant increase [15]. We could observe a positive correlation between the serum levels of sIL-2R and CD25 cell surface expression on peripheral blood lymphocytes. The quantitative correlation stated for soluble and membrane-bound IL-2R expression may be explained by its subsequent shedding without the transmembrane domain of the CD25 molecule [32].
The exact mechanism of the immune modulatory capacity of sIL-2R in vitro and in vivo remains still to be clarified. Here, we suggest two different possible mechanisms of immune modulation by the soluble IL-2R depending on its concentration. In vitro we could previously demonstrate that the neutralizing capacity for IL-2 driven immune responses (like CTLL-proliferation, cytotoxicity to malignant cell lines) is dose-dependent for IL-2R concentrations up to 100 pmol l−1 and is not due to a direct functional interaction between sIL-2R and free IL-2; at this lower concentration, an interaction of sIL-2R with the membrane-bound IL-2R seemed to be possible [18].
Our present in vivo data showed a trend towards decreasing AUC(0,24 h) values for sIL-2R concentrations higher than 1200 pmol l−1. Because of the low affinity of sIL-2R in comparison to the membrane-bound IL-2R, a direct interaction between the sIL-2R and free nonbound IL-2 seemed to be possible only at higher concentrations of the sIL-2R.
In conclusion, the pharmacokinetics of s.c. rhIL-2 in patients with metastatic renal cell carcinoma can be summarized as follows:
[A] Split s.c. injection (every 12 h) of rhIL-2 results in a significantly (P=0.029) higher total AUC(0,24h) than corresponding single dose administered once daily. [B] There were no significant differences between the Cmax and AUC(0,12h) levels after single s.c. administration of 10×106 and 20×106 IU m−2 of rhIL-2, respectively. [C] AUC(0,24h) following s.c. administration of rhIL-2 in two daily doses was comparable with AUC(0,24h) reported after i.v. bolus rhIL-2. [D] The sIL-2R increased significantly (P=0.016) during treatment with rhIL-2, and soluble IL-2R levels over 1200 pmol l−1 seemed to cause a decrease of AUC(0,24h).
References
- 1.Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 2.Atzpodien J, Körfer A, Franks C, Poliwoda H, Kirchner H. Home therapy with recombinant interleukin-2 and interferon-α2b in advanced human malignancies. Lancet. 1990;335:1509–1512. doi: 10.1016/0140-6736(90)93039-r. [DOI] [PubMed] [Google Scholar]
- 3.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lotze MT, Grimm EA, Mazumder EA, Strausser JL, Rosenberg SA. Lysis of fresh and cultured autologous tumour by human lymphocytes cultured in T-cell growth factor. Cancer Res. 1981;41:4420–4425. [PubMed] [Google Scholar]
- 5.Atzpodien J, Körfer A, Evers P, et al. Low-dose subcutaneous recombinant interleukin-2 in advanced human malignancy: a phase II outpatient study. Mol Biother. 1990;2:18–26. [PubMed] [Google Scholar]
- 6.Atzpodien J, Poliwoda H, Kirchner H. Alpha-interferon and interleukin-2 in renal cell carcinoma: studies in nonhospitilized patients. Sem Oncol. 1991;18:108. [PubMed] [Google Scholar]
- 7.Sleijfer DT, Janssen RA, Buter J, de Vries EG, Willemse PH, Mulder NH. Phase II study of subcutaneous interleukin-2 in unselected patients with advanced renal cell cancer on a outpatient basis. J Clin Oncol. 1992;10:1119. doi: 10.1200/JCO.1992.10.7.1119. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JA, Lee DJ, Cox WW, et al. Recombinant interleukin 2 toxicity, pharmacokinetics, and immunomodulatory effects in a phase I trial. Cancer Res. 1987;47:4202–4207. [PubMed] [Google Scholar]
- 9.Konrad MW, Hemstreet G, Hersh EM, et al. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 1990;50:2009–2017. [PubMed] [Google Scholar]
- 10.Gustavson LE, Nadeau RW, Oldfield NF. Pharmacokinetics of Teceleukin (recombinant human interleukin-2) after intravenous or subcutaneous administration to patients with cancer. J Biol Res Modif. 1989;8:440–449. [PubMed] [Google Scholar]
- 11.Voss SD, Hank JA, Nobis CA, Fisch P, Sosman JA, Sondel PM. Serum levels of low affinity interleukin-2 receptor molecule (TAC) during IL-2 therapy reflect systemic lymphoid mass activation. Cancer Immunol Immunother. 1989;29:261–269. doi: 10.1007/BF00199214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin LA, Kurmann CC, Fritz ME, et al. Soluble interleukin-2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135:3172–3177. [PubMed] [Google Scholar]
- 13.Smith KA. Interleukin-2: inception, impact and implications. Sci. 1988;240:1170–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 14.Lassalle P, Sergant M, Delneste Y, Gosset P, Wallaert B, Zandecki M. Levels of soluble IL-2 receptor in plasma from asthmatics. Correlations with blood eosinophilia, lung function, and corticosteroid therapy. Clin Exp Immunol. 1992;87:266–271. doi: 10.1111/j.1365-2249.1992.tb02986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Hänninen E, Körfer A, Hadam M, et al. Biological monitoring of low-dose interleukin-2 in humans: soluble interleukin. 2 receptors, cytokines, and cell surface phenotypes. Cancer Res. 1991;50:6312–6316. [PubMed] [Google Scholar]
- 16.Waldmann T. The IL-2/IL-2-receptor system: a target for rational immune intervention. Immunol Today. 1993;14:264–270. doi: 10.1016/0167-5699(93)90043-K. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi M, Yoshiaki N, Russel SM, et al. Interleukin-2 receptor gamma-chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 18.Zorn U, Dallmann I, Grosse J, Kirchner H, Poliwoda H, Atzpodien J. Soluble interleukin-2 receptors abrogate IL-2 induced activation of peripheral mononuclear cells. Cytokine. 1994;6:358–364. doi: 10.1016/1043-4666(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel G, Woloszczak R, Thomann P. In: TopFit Version 2.0, Pharmacokinetic and pharmacodynamic data analysis system for the PC. Karl Thomae Stuttgart, Fischer G., editors. New York: p. 1993. [Google Scholar]
- 20.Ettinghausen SE, Rosenberg SA. Immunotherapy of murine sarcomas using lymphokine activated killer cells: optimisation of the schedule and route of administration of recombinant interleukin-2. Cancer Res. 1986;46:2784–2792. [PubMed] [Google Scholar]
- 21.Stute N, Furman WL, Schell M, Evans WE. Pharmacokinetics of recombinant human granulocyte-macrophage colony-stimulating factor in children after intravenous and subcutaneous administration. J Pharm Sci. 1995;84:824–828. doi: 10.1002/jps.2600840708. [DOI] [PubMed] [Google Scholar]
- 22.Stute N, Santana VM, Rodman JH, Schell MJ, Ihle JN, Evans WE. Pharmacokinetics of subcutaneous recombinant human granulocyte-macrophage colony-stimulating factor in children. Blood. 1992;79:2849–2854. [PubMed] [Google Scholar]
- 23.Ostenstad B. Soluble interleukin-2 receptor levels in patients with malignant melanoma and renal cell cancer. Acta-Oncol. 1992;31:413–415. doi: 10.3109/02841869209088281. [DOI] [PubMed] [Google Scholar]
- 24.Mueller AR, Platz KP, Wiehe I, et al. Cytokine pattern in patients with infections after liver transplantation. Transpl Int. 1996;1(Supplement):126–131. doi: 10.1007/978-3-662-00818-8_32. [DOI] [PubMed] [Google Scholar]
- 25.Kaden J, Schutze B, May G. A critical analysis of soluble interleukin-2 receptor levels in kidney allograft recipients. Transpl Int. 1996;9(Suppl 1) doi: 10.1007/978-3-662-00818-8_17. [DOI] [PubMed] [Google Scholar]
- 26.Filella X, Blade J, Guillermo AL, Molina R, Rozman C, Ballesta AM. Cytokines (IL-6, TNF-alpha, IL-1alpha) and soluble interleukin-2 receptor as serum tumour markers in multiple myeloma. Cancer Detect Prev. 1996;20(1):52–56. [PubMed] [Google Scholar]
- 27.Kawatani T, Endo A, Tajima F, Ooi S, Kawasaki H. Clinical significance of serum soluble interleukin-2 receptor in chronic myeloproliferative disorders. Int J Hematol. 1997;65:123–128. doi: 10.1016/s0925-5710(96)00554-3. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez-Botran R. Soluble cytokine receptors: their role in immunoregulation. FASEBJ. 1991;5:2567–2574. doi: 10.1096/fasebj.5.11.1868981. [DOI] [PubMed] [Google Scholar]
- 29.Jacques Y, Le Mauff B, Boeffard F, Godard A, Soulillou JP. A soluble interleukin-2 receptor produced by a normal alloreactive human T cell clone binds interleukin-2 with low affinity. J Immunol. 1987;139:2308–2316. [PubMed] [Google Scholar]
- 30.Pizzolo G, Vincenzi C, Vinante F, et al. Highly concentrated urine-purified tac peptide fails to inhibit IL-2-dependent cell proliferation in vitro. Cell Immunol. 1992;141:253–259. doi: 10.1016/0008-8749(92)90144-e. [DOI] [PubMed] [Google Scholar]
- 31.Kondo N, Kondo S, Shimizu A, Honjo T, Hamuro J. A soluble ‘Anchorminus’ interleukin-2 receptor suppress in vitro interleukin-2 mediated immune response. Immunol Lett. 1988;19:299–308. doi: 10.1016/0165-2478(88)90159-9. [DOI] [PubMed] [Google Scholar]
- 32.Josimovicz-Alasevic O, Herrmann T, Diamantstein T. Demonstration of two distinct forms of released low-affinity type interleukin-2 receptors. Eur J Immunol. 1988;18:1855–1857. doi: 10.1002/eji.1830181133. [DOI] [PubMed] [Google Scholar]
- 33.Chilosi M, Semenzato G, Cetto A, et al. Soluble interleukin-2 receptors in the sera of patients with hairy cell leukemia: relationship with the effect of recombinant α-interferon therapy on clinical parameters and natural killer in vitro activity. Blood. 1987;70:1530–1535. [PubMed] [Google Scholar]
- 34.Symons JA, Wood NC, Di Giovine FS, Duff GW. Soluble IL-2 receptor in rheumatoid arthritis. Correlation with disease activity, IL-2 and IL-2 inhibition. J Immunol. 1988;141:2612–2618. [PubMed] [Google Scholar]
- 35.Treiger BF, Leonard WJ, Svetlik P, Rubin LA, Nelson DE, Greene WC. A secreted form of the human interleukin-2 receptor encoded by an ‘anchor minus’ cDNA. J Immunol. 1986;136:4099–4105. [PubMed] [Google Scholar]