Abstract
Aims
To determine the induction effect of rifampicin on the activity of 4′-hydroxylase in poor metabolizers (PMs) with m1 mutation of S-mephenytoin 4′-hydroxylation and the relationship of the effect with gene dose.
Methods
Seven extensive metabolizers (EMs) of S-mephenytoin 4′-hydroxylation and five PMs with m1 mutation were chosen to take rifampicin 300 mg day−1 orally for 22 days. Prior to and after rifampicin treatment, each subject was given racemic mephenytoin 100 mg. The 4′-hydroxymephenytoin (4′-OH-MP) excreted in the 0–24 h urine and mephenytoin S/R ratio in the 0–8 h urine were determined by h.p.l.c. and GC, respectively.
Results
In all EMs, the excretion of 4′-OH-MP in the 0–24 h urine was increased by 146.4±17.9%, 0–8 h urinary mephenytoin S/R ratio was decreased by 77.3±8.8%, the percentage increase in the 0–24 h excretion of 4′-OH-MP in those CYP2C19 homozygous (wt/wt) was greater than that in those heterozygous (wt/m1 and wt/m2 ) (203.9±42.5%vs 69.6±4.1%). 0–8 h urinary mephenytoin S/R ratio of those PMs with m1 mutation was decreased by 9.6%, the amount of 4′-OH-MP excreted in the 0–24 h urine was increased by 80.1±48.0%.
Conclusions
The activity of 4′-hydroxylase of PMs with m1 mutation of S-mephenytoin 4′-hydroxylation can be induced by rifampicin and the inducing effect of rifampicin on 4′-hydroxylase is gene dependent.
Keywords: S-mephenytoin, rifampicin, 4′-hydroxylase, m1 mutation, gene
Introduction
The enzyme (CYP2C19) that 4′-hydroxylates S- mephenytoin (S-MP) is a genetically polymorphic cytochrome P450. Based on the activity of CYP2C19, individuals are phenotyped as extensive metabolizers (EM) and poor metabolizers (PM) [1]. S-MP is extensively metabolized in EMs; this results in a lower S/R ratio measured in EMs than that in PMs. The PM phenotype is inherited as a Mendelian autosomal recessive trait involving two alleles at a single gene locus [2, 3]. Recently, two genetic mutations (m1 and m2 ) have been found to result in PM of S-MP [4, 5]. The m1 mutation, caused by a single base pair (G→A) mutation in exon 5 corresponding to base pair 681 of the cDNA, creates an aberrant splice site. This defect accounts for 75–85% of Caucasian and Japanese PMs. The m2 mutation consisting of a G→A transition at base 636 has been found only in Oriental populations [6]. It is well known that some of the enzymes in P4502C subfamily (including CYP2C19) can be induced in both animals and humans; also, chronic use of mephenytoin causes autoinduction [7]. Treating EMs and PMs of S-MP with rifampicin, using mephenytoin as a probe, we previously reported that CYP2C19 activity was inducible in EMs but not in PMs [8]. Recently we found that some of the PMs of S-MP whose genotypes were defined as m1/m1 or m1/m2 excreted as much as 8% of the dose as 4′-OH-MP. In addition, the S/R mephenytoin ratio of those m1 mutation heterozygous was lower than that of m2 mutation heterozygous [9]. These data suggest that the activity of 4′-hydroxylase of those heterozygous with m1 mutation is different from that of heterozygous with m2 mutation. Moreover, the mephenytoin S/R ratio of the heterozygous (wt/m1 and wt/m2 ) is slightly higher than that of homozygous (wt/wt), indicating that genetics affects the 4′-hydroxylation of S-MP [10]. Therefore, we hypothesize that the activity of 4′-hydroxylase in PMs with m1 mutation may not be lost completely and consequently its activity in these PMs should be inducible and the induction may be related to gene dose. The study was designed to test this hypothesis.
Methods
Seven male Chinese EMs of S-MP and five PMs with m1 mutation whose genotype was defined in previous studies, aged 19 to 22 (21±1 years, mean±s.d.), weighing 54 to 75 kg (58.9±6.2 kg) were enrolled in the study. No subject had had recent illness, and none had taken drugs for at least 2 weeks prior to or during the study. No subject had any abnormalities on physical examination or any biochemical evidence of renal or hepatic disorders. Four of the EMs were genotyped as homozygous (wt/wt), three as heterozygous (two as wt/m1 and one as wt/m2 ); four of the PMs were genotyped as m1/m1, one as m1/m2. The study protocol was approved by the Ethics Committee of Hunan Medical University and written consent was obtained from the subjects.
After an overnight fast, all the subjects took 100 mg racemic mephenytoin (Mesantoin®, Sandoz Pharmaceuticals, Inc.). 0–8 h and 8–24 h urine were collected and the volume was measured. Urine samples were stored at −15° C until assayed. From the second day on, every subject received 300 mg rifampicin (Rifampicin Capsule, 150 mg/capsule, pitch number: 950106, Xinyang Pharmaceuticals of Henan Province, PRC) daily for 22 days and racemic mephenytoin was again administered along with the final dose of rifampicin, urine samples were collected and stored in the same way as mentioned above.
The 0–8 h urinary mephenytoin S/R ratio was measured by gas chromatography [11]. The amount of 4′-OH-MP excreted in the 0–8 h and 8–24 h urine was determined by high performance liquid chromatography [12].
The data were analyzed by paired t-test and rank sum test with P<0.05 as the minimal level of statistical significance.
Results
Treatment with rifampicin decreased the 0–8 h urinary S/R-MP ratio and increased the excretion of 4′-OH-MP in the 0–24 h urine in all EMs. The 0–8 h urinary S/R-MP ratio was decreased by 77.3±8.8% (P<0.01) and the excretion of 4′-OH-MP in the 0–24 h urine was increased by 146.4±17.9% (P<0.01) (Figure 1). Taking into account of the genotype, the percentage increase in the 0–24 h excretion of 4′-OH-MP in CYP2C19 homozygous for wt (203.9±42.5%) was significantly greater than that in heterozygous for either one m1 or one m2 (69.6±4.1%, P<0.05) (Table 1).
Figure 1.
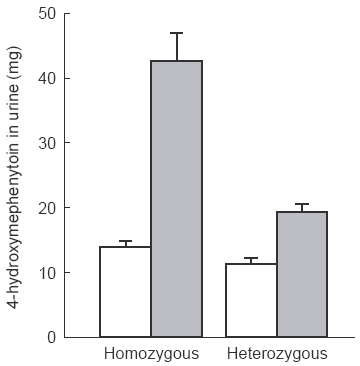
Comparison of changes in the 0–24 h urinary excretion of 4′-OH-MP between homozygous (wt/wt) and heterozygous (wt/m1 and wt/m2) EMs (P<0.05, homo- vs hetero-). Mean data, vertical bars s.d. □ pre-rifampicin,  post-rifampicin.
post-rifampicin.
Table 1.
The effect of rifampicin on the 0–8 h urinary mephenytoin S/R ratio and 0–24 h urinary excretion of 4′-OH-MP in the homo- and heterozygous of EMs and PMs with m1 mutation. Results are expressed as mean±s.d.
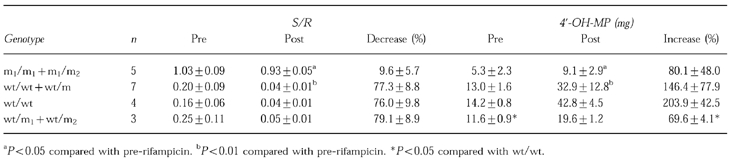
After treatment with rifampicin, 0–8 h urinary mephenytoin S/R ratio in PMs with m1 mutation was decreased by 9.6±5.7% (P<0.05), and the amount of 4′-OH-MP excreted in the 0–24 h urine was increased by 80.1±48.0% (P<0.05).
The 0–8 h urinary S/R-MP ratio in EMs was lower than that in PMs and the 0–24 h excretion of 4′-OH-MP was higher in EMs than that in PMs whether rifampicin was treated or not.
Discussion
Rifampicin is a potent unspecific inducer of hepatic P450 oxidative enzymes. When 300–600 mg rifampicin was given daily for 2–4 weeks, the half-life of most drugs was reduced [3]. In the study of rifampicin interacting with propranolol, Branch et al. [14] found that after 3 weeks medication of rifampicin and propranolol, further increases in the dose of rifampicin did not cause a further increase in enzymatic induction. Therefore, we dosed subjects with rifampicin for 22 days to obtain the maximal inducing effect.
The fact that gene dose has an effect on drug metabolism has been reported previously. Broly et al. [15] described that MR of heterozygous EMs of CYP2D6 is higher than that of homozygous EMs. We also found that gene dose slightly affects the metabolism of S-MP [9, 10]. In this study, we demonstrated that 0–24 h urinary excretion of 4′-OH-MP in the homozygous was significantly more than that in the heterozygous, showing a gene dose effect on the metabolism of S-MP. The present study indicates that the amount of 4′-OH-MP excreted in the 0–24 h urine in homozygous for wt was increased by 203.9±42.5%, while that of heterozygous for one either m1 or m2 was only increased by 69.6±4.1%. These data suggest for the first time that the induction of rifampicin on 4′-hydroxylase is gene dose dependent.
In this study, we also found that 0–8 h urinary S/R-MP ratio in PMs with m1 mutation was decreased by 9.6±5.7%, the amount of 4′-OH-MP excreted in the 0–24 h urine was increased by 80.1±48.0%. This shows that the activity of 4′-hydroxylase in PMs with m1 mutation can be induced by rifampicin. Since m1 mutation results in functionally inactive CYP2C19 in PMs, the induced 4′-hydroxylase should be a non-CYP2C19 4′-hydroxylase. Goldstein & de Marais [16] reported that in recombinant human CYP2C yeast microsomes, though the rate of 4′-hydroxylation of S-MP by CYP2C9 was low, it was detectable. It is not certain whether CYP2C9 is active in these PMs with m1 mutation, and thus can be induced by rifampicin or whether the enzymatic activity of CYP2C9 in PMs with m1 mutation is different from that in PMs with m2 mutation. The results from this study are not consistent with data reported previously [8] in which rifampicin treatment had little effect on mephenytoin S/R ratio and urinary excretion of 4′-hydroxymephenytoin in PMs. A possible explanation for this discrepancy is that in those PMs, m1 mutation may not be the predominant mutation.
In conclusion, results of this study show that the 4′-hydroxylase in PMs with m1 mutation can be induced by rifampicin, the inducing effect of rifampicin on 4′-hydroxylase is gene dose dependent.
Acknowledgments
This work was supported by the National Natural Science Foundation of China grant 39330230 and by China Medical Board grant 92-568.
References
- 1.Kupfer A, Desmond P, Patwardham R, Schenker S, Branch RA. Mephenytoin hydroxylation deficiency: kinetics after repeated doses. Clin Pharmacol Ther. 1984;35:33–39. doi: 10.1038/clpt.1984.5. [DOI] [PubMed] [Google Scholar]
- 2.Inaba T, Jurima M, Kalow W. Family studies of mephenytoin hydroxylation deficiency. Am J Hum Gen. 1986;38:768–772. [PMC free article] [PubMed] [Google Scholar]
- 3.Ward SA, Goto F, Nakamura K, Jacqz E, Wilkinson GR, Branch RA. S-Mephenytoin 4-hydroxylase is inherited as an autosomal-recessive trait in Japanese families. Clin Pharmacol Ther. 1987;42:96–99. doi: 10.1038/clpt.1987.114. [DOI] [PubMed] [Google Scholar]
- 4.de Morais SMF, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-Mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 5.de Morais SMF, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of S-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594–598. [PubMed] [Google Scholar]
- 6.Goldstein JA, Faletto MB, Romkes-Sparks M, et al. Evidence that CYP2C19 is the major (S)-mephenytoin 4′-hydroxylase in humans. Biochemistry. 1994;33:1743–1752. doi: 10.1021/bi00173a017. [DOI] [PubMed] [Google Scholar]
- 7.Wedlund PJ, Aslanian WS, Jacqz E, McAllister CB, Branch RA, Wilkinson GR. Phenotypic differences in mephenytoin pharmacokinetics in normal subjects. J Pharmacol Exp Ther. 1985;234:662–669. [PubMed] [Google Scholar]
- 8.Zhou HH, Anthony LB, Wood AJJ, Wilkinson GR. Induction of polymorphic 4′-hydroxylation of S-mephenytoin by rifampicin. Br J Clin Pharmacol. 1990;30:471–475. doi: 10.1111/j.1365-2125.1990.tb03799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Morais SMF, Goldstein JA, Xie HG, et al. Genetic analysis of the S-mephenytoin polymorphism in a Chinese population. Clin Pharmacol Ther. 1995;58:405–411. doi: 10.1016/0009-9236(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 10.Xiao ZS, Xie HG, He N, Huang SL, Xu ZH, Zhou HH. The effect of gene dose on the activity of S-mephenytoin hydroxylase. J Clin Med. 1996;76:389–390. [Google Scholar]
- 11.Huang SL, Xie HG, Wang W, Xu ZH, Jiang CH, Zhou HH. Application of gas chromatography to determine the concentration of S-mephenytoin and R-mephenytoin in human urine. Chromatography. 1997;15:277–279. [Google Scholar]
- 12.Xie HG, Huang SL, Zhou HH. High-performance liquid chromatographic determination of urinary 4′-hydroxymephenytoin, a metabolic marker for the hepatic enzyme CYP2C19, in humans. J Chromatography. 1995. pp. 1–7. [DOI] [PubMed]
- 13.Shawn MB, Anne MB, Timothy HS. Update on rifampicin drug interaction II. Arch Intern Med. 1992;152:711–716. [PubMed] [Google Scholar]
- 14.Branch RA, Herman RJ. Enzyme induction and β-adrenergic receptor blocking drugs. Br J Clin Pharmacol. 1984;17:77S–84S. doi: 10.1111/j.1365-2125.1984.tb02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broly F, Gaedigk A, Heim M, Eichelbaum M, Morike K, Meyer UA. Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA and Cell Biology. 1991;10:545–558. doi: 10.1089/dna.1991.10.545. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein JA, de Marais SMF. Biochemistry and molecular biology of the human CYP2C subfamily. Pharmacogenetics. 1994;4:285–299. doi: 10.1097/00008571-199412000-00001. [DOI] [PubMed] [Google Scholar]


