Abstract
Aims
Postoperative nausea and vomiting (PONV) may be exacerbated by postoperative opioid analgesics and may limit patients’ successful use of these medications when used with patient controlled analgesia (PCA). We tested the hypothesis that the rapid change in blood morphine concentration associated with PCA bolus delivery contributed to PONV, and that prolonging its delivery to a brief infusion would result in decreased PONV.
Methods
Patients, who were receiving morphine for pain relief via patient-controlled analgesia (PCA) after total abdominal hysterectomy, received 1 mg morphine sulphate incremental doses either over 40 s with a 5 min lockout interval or over 5 min delivery with a 1 min lockout interval. Episodes of nausea, retching and vomiting, along with the use of morphine and the pain relief obtained, were recorded.
Results
Data from 20 patients in each group were analysed. Contrary to expectations, most patients in both groups reported nausea postoperatively. Those patients receiving morphine over 5 min experienced more episodes of emesis (36) than those receiving the dose over 40 s (17). Most patients receiving the 40 s doses vomited in the first 12 h (median time 8 h), while those receiving the 5 min doses vomited between 12 and 24 h (median time 19 h) (P=0.01). There were no differences between groups in the visual analogue pain scores or use of morphine between groups.
Conclusions
Reasons for these unexpected findings remain speculative. The high incidence of PONV appears to be inherently high in gynaecological surgery patients and standard antiemetic medication regimens appear to be poorly efficacious. Reasons for the differences in the time-course of emetic episodes between the two groups may be related to differences in the time-course of central opioid receptor occupancy.
Keywords: patient controlled analgesia, postoperative nausea and vomiting, pharmacokinetics, dosage regimens
Introduction
Postoperative nausea and vomiting (PONV) is a relatively common, troublesome, and potentially hazardous complication of surgery. Its incidence in the general surgical population has been estimated to be between 8–92% [1] although most estimates are around 20–30% [2, 3]. Despite the wide variety of available antiemetic medications, current treatment of PONV is considered to be poor [4–7]. It is thought that in the patient-controlled analgesia (PCA) environment, nausea and vomiting may limit patients’ use of opioid analgesics; patients may in fact use PCA to balance pain against side-effects such as nausea [8].
Clearly, minimising PONV is an important clinical goal and most strategies to alleviate it have revolved around the use of antiemetic medication, especially in patients receiving opioid analgesics for postoperative pain management. It has long been speculated that rapid changes in blood opioid concentration are more likely to cause unpleasant side-effects than more gradual changes in concentration [9] but simple manoeuvres to alleviate PONV, such as altering the mode of analgesic drug administration, appear not to have been investigated. Some devices used for PCA allow the duration over which a dose is delivered to be either ‘bolus’ (usually delivered over around 40 s) or over a longer period, typically 5 min. Increasing the bolus dose delivery duration time will decrease the rate of change of blood drug concentration and the maximum blood-drug concentration (Cmax) whilst prolonging the time to Cmax (tmax). This study was performed to test the hypothesis that increasing the duration of delivery of morphine would decrease the incidence of PONV.
This work was presented as a poster at the 11th World Congress of Anaesthesiologists, Sydney April 17, 1996.
Methods
This study was approved by the Medical Research Ethics Committee (MREC) of the Royal North Shore Hospital.
Subjects
Forty-eight patients aged between 34 and 74 years (mean age 47 years; s.d.=8) undergoing total abdominal hysterectomies were selected for the study. All patients approached agreed to participate. All patients had similar systemic pathology (ASA 1–2) and had renal and liver functioning within normal physiological limits. Patients who could not understand the English language or the concept of PCA, those with a history of mental illness and those receiving monoamine oxidase inhibitors (MAOI) within 14 days prior to the study were excluded from selection. Four patients were withdrawn from the study: one patient was not using PCA because she had very little pain, one patient disliked morphine and requested pethidine, one patient underwent a vaginal rather than abdominal hysterectomy, and one patient underwent a laparotomy. Two patients received intra- or post-operative ketorolac, a third patient received ondansetron and another patient had insufficient data recordings taken. These patients were not included in the statistical analysis.
Procedure
Prior to commencing the study, pharmacokinetic simulation was performed to show the differences in predicted morphine plasma concentrations from delivering 1 mg morphine sulphate over 40 s or over 5 min. The simulation was based on data obtained from our work in progress in which arterial plasma concentrations were measured for 6 h after i.v. administration of 2 and 4 mg morphine sulphate [10]. The serial plasma morphine concentration-time data were fitted by a three compartment (central or plasma containing, peripheral rapidly equilibrating, peripheral slowly equilibrating) open model using Marquardt's algorithm implemented in EDFAST software on a personal computer [11]. The simulations are shown (Figure 1) for 1 mg of morphine sulphate. Inspection of Figure 1 indicates that a two-fold higher morphine Cmax is predicted when administered over 40 s compared with 5 min. The fraction of dose predicted distributed to the rapidly equilibrating peripheral compartment is similar for both deliveries but tmax is, of course, delayed when the morphine is delivered over 5 min; neither distribution to the deep compartment and elimination are detectably different for both groups over the simulated time course. The parameters used for the simulations were: initial dilution volume Vc=16 l; elimination rate constant kel=0.08 min−1; rapidly equilibrating rate constants k12=0.5 min−1 and k21=0.2 min−1; slowly equilibrating rate constants k13=0.09 min−1 and k31=0.008 min−1; total apparent volume of distribution Vss=236 l; mean total body clearance CL=1.28 l min−1 [10].
Figure 1.
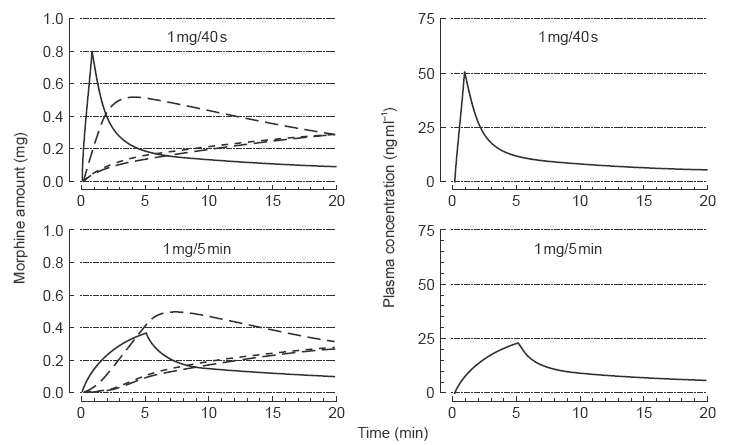
Pharmacokinetic modelling of morphine sulphate delivered as 1 mg over 40 s (upper panels) and over 5 min (lower panels). Left panels show amounts of morphine in the various compartments of the three compartment open mamillary model; right panels show concentrations of morphine in the plasma-containing central compartment. [The parameters used for the simulations were Vc=16l; ke=0.08 min−1; k12=0.5 min−1; k21=0.2 min−1; k13=0.09 min−1; k31=0.008 min−1; Vss=236 l; CL=1.28 l min−1].
Patients were selected daily from the gynaecological operating lists if they met the entry criteria. They were seen by the same researcher (AW) on the evening before surgery and informed about the study. If informed consent was obtained, they were then given standard instructions on how to use PCA (Appendix 1). Anaesthesia and surgery proceeded according to methods practised by the relevant attending staff. The choice of anaesthetic agents was at the discretion of the attending anaesthetist, however there were no systematic differences between groups in anaesthesia or intraoperative management. The anaesthetic essentially consisted of induction with thiopentone or propofol followed by maintenance with isoflurane/nitrous oxide/oxygen, muscle relaxation with vecuronium or atracurium, intraoperative antinociception with morphine, and antagonism of residual muscle relaxation with atropine/neostigmine. Anaesthetic records indicated that of the 20 patients who were later to receive the 40 s dose duration, 15 had anaesthesia induced with thiopentone; of those who were later to receive the 5 min dose duration, 14 had anaesthesia induced with thiopentone. PCA was initiated immediately postoperatively in the recovery room using Graseby 1205–0002 PCAS pumps. All patients were titrated to comfort with morphine according to the standard practice in this hospital and then received PCA-morphine with the initial incremental dose being 1 mg. If pain relief was not sufficient, the incremental dose could be increased at any time during the postoperative period. Patients were randomly assigned (computer generated numbers) to then receive the bolus dose either as a bolus dose with a 5 min lockout or over 5 min with a 1 min lockout interval. No patient received a background infusion.
Having left the recovery room, the patients were returned to their ward where standard nursing observations were maintained. In addition, 2 hourly charts with standard 10 cm visual analogue scale (VAS) (Appendix 2) for pain and nausea as a side-effect were recorded from the distance (in mm) marked on the scale. When the patient was sleeping this was recorded as ‘0’ (no pain or nausea). Morphine use and number of antiemetic drug doses given were taken from the patients’ notes at completion of PCA.
Standard orders for antiemetic medications, which are printed on the back side of the hospital PCA chart, are as follows: ‘Metoclopramide 10 mg i.v. 4th hourly prn. If ineffective, give droperidol 0.25 mg i.v. and repeat once as needed’. The incidence of nausea, retching and vomiting were recorded. Definitions of nausea, retching and vomiting were taken from Korttila [12]. Total nausea scores were determined as univariate parameters for each patient as the sum of the scores during the study period. Retching and vomiting were rated in intensity as ‘none, mild—it occurs 1–2 times, moderate—it occurs 3–5 times, severe—it occurs more than 5 times’. These were assigned numerical values of 0, 1, 2, and 3 for further analysis of symptom intensity.
Statistical analyses
The mean and total scores for pain, nausea, retching and vomiting were calculated at 12, 24, 36, and 48 h. Data were analysed using Student's t-tests, Median test, Mann-Whitney U tests and Fishers exact tests, as appropriate. P<0.05 was taken as statistically significant. A group size of 40 subjects was sufficient to detect a difference between groups in VAS or in symptom intensity equal to 0.62 standard deviations from the mean with a power of 0.80.
Results
The age of patients did not differ significantly between groups (Student's t-test).
Incidence of nausea, retching and vomiting
Of the patients treated with the 40 s dose delivery 90% reported nausea, 25% retching and 20% vomiting during the observation period. Of the patients treated with the 5 min dose delivery, 100% reported nausea, 45% reported retching and 50% reported vomiting. Fisher's Exact test indicated no significant differences in the incidence of nausea, retching or vomiting between the two groups. However, patients receiving morphine over 5 min had more emetic episodes (36) and these occurred later (median time 19 h) than in patients receiving morphine over 40 s (17 episodes, median time 8 h) (Median test, P=0.01). The episodes of emesis (retching and vomiting) are plotted with individual patients’ cumulative morphine use (Figure 2) and show clearly that most patients receiving the dose over 40 s vomited early in the postoperative period, while the majority of patients receiving the dose over 5 min vomited at between 12 and 24 h postoperatively.
Figure 2.
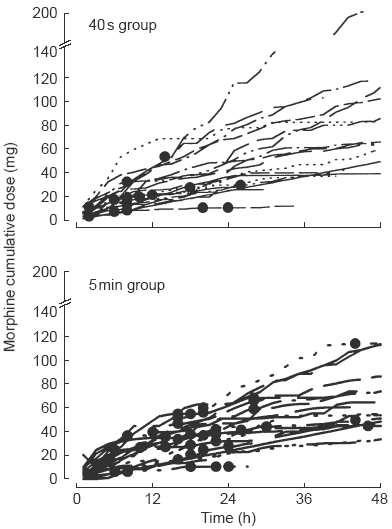
Individual patients’ cumulative dose of morphine sulphate by intravenous PCA with 1 mg incremental doses delivered over 40 s (upper panel) or 5 min (lower panel). Emetic episodes are indicated as dots.
Intensity of nausea, retching and vomiting
The total scores for nausea across the entire post surgical period between the two groups did not differ (Mann-Whitney U test) (Figure 3). Nausea scores at 12, 24, 36 or 48 h for either group were not correlated with the cumulative dose of morphine.
Figure 3.
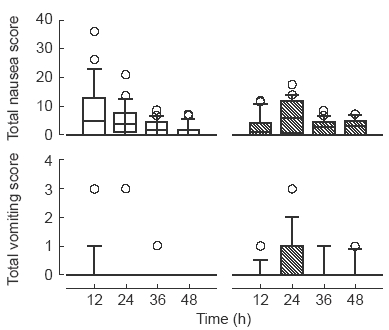
Box and whisker plots showing total nausea scores (upper panels) and total vomiting scores (lower panels). Patients received morphine sulphate by intravenous PCA with 1 mg incremental doses delivered either over 40 s (left panels, □) or 5 min (right panels, ). Data are shown as median with boxes of 25 and 75 percentiles, whiskers of 10 and 90 percentiles and data (circles) outlying these, for 12 h time segments.
). Data are shown as median with boxes of 25 and 75 percentiles, whiskers of 10 and 90 percentiles and data (circles) outlying these, for 12 h time segments.
As it was difficult to distinguish between retching and vomiting in the gastrically empty postoperative patient, retching and vomiting scores were combined and referred to as ‘total vomiting’. A Mann-Whitney U test indicated that the total vomiting scores over the entire postoperative period were significantly higher (P=0.008) in the patients receiving the dose over 5 min (median score=1; range=0 to 7) than those receiving the dose over 40 s (median score=0; range=0 to 6). A Mann-Whitney U test also indicated that the mean vomiting scores over the entire postoperative period were significantly higher (P=0.009) in the patients receiving the dose over 5 min (median score=0.09; range=0 to 1.2) than those receiving the dose over 40 s (median score=0; range=0 to 1.8). To further examine the pattern of emesis, comparisons of the vomiting scores were made at 12 h time periods. Patients receiving the dose over 5 min had significantly higher total vomiting scores at 24 (P=0.02), 36 (P=0.05) and 48 (P=0.04) hours (Mann-Whitney U test) (Figure 3).
Number of antiemetic doses given
There were no significant differences between groups in the number of antiemetic doses given over 48 h (Fishers exact test). There was no significant difference in the median number of antiemetic doses between the patients having dose over 40 s (2, range=0 to 8), and for the patients having doses over 5 min (3, range=0 to 6). Similarly, there were no differences between groups in the times to the first antiemetic or in the number of antiemetic doses given in the first 12 h (Mann-Whitney U test).
Pain scores and morphine use
The average pain scores of both groups decreased over the postoperative period (Figure 4) but there were no significant differences in pain scores between groups across the entire postoperative period (Mann-Whitney U test). Similarly, the average morphine use of both groups decreased over the postoperative period, but there were no significant differences in morphine use between groups at 12, 24, 36 and 48 h after surgery (Figure 4).
Figure 4.
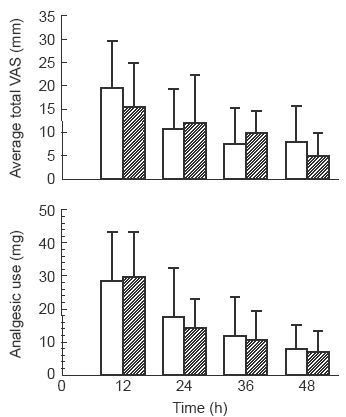
Mean and s.d. of total pain scores (VAS in mm) for groups receiving intravenous PCA with 1 mg incremental doses of morphine sulphate delivered either over 40 s (□) or over 5 min ( ) (upper panel). Mean and s.d. of morphine sulphate use by patients receiving intravenous PCA with delivery over 40 s or over 5 min (lower panel). Data for both panels are for 12 h time segments.
) (upper panel). Mean and s.d. of morphine sulphate use by patients receiving intravenous PCA with delivery over 40 s or over 5 min (lower panel). Data for both panels are for 12 h time segments.
Discussion
Both intuition and pharmacokinetic modeling predicted that the initial morphine concentrations in the central or blood-containing compartment would be higher and reached more quickly when morphine was delivered over 40 s rather than over 5 min. Since nausea and vomiting are thought to be governed centrally, it was hypothesized that rapid changes in blood morphine concentrations could contribute to increased nausea and vomiting. It was therefore proposed that delivering PCA morphine as a 5 min infusion as opposed to a 40 s dose would reduce the incidence of postoperative emesis thereby providing a simple manoeuvre with a useful therapeutic outcome. The findings of the study, however, did not support this hypothesis.
Firstly, the incidence of nausea was found to be very high in both treatment groups, with almost all patients reporting some nausea following surgery. Although this is consistent with some reports of other investigators [1], these values are higher than those commonly reported in the literature, as well as higher than those found previously in general surgical patients having abdominal surgery in this institution and studied with a different protocol [13]. One explanation for the high incidence could be that patients were asked about their nausea very frequently (each hour) and it may be that they reported it more readily. Alternatively, frequent questioning about emesis may have been suggestive thereby sensitizing patients to nausea. This seems unlikely since many patients experienced only one or two incidents of nausea and then recovered fully. Other patients appeared to have a low level of nausea quite frequently (i.e. VAS of 1–3), with higher scores on occasion. The higher scores reported tended to follow incidents such as movement or food intake, while the lower levels may have reflected a general feeling of ‘unwellness’ experienced by patients whilst in hospital. This could be due to the combination of hospital experiences such as preoperative fasting, medications, surgical intervention and stress superimposed upon the emotional impact of the (hysterectomy) surgery. Given the inability of most antiemetics to eliminate nausea completely, the findings of this study indicate the importance of considering frequency, intensity and duration of nausea, in addition to incidence, when making clinical decisions concerning prophylactic antiemetic treatments. It is emphasized that with the exception of prolonging to 5 min the dose of morphine delivered by PCA in one group of patients, the postoperative and antiemetic procedures adopted in the study conformed to standard practice at this teaching hospital. A high incidence of PONV, as observed in this study upon investigation, may be a common but under-reported occurrence in patients undergoing major gynaecological surgery [14].
Secondly, patients receiving PCA morphine delivered over 5 min experienced a greater intensity of retching and vomiting than patients receiving PCA morphine delivered over 40 s. In addition to this, the timing of emetic episodes appeared different for the two groups of patients; patients receiving the morphine over 40 s reported most vomiting in the first 12 h after surgery (median time 8 h), whereas those receiving the morphine over 5 min reported most vomiting from 12 to 24 h postoperatively (median time 19 h) (Figure 3). Given that patients’ ages, anaesthetic and other intraoperative regimens, pain scores and analgesic use patterns were all similar, reasons for these findings are unclear. Moreover, a similar number of operations were performed by the same attending surgeons in each group.
The number, timing and type of antiemetic medication doses given to patients in both groups were similar. Also, the time at which antiemetics were given was investigated, given that this may have explained the different timing of emesis between groups. Patients who complained of nausea soon after surgery may have been given a relatively long-acting antiemetic (metoclopramide) which would have continued to have effect into the second 12 h period after surgery. Patients in the group receiving morphine over 5 min may not have felt nausea in that initial period and therefore not asked for or received antiemetics and thus, would not have had effective antiemetic cover for the next 12 h period. However, there were no differences in the timing of first and second antiemetics given to patients in either group.
In rationalizing the rejection of our hypothesis, we speculate that there may be a critical blood or tissue morphine concentration (or receptor occupancy) above which patients experience nausea. A bolus dose would mean that the concentration rapidly exceeds this level but then also rapidly falls below it. A dose delivered over 5 min would result in a slower increase and decrease in the blood drug concentration but the concentration may remain above the critical ‘nausea’ level for longer. However, this would not explain the differences in the timing of emetic episodes between groups unless there is some combination of a minimum blood drug concentration for nausea and some other cumulative effect of the opioid.
In summary, the findings of this study remain unexplained. The administration of PCA morphine over 5 min was associated with an increase in the intensity of retching and vomiting compared with patients receiving PCA morphine over 40 s. Patients receiving the dose more slowly also experienced their emetic episodes later in the postoperative period as opposed to patients receiving a bolus who developed nausea and vomiting immediately postoperatively.
Acknowledgments
The authors wish to thank the anaesthetists and the staff of ward 10A of the Royal North Shore Hospital for their cooperation and help with this study.
Appendix 1: Standard instructions for PCA use
Good afternoon. My name is .................... and I am here to talk to you about management of your pain after your surgery tomorrow.
After your surgery I am sure you are aware you will experience some pain or discomfort. What I think your anaesthetist would like to do for your pain management is to give you something called PCA. Have you heard of PCA? PCA stands for Patient-Controlled Analgesia. With the PCA pump, you will be able to control your own pain by pressing this button. Because only you, the patient know how much pain you are in, the PCA machine allows you to have control over your pain relief. The PCA machine will sit next to your bed and will be connected to you by a small needle in the back of your hand. You will also have this button in your other hand. When you press the button the pump will deliver one bolus dose of pain relieving medication to you. So, when you have pain, you should press the button and you will receive one dose of pain relieving medication. It is very important that you keep yourself comfortable after your surgery. If your pain is well controlled and you are comfortable, you are better able to move, breathing is easier, and you may be able to go home quicker.
After your surgery we will ask you about your pain. We will ask you to rate your pain between 0 and 10, where 0=‘no pain’ and 10=‘worst possible pain’. Do you understand?
It is virtually impossible to use the button too much and you will not overdose when using the PCA pump. There is a special safety feature called a lockout time, during which no pain relieving medication will be delivered to you. The lockout time stops the machine from delivering a bolus dose too soon after the last dose. The PCA machine will only deliver pain relieving medication to you when it is safe to do so.
Any questions?
Appendix 2: Side-effect and VAS pain chart
Pain
No pain-----------------------Worst possible pain
Nausea
Nausea is a subjective sensation in which the patient describes ‘feeling sick’ or the desire to vomit.
Nausea should be rated on a VAS :(ask patients for an overall rating since being in the recovery room)
No nausea-------------------------Extreme nausea
Retching
Retching is distinguished from vomiting by the absence of stomach contents. Retching is when no stomach contents are expelled.
Retching should be rated as: (Please answer by circling the appropriate choice)
None
Mild – it occurs 1–2 times
Moderate – it occurs 3–5 times
Severe – it occurs more than 5 times
Vomiting
Vomiting is defined as the expulsion of even the smallest amount of stomach contents.
Vomiting should be rated as: (Please answer by circling the appropriate choice)
None
Mild – it occurs 1–2 times
Moderate – it occurs 3–5 times
Severe – it occurs more than 5 times
Pruritus
Pruritus should be rated as: (Please answer by circling the appropriate choice)
None
Mild – patient mentioned only when asked.
Moderate – patient complained of itch (no
medication required).
Severe – patient required medication for itch.
Sedation
Awake
Drowsy, asleep, easily rousable
Very drowsy, difficult to rouse
Unable to rouse, reversal procedures initiated
Other side-effects
Please record any other side-effects
....................................................................................
....................................................................................
....................................................................................
References
- 1.Camu F, Lauwers MH, Verbessem D. Incidence and aetiology of postoperative nausea and vomiting. Eur J Anaesthesiol. 1992;9(Suppl 6):25–31. [PubMed] [Google Scholar]
- 2.Lerman J. Surgical and patient factors involved in postoperative nausea and vomiting. Br J Anaesth. 1992;69(Suppl 1):24S–32S. doi: 10.1093/bja/69.supplement_1.24s. [DOI] [PubMed] [Google Scholar]
- 3.Watcha MF, White PF. Postoperative nausea and vomiting. Its etiology, treatment, and prevention. Anesthesiology. 1992;77:162–184. doi: 10.1097/00000542-199207000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Kapur PA. The big ‘little problem’. Anesth Analg. 1991;73:243–245. doi: 10.1213/00000539-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Rowbotham DJ. Current management of postoperative nausea and vomiting. Br J Anaesth. 1987;67:46S–59S. doi: 10.1093/bja/69.supplement_1.46s. [DOI] [PubMed] [Google Scholar]
- 6.Woodhouse A, Mather LE. Nausea and vomiting and vomiting in the postoperative patient-controllead analgesia environment. Anaesthesia. 1997;52:770–775. doi: 10.1111/j.1365-2044.1997.144-az0148.x. [DOI] [PubMed] [Google Scholar]
- 7.Semple P, Madej TH, Wheatley RG, Jackson IJB, Stevens J. Transdermal hysocine with patient-controlled analgesia. Anaesthesia. 1992;47:399–401. doi: 10.1111/j.1365-2044.1992.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 8.Harrison DM, Sinatra R, Morgese L, Chung JH. Epidural narcotic and patient-controlled analgesia for post-cesarean section pain relief. Anesthesiology. 1988;68:454–457. doi: 10.1097/00000542-198803000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Mather LE. Pharmacokinetic and pharmacodynamic factors influencing the choice, dose and route of administration of opiates for acute pain. Clinics in Anaesthesiology. 1983;1:17–40. [Google Scholar]
- 10.Ward ME, Woodhouse A, Mather LE, et al. Morphine pharmacokinetics after pulmonary administration from a novel aerosol inhalation delivery system. Clin Pharmacol Ther. (in press). [DOI] [PubMed]
- 11.Sebalt RJ, Kreeft J. Efficient pharmacokinetic modelling of complex clinical dosing regimens; the universal elementary dosing regimen and computer algorithm EDFAST. J Pharm Sci. 1987;76:93–100. doi: 10.1002/jps.2600760202. [DOI] [PubMed] [Google Scholar]
- 12.Korttila K. The study of postoperative nausea and vomiting. Br J Anaesth. 1992;69(7 Suppl. 1):20S–23S. doi: 10.1093/bja/69.supplement_1.20s. [DOI] [PubMed] [Google Scholar]
- 13.Woodhouse A, Hobbes AFT, Mather LE, Gibson M. A comparison .of morphine, pethidine and fentanyl in the postsurgical patient-controlled analgesia (PCA) environment. Pain. 1996;64:115–121. doi: 10.1016/0304-3959(95)00082-8. [DOI] [PubMed] [Google Scholar]
- 14.Paech MJ, Pavy TJG, Evans SF. Single-dose prophylaxis for postoperative nausea and vomiting after major abdominal surgery: ondansetron versus droperidol. Anaesth Intens Care. 1995;23:548–534. doi: 10.1177/0310057X9502300503. [DOI] [PubMed] [Google Scholar]


