Abstract
Under selective pressure from host cytotoxic T lymphocytes, many viruses have evolved to downregulate major histocompatibility complex (MHC) class I and/or T-cell costimulatory molecules from the surface of infected cells. Kaposi's sarcoma-associated herpesvirus (KSHV) encodes two proteins, MIR-1 and MIR-2, that serve this function during lytic replication. In vivo, however, KSHV exists in a predominantly latent state, with less than 5% of infected cells expressing discernible lytic gene products. Thus, mechanisms of immune evasion that depend on genes expressed only during lytic replication are unlikely to be active in most KSHV-infected cells. As a result, we searched for evidence of similar defensive strategies extant during latency, employing culture systems that strongly favor latent KSHV infection. We measured cell surface levels of immunomodulatory proteins on both primary dermal microvascular endothelial cells (pDMVEC) infected through coculture with induced primary effusion lymphoma cells and telomerase-immortalized DMVEC infected directly with cell-free virus. Employing a panel of antibodies against several endothelial cell surface proteins, we show that de novo infection with KSHV leads to the downregulation of MHC class I, CD31 (PE-CAM), and CD54 (ICAM-I) but not CD58 (LFA-3) or CD95 (Fas). Furthermore, flow cytometry with a fluorescently labeled monoclonal antibody to the latency-associated nuclear antigen (LANA) revealed that downregulation occurred predominantly on KSHV-infected (LANA-positive) cells. Although the vast majority of infected cells displayed this downregulation, less than 1% expressed either immediate-early or late lytic proteins detectable by immunofluorescence. Together, these results suggest that downregulation of immunomodulatory proteins on the surface of target cells may represent a constitutive mode of immune evasion employed by KSHV following de novo infection.
Herpesviruses constitute a family of human pathogens that persist indefinitely in the host organism and often contribute to a variety of disease states. Viral persistence depends on the ability of herpesviruses to establish a latent infection within cells. This process requires the successful evasion of host immune defenses, which usually includes the restricted expression of viral genes during latency. Although such restriction lowers their overall antigenic profile, herpesviruses must employ additional mechanisms to ensure prolonged protection from host immune responses. One such mechanism involves the downregulation of immunoregulatory proteins, such as major histocompatibility complex (MHC) class I, from the surface of infected cells. This serves to limit their recognition by cytotoxic T lymphocytes (CTLs) and dampen the inflammatory response to viral infection. Examples of human herpesvirus proteins that perform this function include the ICP47 protein of herpes simplex virus 1 (2, 25, 75), the interleukin-10 protein of Epstein-Barr virus (17, 59, 76), and the US2, US3, US6, and US11 proteins of human cytomegalovirus (1, 5, 30, 52, 68).
Kaposi's sarcoma-associated herpesvirus (KSHV) is a recently discovered member of the gamma (lymphotropic) subfamily of herpesviruses and is responsible for several distinct diseases in humans, including Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman's disease (9, 10, 21, 45). While pathologically diverse, these disorders are all associated with an immunocompromised state and pose a significant threat to human immunodeficiency virus-infected individuals and solid-organ transplant recipients worldwide. In contrast, KSHV infection in healthy patients is usually asymptomatic. In all infected individuals, KSHV elicits a humoral and cellular host immune response directed against both lytic and latent proteins of the virus (3, 6, 32, 35, 44, 71), yet this response, even in healthy persons, is unable to eradicate KSHV from the body. This suggests that KSHV, like other human herpesviruses, possesses the ability to evade immune responses during infection.
Recent studies have identified several KSHV lytic proteins that exert potential immunoregulatory roles during lytic replication. These include inhibition of apoptosis by viral Bcl-2 (63) and open reading frame (ORF) K7 (70), complement deregulation by ORF 4 (64), Th2-type polarization by viral MIP-II (72), and inhibition of the interferon antiviral response by viral interferon response factor 1 (vIRF-1) (24), vIRF-3 (42), and viral interleukin-6 (11). Furthermore, KSHV also encodes two immediate-early proteins, MIR-1 (encoded by ORF K3) and MIR-2 (encoded by ORF K5), that downregulate immunoregulatory proteins such as MHC class I and ICAM-1 from the surface of cells to limit their recognition by immune cells (14, 15, 26-28, 61). KSHV, however, most often follows the general paradigm of gammaherpesvirus infection, namely a primarily latent phase of infection marked by a highly restricted pattern of viral gene expression. The prevalence of KSHV-infected cells undergoing lytic (productive) infection both in vitro and in vivo is typically low (1 to 5%), with the remaining infected cells harboring the virus in its latent form (19, 33, 49, 62, 65, 67, 77). Thus, mechanisms of immune evasion contingent upon genes expressed solely during the lytic cycle would protect only a small fraction of KSHV-infected cells. It follows, therefore, that KSHV may additionally require mechanisms of evasion that are active during latency.
Several KSHV latent proteins have likewise been implicated in viral immune evasion. These include disruption of p53 by latency-associated nuclear antigen (LANA) (23) and LANA2 (55), inhibition of apoptosis by viral FLIP (20), downregulation of the B-cell receptor by ORF K1 (39), and deregulation of the interferon response by vIRF-II (8). Additionally, work by Brander et al. has shown that KSHV-infected PEL cells express lower levels of MHC class I and possess increased resistance to CTLs compared with Epstein-Barr virus-infected or primary B cells (7). However, the relevance of these findings to de novo infection of endothelial cells with intact KSHV remains unknown.
Therefore, we investigated the potential viral immune evasion strategies of KSHV in two endothelial cell culture systems that strongly favor latent infection with KSHV. Specifically, we tested telomerase-immortalized dermal microvascular endothelial cells (DMVEC) infected with concentrated virus and primary DMVEC (pDMVEC) infected via coculture with induced BCBL-1 cells, a PEL line harboring KSHV. With a panel of antibodies against numerous endothelial cell surface proteins, we examined these immortalized and primary cells for signs of immune modulation following de novo infection with KSHV.
MATERIALS AND METHODS
Cell culture.
BCBL-1 and BJAB cells were grown in RPMI 1640 medium (Gibco, Rockville, Md.) supplemented with 10% fetal bovine serum, 10 mM HEPES (pH 7.5), 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mM l-glutamine, 0.05 mM β-mercaptoethanol, and 0.02% (wt/vol) sodium bicarbonate. Cells were maintained at 2.5 × 105 to 8.0 × 105/ml and were used for up to 2 months after the thaw date. pDMVEC were obtained from Clonetics Corporation (Walkersville, Md.) at low passage and grown in EGM-2 MV medium (Clonetics) with 50 U of penicillin per ml and 50 μg of streptomycin per ml substituted for amphotericin B and gentamicin.
Creation of T4 TIME cells.
Telomerase-immortalized microvascular endothelial (T4 TIME) cells were generated from pDMVEC with the stable incorporation of the human telomerase gene (hTERT) by retroviral transduction. Briefly, the pBABE retroviral plasmid containing the hTERT gene (a gift from R. Weinberg) was packaged into a Moloney murine leukemia virus pseudo typed with the vesicular stomatitis virus glycoprotein G. This virus was then transduced into pDMVEC by spinfection for 2 h at 1,000 × g, and stable clones were selected in the presence of 0.5 μg of puromycin per ml for 1 month. hTERT expression was then confirmed with the Trapeze telomerase detection kit (Intergen, Manhattan N.Y.). One of the resultant clones, T4, expressed high levels of telomerase, as determined by telomerase repeat amplification analysis and has survived, to date, over 80 passages without detectable changes in morphology, growth rate, or susceptibility to infection with KSHV compared with early-passage T4 TIME cells (data not shown).
Coculture infection of pDMVEC cells with KSHV.
pDMVEC at early passage (<15 passages) were infected by coculture with the primary effusion lymphoma cell line BCBL-1, based on methods described by Sakurada et al. (58). Briefly, BCBL-1 cells were induced with a 12-h exposure to 20 ng of O-tetradecanoyl phorbol 13-acetate per ml and 300 μM sodium butyrate and, after 3 days, cocultured directly with pDMVEC at various ratios for 12 h in RPMI 1640 medium supplemented with 10% fetal bovine serum and 100 μg of endothelial cell growth supplement per ml (BD Pharmingen, San Jose, Calif.). The pDMVEC were then washed vigorously to remove the BCBL-1 cells and cultured for an additional day in EGM-2 endothelial cell medium lacking hydrocortisone. We found that low levels of hydrocortisone, normally present in EGM-2 endothelial growth medium, led to a modest two- to threefold induction of immediate-early lytic protein expression in the immunofluorescence assay (data not shown). As a result, we eliminated hydrocortisone from the growth medium in all short-term experiments to minimize the levels of spontaneous lytic reactivation. For controls, pDMVEC were cocultured with uninduced BCBL-1 cells or BCBL-1 cells induced in the presence of 0.5 mM phosphonoformic acid (Sigma Aldrich, St. Louis, Mo.).
Cell-free virus infection of T4 TIME cells.
BCBL-1 cells were induced as described above, and virus was collected from the supernatant on the sixth day after induction by centrifugation at 13,000 × g for 3 h. The viral pellet was resuspended at 100th the original volume in EGM-2 MV culture medium lacking hydrocortisone, and aliquots were frozen at −80°C. A small aliquot of concentrated virus was used to determine the number of viral genome equivalents by Southern blot analysis with a fluorescently labeled single-stranded KSHV DNA probe complementary to ORF 73 (Random Primer DNA chemiluminescence kit; New England Nuclear, Boston, Mass.). Approximately 5,000 viral genome equivalents per endothelial cell were then used to infect T4 TIME cells in the presence of 8 μg of Polybrene per ml (Sigma Aldrich) for 2 h. Hydrocortisone was omitted from the culture medium 24 h before addition of the virus. For controls, a sister plate was infected with an equivalent amount of virus that was first heat inactivated for 30 min at 65°C or UV-inactivated at 40 W for 60 min with 254-nm UV light. Cells were then washed to remove the Polybrene and incubated for 2 days in fresh EGM-2 medium lacking hydrocortisone.
Flow cytometry.
pDMVEC and T4 TIME cells were infected with KSHV as described above and harvested for flow cytometry at the indicated times. Cells were detached from the plate with a 0.25% trypsin-EDTA solution (Clonetics), and 0.5 × 106 cells per sample were then transferred to a 96-well plate after assessing cell count and viability by trypan blue exclusion. For surface staining, samples were washed twice with 1× PBSA (1× phosphate-buffered saline [PBS] with 0.09% sodium azide) and blocked for 30 min with antibody staining buffer (3% fetal bovine serum in PBSA). Cell surface staining was then carried out for 1 h at 4°C in the dark.
All cell surface antibodies and isotype controls were obtained from BD Pharmingen and used at the recommended dilution of 0.25 μg of antibody/106 cells in antibody staining solution. Apoptotic cells were identified with the annexin V fluorescein isothiocyanate apoptosis detection kit as described by the manufacturer (BD Pharmingen). Cells were then washed twice with cold 1× PBSA and permeabilized for nuclear staining with the paraformaldehyde-saponin-based bromodeoxyuridine Fix/Perm kit from BD Pharmingen as described by the manufacturer. Nuclear staining to identify KSHV-infected cells was performed with a rat monoclonal antibody to the KSHV LANA protein (Applied Biosciences Inc., Columbia, Md.) conjugated to the fluorochrome Alexa 488 per the manufacturer's protocol (Molecular Probes, Eugene, Oreg.) and used at a 1:1,000 dilution in BD Pharmingen Perm/Wash buffer supplemented with 3% fetal bovine serum. Flow cytometry was performed with a FACSCalibur flow cytometer and analyzed with Cell Quest Pro software (Becton Dickinson, Bedford, Mass.). Prior to analysis, all samples were gated by forward and side scatter to eliminate dead cells.
Immunofluorescence.
T4 TIME cells and pDMVEC (with and without KSHV) were grown overnight on Becton Dickinson eight-well culture slides coated with fibronectin. The slides were then fixed in 95% ethanol-5% acetic acid for 10 min at room temperature and permeabilized in PBS with 0.5% Triton X-100 for 20 min at room temperature as described by Moses et al. (46). Nonspecific binding was blocked by incubating the cells with 10% goat serum supplemented with 1% glycine and 3% bovine serum albumin and then with the appropriate primary antibody diluted in blocking buffer for 60 min. To determine the extent of KSHV infection, cells were assayed for LANA expression with Alexa 488-conjugated monoclonal antibody to LANA at a 1:1,000 dilution. Rabbit polyclonal antibodies to RTA (ORF 50), (a gift from D. Lukac), ORF 45 (a gift from Y. Yuan), MIR-1 (ORF K3) and MIR-2 (ORF K5) (both gifts from G. Hayward), MCP (ORF 25) and SCAF (ORF 17.5) were all used at 1:500 except MIR-2, which was used at 1:1,000. Following staining with primary antibodies, cells were washed twice with 1× PBS and then incubated with goat anti-rabbit immunoglobulin conjugated to Texas Red (Jackson Labs, West Grove. Pa.) diluted 1:150 for 60 min in the dark. Cells were then washed twice with 1× PBS and subsequently stained with 0.5 μg of 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma) per ml in 180 mM Tris (pH 7.5) for 30 min at 4°C in the dark. The slide was then coated with Antifade mounting solution (Biomeda, Foster City, Calif.) and sealed with a coverslip.
Paracrine experiments.
Medium was harvested from T4 TIME cells 48 h after infection with cell-free virus (as described above). The medium was cleared of cell debris and viral particles by ultracentrifugation at 75,000 × g for 30 min in an SW-55Ti rotor (Sorvall, Newtown, Conn.). Naïve T4 TIME cells were then incubated with the virus-free supernatant for 48 h and prepared for flow cytometry as described above. Naïve T4 TIME cells incubated with medium from mock-infected T4 TIME cells prepared similarly served as controls.
RESULTS
KSHV infection of telomerase-immortalized endothelial cells.
To investigate potential KSHV-specific immune evasion strategies, we assessed changes in the expression of specific cell surface proteins following de novo infection of endothelial cells with KSHV. We focused our initial efforts on DMVEC because they represent a probable target cell of KSHV within Kaposi's sarcoma lesions in vivo (40, 57, 69). Previous attempts at infecting similar cells with KSHV, however, have resulted in initial infection rates of less than 5% (22, 53). Therefore, we took advantage of recent findings indicating that human pDMVEC first immortalized with the human telomerase gene (hTERT) support higher rates of infection with concentrated KSHV (38). We created hTERT-immortalized microvascular endothelial cells (TIME cells) through the retroviral insertion of hTERT into pDMVEC and the subsequent selection of antibiotic-resistant colonies (see Materials and Methods).
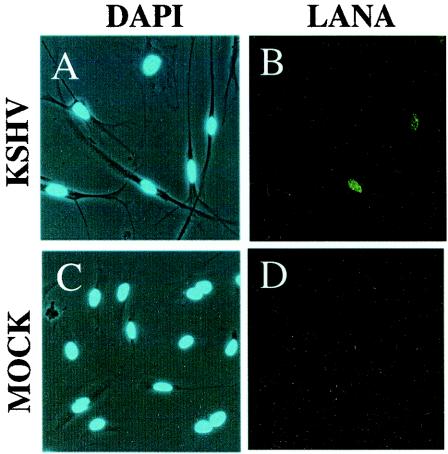
Infection of one of the resultant clones, T4, with the concentrated viral supernatant from the KSHV-infected PEL line BCBL-1 resulted in a dramatic morphological change in many of the target endothelial cells to a spindle-like phenotype within 24 h (Fig. 1A). This change is characteristic of KSHV infection of endothelial cells (13, 46) and coincided closely with the onset of latent gene expression, as evidenced by the detection of LANA by the immunofluorescence assay (Fig. 1B). In contrast, incubation of T4 cells with either UV-treated (not shown) or heat-inactivated KSHV caused neither LANA staining nor morphological changes associated with infection (Fig. 1C and D).
FIG. 1.
Morphological changes associated with infection of T4 TIME cells by KSHV. KSHV-infected (A) and mock-infected (C) T4 TIME cells were identified by phase microscopy, with superimposed DAPI staining of the nuclei 48 h after infection. (B and D) Cells from panels A and C, respectively, stained for LANA expression and visualized by fluorescence microscopy.
The proportion of cells infected with KSHV correlated with the amount of input virus, determined by viral genome copy number (see Materials and Methods). However, with viral concentrations sufficient to generate 100% infection of all of the target cells, we often observed excessive cell death. To avoid this issue, we optimized the initial infection level in most experiments to approximately 50% by maintaining a constant ratio of KSHV genome copy number to target cell of approximately 5,000:1. This high ratio may reflect the small proportion of DNA-containing particles that are infectious virions as well as the relatively low susceptibility of endothelial cells to KSHV infection (C. Tomescu and D. H. Kedes, unpublished observations). With initial infection rates of approximately 50%, the number of infected (LANA-positive) cells remained stable for several days. However, the proportion of KSHV-infected cells began to decrease with continued culture. This reduction began approximately 4 days postinfection, so that by 3 weeks fewer than 15% of the cells remained positive (data not shown). This reduction may reflect either loss of the virus from within infected endothelial cells over time or a growth advantage of uninfected over infected cells within the culture. As a result, we restricted most of our analyses to the first 72 h postinfection.
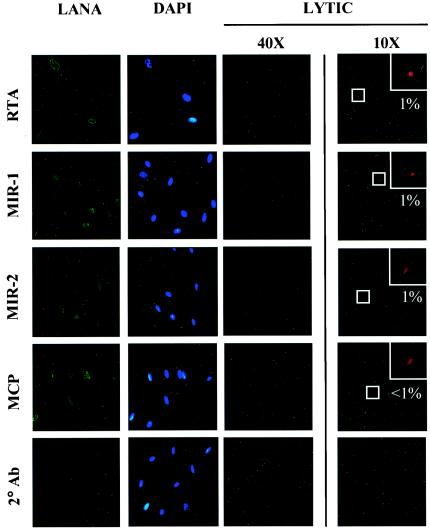
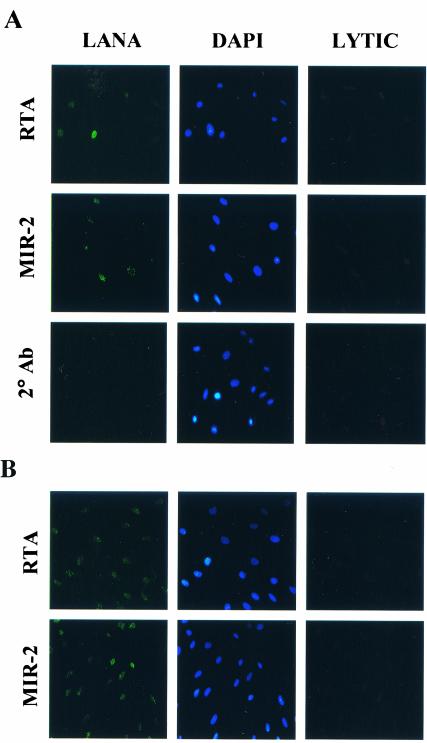
We next characterized the frequency of viral lytic gene expression among the newly infected T4 TIME cells. Lagunoff and colleagues reported that incubation of a similarly derived TIME cell line with KSHV resulted in predominantly latent infection, with lytic protein expression confined to less than 1% of infected cells (38). To explore whether our T4 TIME cells displayed a similar latent bias after infection, we assayed for KSHV lytic protein expression by immunofluorescence with antibodies specific for four immediate-early lytic gene products, ORF 50 (RTA), ORF 45, K3 (MIR-1), and K5 (MIR-2), as well as two late lytic gene products, ORF 17.5 (SCAF) and ORF 25 (MCP). We also costained samples for latent protein expression with a monoclonal antibody to LANA to identify the KSHV-infected cells in the culture.
With this approach, we observed a relative paucity of both immediate-early and late lytic protein expression in the great majority of KSHV-infected (LANA-positive) cells (Fig. 2, third and fourth columns). Specifically, immunofluorescence assays detected the immediate-early lytic proteins RTA, MIR-1, and MIR-2 in only 1% of KSHV-infected T4 TIME cells (Fig. 2, fourth column). ORF 45 antibodies gave similar results (data not shown), while even fewer cells demonstrated late lytic proteins such as SCAF (not shown) and MCP (Fig. 2, fourth column). This last finding may reflect premature termination of the lytic cascade prior to expression of late lytic proteins in the few cells undergoing spontaneous lytic reactivation. Under higher magnification, reactivity with antibodies to lytic proteins in most KSHV-infected cells was indistinguishable from background levels with secondary antibody alone (Fig. 2, third column) despite characteristic LANA staining (Fig. 2, first column). In parallel assays, the same set of lytic antibodies reacted with 10 to 15% of similarly induced BCBL-1 cells (not shown), reflecting the higher levels of lytic reactivation that characterize this PEL line (54, 62, 67). Together, these results argue that KSHV infection of T4 TIME cells, in the absence of inducing agents, is predominantly latent. Nevertheless, we cannot rule out the distinct possibility that low levels of lytic proteins, expressed in quantities below the sensitivity of our immunofluorescence assay, are present in some cells following KSHV infection.
FIG. 2.
KSHV-infected T4 TIME cells stained for latent and lytic protein expression and analyzed by immunofluorescence with the indicated antibodies 48 h after infection. The first, second, and third columns represent immunofluorescence assays at high magnification (40×) of a single field for LANA, DAPI, and the indicated lytic protein, respectively. Note that the background reactivity of KSHV-infected T4 TIME cells to the indicated lytic antibodies was indistinguishable from that with secondary antibody alone. The fourth column represents a parallel culture of infected cells stained with the same antibodies at low magnification (10×), with insets showing magnified views of the indicated (boxed) reactive cell. Values represent the percentage of KSHV-infected (LANA-positive) cells that exhibited reactivity to the corresponding lytic antibody.
Downregulation of MHC class I, PE-CAM, and ICAM-1 after de novo infection of T4 TIME cells with KSHV.
To investigate potential KSHV-specific immune evasion strategies that might arise following initial infection, we examined newly infected T4 TIME cells with a panel of antibodies to endothelial cell surface proteins involved in immune recognition (12, 43, 66, 73, 74). This analysis included monoclonal antibodies to MHC class I, MHC class II, CD31 (PE-CAM), CD40, CD54 (ICAM-1), CD58 (LFA-3), CD80 (B7.1), CD86 (B7.2), and CD95 (Fas). While four of these nine proteins, MHC class II, B7.1, B7.2, and CD40, were only poorly expressed on T4 TIME cells, the five remaining proteins were well represented prior to KSHV infection.
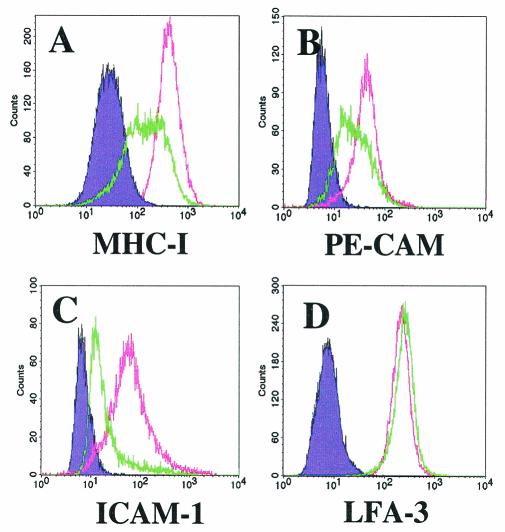
We monitored the cell surface expression of this set and observed a significant downregulation of three of the proteins shortly after infection. Included among these was MHC class I, which was distinctly downregulated within 48 h of KSHV infection (Fig. 3A). In contrast, incubation of T4 TIME cells with heat- or UV-inactivated virus led to no change in MHC class I levels compared with uninfected, naïve cells (data not shown). The number of cells with downregulated MHC class I varied proportionally with the amount of input virus and, in turn, with the level of infection as reflected by LANA reactivity in immunofluorescence assays (not shown). Furthermore, flow cytometry of cultures that were approximately 50% infected (LANA positive) most often demonstrated a widened distribution of MHC class I signal rather than a biphasic curve with two distinct populations, indicating that the extent of downregulation per cell varied considerably (Fig. 3A). Nevertheless, the mean KSHV-associated reduction in MHC class I ranged between two and fivefold compared to mock-infected control cultures in four separate experiments. These measurements, however, represent a minimal estimate, since approximately 50% of the cells in the cultures were uninfected.
FIG. 3.
Downregulation of immunoregulatory proteins on T4 TIME cells following infection with KSHV. Flow cytometric analysis of KSHV-infected (green line) and mock-infected (pink line) T4 TIME cells stained for surface protein expression with the indicated antibodies 36 h after infection. The shaded purple histogram indicates the isotype control. Note that ICAM-1 downregulation was unusually pronounced in this experiment. Mock-infected cells were incubated with heat-inactivated KSHV; UV-inactivated KSHV gave similar results.
In addition to MHC class I, KSHV infection of T4 TIME cells also led to surface downregulation of PE-CAM (CD31) and ICAM-1 (CD54) (Fig. 3B and C, respectively). These two proteins, normally expressed on endothelial cells, provide adhesion and costimulatory signaling to CTLs during T-cell-endothelial cell interactions (43, 74). Furthermore, inhibition of these signaling pathways greatly reduces the ability of CTLs to kill target cells (12, 73). In contrast, KSHV infection of T4 TIME cells led to no appreciable change in the level of the cell surface protein LFA-3 (CD58) (Fig. 3D) while slightly increasing the levels of another marker, Fas (CD95) (data not shown). These findings argue that the downregulation of MHC class I, ICAM-1, and PE-CAM during infection is specific and not merely a reflection of global or indiscriminant downregulation.
To investigate the kinetics of immunoregulatory protein downregulation, we measured cell surface expression of MHC class I, PE-CAM, ICAM-1, and LFA-3 on T4 TIME cells at 12-h intervals following de novo infection with KSHV. For MHC class I, downregulation was not evident at 0 (not shown) or 12 h postinfection (Fig. 4A). However, downregulation was apparent by 24 h and reached its peak by 48 h (Fig. 4A). In contrast, LFA-3 levels remained unchanged or slightly elevated throughout this period (Fig. 4B). PE-CAM and ICAM-1 downregulation kinetics (not shown) were similar to those of MHC class I. For all three proteins, cell surface levels at 72 h (not shown) postinfection were similar to those at 48 h, suggesting that a new, lower expression set point may have been established. By 96 h postinfection, however, the percentage of LANA-positive cells within the culture began to decrease (see above). This led to a concomitant reduction in the number of cells that exhibited downregulation of immunoregulatory proteins, although the profile of proteins affected on these individual cells was unchanged from that at the earlier times. Apoptosis was not directly responsible for a decrease in the proportion of cells downregulating immunoregulatory proteins, since greater than 97% percent of all cells exhibiting downregulation were viable, as evidenced by a lack of annexin V reactivity (data not shown).
FIG. 4.
Kinetics of MHC class I downregulation following KSHV infection of T4 TIME cells. Flow cytometric analysis of KSHV-infected (green line) and mock-infected (pink line) T4 TIME cells stained with antibodies to MHC class I (A) and LFA-3 (B) at the indicated times postinfection. The results at 0 and 72 h (not shown) were identical to those at 12 and 48 h, respectively. Note that the levels of KSHV infection per cell and, as a result, the number of cells downregulating MHC class I were slightly lower in this experiment than in most others (see, for example, Fig. 3).
At all times, the proportion of cells exhibiting surface protein downregulation correlated directly with the proportion of KSHV-infected (LANA-positive) cells within the culture (data not shown). These findings suggest that direct infection with KSHV may be required to induce downregulation on cells within the mixed culture. However, they do not exclude the possibility that downregulation may be variable on infected cells and that soluble paracrine factors or direct cell-to-cell contact may contribute to similar changes on uninfected neighboring cells.
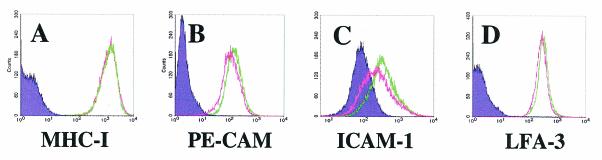
To investigate the possibility of a paracrine effect, we incubated naïve T4 TIME cells for 48 h with virus-cleared medium removed from a culture of T4 TIME cells infected with KSHV 48 h earlier (see Materials and Methods). Flow cytometry of cells treated with this conditioned medium, however, revealed no downregulation of any of the cell surface proteins that we tested (Fig. 5). Instead, treatment of naïve cells with conditioned medium from KSHV-infected cells led to a modest upregulation of ICAM-1 and PE-CAM (Fig. 5). These findings may reflect a response to proinflammatory cytokines potentially released by cells within the KSHV-infected culture. In any case, these results indicate that soluble factors are not sufficient to elicit downregulation in uninfected cells. However, cell-to-cell contact, as a mechanism of downregulation, remained a formal possibility. Addressing this question required a method to distinguish infected from uninfected cells within a mixed population while simultaneously measuring levels of cell surface proteins.
FIG. 5.
Absence of soluble paracrine effects in downregulation of immunoregulatory proteins by KSHV. (A to D) Flow cytometric analysis of naïve T4 TIME cells incubated with virus-free medium from KSHV-infected (green line) or mock-infected (pink line) T4 TIME cells and stained with the indicated antibodies.
Development of flow cytometric approach to identify KSHV-infected cells.
Since the downregulation of immunomodulatory proteins by KSHV did not appear to involve paracrine effects, we next tested if the effects were dependent on direct infection with KSHV. To investigate this possibility, we developed a flow cytometric approach, similar to that described by Kellam et al., to identify KSHV-positive cells following de novo infection (37). As a marker of infection, we chose to monitor the expression of LANA because it is an abundant viral protein expressed in all KSHV-infected cells (33, 34, 50). We first conjugated the highly reactive fluorochrome Alexa 488 to an anti-LANA rat monoclonal antibody to identify LANA-expressing cells in culture and then devised a fixative process to detect LANA expression in the nucleus while preserving membrane integrity for cell surface analysis (see Materials and Methods).
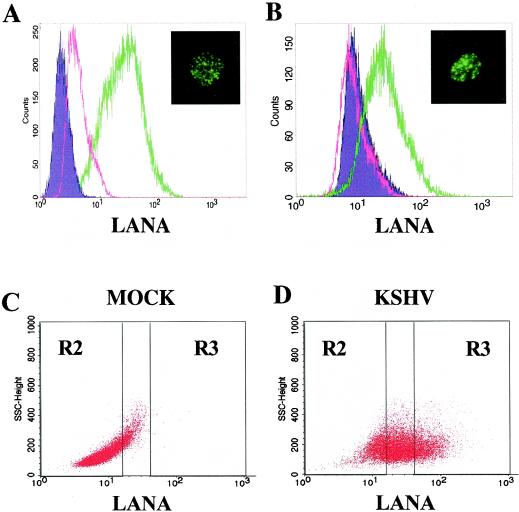
To validate this approach, we tested the ability of this conjugated antibody to detect LANA expression within the KSHV-infected BCBL-1 cell line by flow cytometry. Greater than 95% of BCBL-1 cells were positive for LANA with this technique, while the reactivity of the same antibody with the KSHV-uninfected B-cell line BJAB was minimal (Fig. 6A). Furthermore, fluorescent microscopy revealed that BCBL-1 cells prepared for flow cytometric analysis with the fluorescently labeled LANA antibody exhibited the speckled nuclear staining pattern that is characteristic of the LANA immunofluorescence assay (Fig. 6A, inset) (34-36, 50). We next used this approach to identify newly infected T4 TIME cells following KSHV infection. LANA-specific flow cytometry detected evidence of KSHV infection of T4 TIME cells within 24 h of their incubation with the virus (Fig. 6B). Dot plot analysis demonstrated a marked shift in fluorescence in a subset of the cells that stained brightly for LANA (Fig. 6D, gate R3). The mean fluorescence intensity of infected cells was also well above the background of mock-infected cells (Fig. 6C).
FIG. 6.
Flow cytometry of LANA staining in KSHV-infected B cells and T4 TIME cells. (A) KSHV-infected BCBL-1 cells (green line) and uninfected control BJAB cells (pink line) were stained with a fluorescently labeled monoclonal antibody targeting LANA. Inset: A typical KSHV-infected BCBL-1 cell after LANA staining for flow cytometry but visualized by fluorescence microscopy. (B) KSHV-infected T4 TIME cells (green line) and mock-infected T4 TIME cells (pink line) stained for LANA as in panel A. Inset: A typical KSHV-infected T4 TIME cell after LANA staining for flow cytometry but visualized by fluorescence microscopy. (C) Dot plot analysis of LANA expression in mock-infected T4 TIME cells. (D) Dot plot analysis of LANA expression in KSHV-infected T4 TIME cells. Gates R2 and R3 represent cells after virus exposure with the least (bottom 25%) and the greatest (top 25%) reactivity with LANA monoclonal antibody, respectively. Gate R1 was used to isolate live cells by forward and side scatter (not shown). Note that essentially no mock-infected T4 TIME cells fell within the R3 gate.
In subsequent experiments, we compared cells with the highest (top 25%) LANA signal (Fig. 6D, gate R3) to those with the lowest (bottom 25%) (Fig. 6D, gate R2) to unambiguously delineate any potential differences in the surface protein expression profile of infected versus uninfected cells. The latter group stained with the same low intensity as naïve cells and therefore likely represented only background staining. This strict gating approach allowed us to focus on KSHV-infected (LANA-positive) cells in the culture while minimizing potentially confounding effects from background fluorescence.
Of note, in determining absolute infection rates, this flow cytometric technique, although specific for KSHV-infected cells, was less sensitive than parallel immunofluorescence assays with the same anti-LANA monoclonal antibody. This difference likely reflects the advantage of the immunofluorescence assay over flow cytometry in distinguishing between the distinct intranuclear, punctate LANA staining pattern and nonspecific background fluorescence. As a result, we used an anti-LANA immunofluorescence assay in each experiment to more accurately assess overall infection rates.
Downregulation of MHC class I, PE-CAM, and ICAM-1 on KSHV-infected T4 TIME cells.
We next investigated whether the cells that exhibited immunoregulatory protein downregulation tracked with the population that gated as LANA positive by flow cytometry. KSHV infection of T4 TIME cells followed by staining for both intranuclear LANA and surface proteins demonstrated that increased expression of LANA generally correlated with lower expression of MHC class I, shifting the population from the upper left quadrant (low LANA/high MHC class I) to the lower right quadrant (high LANA/low MHC class I) in flow cytometry analyses (Fig. 7B). A similar association was also evident between LANA expression and the downregulation of PE-CAM (Fig. 7D) and ICAM-1 (Fig. 7F), although ICAM-1 downregulation was also present in cells with intermediate to low LANA expression. In contrast, LFA-3 surface expression remained essentially unchanged in the same population, showing no correlation with LANA expression (Fig. 7H).
FIG. 7.
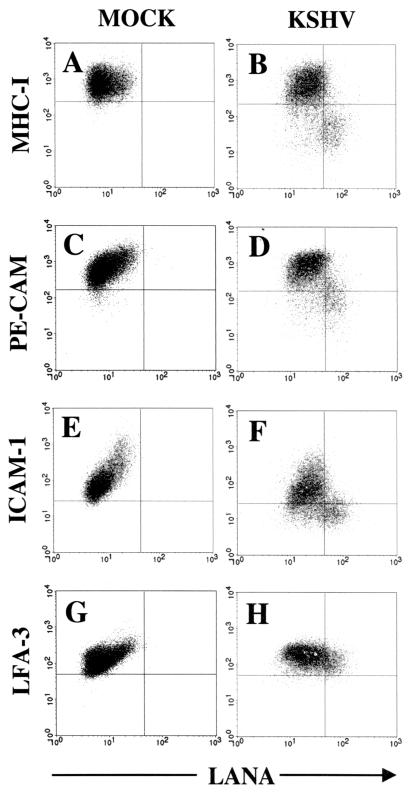
Association between LANA expression (x axis) and downregulation of immunoregulatory proteins (y axis) in T4 TIME cells. (A to H) Flow cytometry of mock-infected and KSHV-infected cells following membrane and nuclear staining with the indicated antibodies 48 h after infection. Note the shift in the cell population in KSHV-infected samples from the upper left quadrant to the lower right quadrant for MHC class I, PE-CAM, and ICAM-1 but not LFA-3. Quadrants were set based on mock-infected control samples for the indicated surface antibodies and isotype controls for LANA staining.
Although these data suggested a trend between level of LANA expression and downregulation of MHC class I, PE-CAM, and, to a lesser extent, ICAM-1, the lack of signal intensity from intranuclear LANA fluorescence in our flow cytometry assay prevented a more definitive assessment of this relationship. Specifically, the LANA flow cytometry assay was unable to separate sufficiently infected cells with intermediate levels of LANA signal from uninfected cells with similar but nonspecific background fluorescence (note the overlap in LANA staining between KSHV-infected and mock-infected cells in Fig. 6B.) Therefore, in subsequent experiments, we used the strict gating approach shown in Fig. 6D to compare downregulation between the cells exhibiting the greatest (top 25%) and the least (bottom 25%) LANA fluorescence intensity and excluded the remainder of the cells for which the LANA signal overlapped with background staining exhibited on mock-infected cells. This gating focused the comparison between the populations that most likely represented infected and uninfected cells.
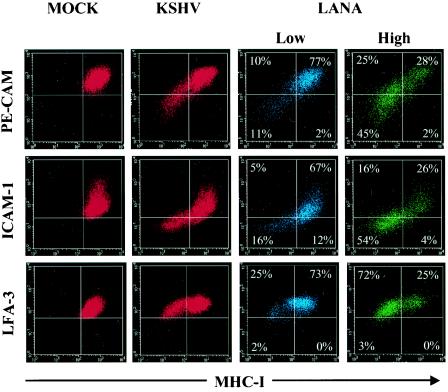
Using four-color flow cytometric staining, we identified a close association between the downregulation of MHC class I and the downregulation of both PE-CAM and ICAM-1 (but not LFA-3) following KSHV infection, suggesting that these processes arise on a similar population of cells (Fig. 8, second column, lower left quadrants). To determine whether this population comprised infected or uninfected cells in the mixed culture, we stained T4 TIME cells for both cell surface protein expression and intranuclear LANA expression and gated on cells with the highest (top 25%) and lowest (bottom 25%) LANA signal as described above. We found that the bulk of the KSHV-infected population (highest LANA expressers) exhibited downregulation of MHC class I, PE-CAM, and ICAM-1, as evidenced by a shift in the cell population from the upper right quadrant to the lower left quadrant (Fig. 8, compare the third and fourth columns). Approximately 70% of KSHV-infected (LANA-positive) cells exhibited downregulation of MHC class I, while less than 3% of the same population showed downregulation of LFA-3 (Fig. 8, fourth column). In addition, codownregulation of MHC class I with PE-CAM and ICAM-1 was pronounced on the high LANA expressers but only minimal on the low LANA expressers (Fig. 8, compare the third and fourth columns, lower left quadrants). Together, these results indicate that the downregulation of immunoregulatory proteins occurs preferentially on cells directly infected with KSHV (highest LANA expressers).
FIG. 8.
Downregulation of immunoregulatory markers on KSHV-infected but not mock-infected T4 TIME cells. Mock-infected (first column) and KSHV-infected (second column) T4 TIME cells were stained for surface protein expression with the indicated antibodies 48 h postinfection. The third and fourth columns show downregulation of immunoregulatory proteins on cells from the second column but first gated on the lowest (bottom 25%, blue cells) or the highest (top 25%, green cells) LANA-expressing cells (see Fig. 6D). Quadrants were set based on mock-infected control samples for the indicated surface antibodies. Values represent the percentage of the gated cell population present within each quadrant.
Primary DMVEC infected by coculture display similar patterns of MHC class I, PE-CAM, and ICAM-1 downregulation.
We next asked if KSHV infection of pDMVEC would recapitulate the pattern of immunomodulatory protein downregulation we observed following direct infection of T4 TIME cells. To circumvent the inefficiency of pDMVEC infection with cell-free virus, we modified a coculture technique first reported by Sakurada and coworkers, demonstrating that direct incubation of BCBL-1 cells with primary human umbilical vein endothelial cells (HUVEC) resulted in high-efficiency KSHV infection (58). In brief, we incubated BCBL-1 cells with O-tetradecanoyl phorbol 13-acetate and sodium butyrate for 12 h to initiate lytic replication and, after 3 days (the earliest time at which we detected released infectious virions [47; C. M. O'Connor and D. H. Kedes, unpublished results]), cocultured these cells with adherent pDMVEC for 12 h in medium modified to support the growth of both cell types (see Materials and Methods).
Infection of pDMVEC with the coculture method resulted in a dramatic morphological change and the onset of LANA expression within 24 h that was reminiscent of KSHV-infected T4 TIME cells (not shown). These changes were specific to infection with KSHV rather than general effects of the coculture system, since incubation with either uninduced BCBL-1 cells or BCBL-1 cells induced in the presence of the herpesvirus DNA replication inhibitor phosphonoformic acid induced no discernible change in the pDMVEC target cells (not shown). We were able to vary the proportion of infected cells by adjusting the ratio of induced BCBL-1 cells to pDMVEC within each coculture. For most experiments, we optimized infection rates of the pDMVEC to approximately 50% with a 10-fold excess of induced BCBL-1 cells, mimicking the infection rates we achieved in T4 TIME cells infected with approximately 5,000 genome copies per cell (see above).
Results from flow cytometric analyses of newly infected pDMVEC cells paralleled the patterns of MHC class I downregulation (Fig. 9A) in KSHV-infected T4 TIME cells, with again little to no change in surface expression of LFA-3 (Fig. 9B). As with the morphological changes we described above, downregulation was specific to infection by KSHV, since incubation with either uninduced BCBL-1 cells (not shown) or BCBL-1 cells induced in the presence of phosphonoformic acid led to no such reduction on the pDMVEC target cells (Fig. 9A). PE-CAM and ICAM-1 were likewise downregulated by KSHV following coculture infection, and their downregulation tracked with MHC class I downregulation (Fig. 9C and D, lower left quadrants). In addition, LANA costaining by flow cytometry revealed an association between high levels of LANA expression and the downregulation of MHC class I (Fig. 9E), PE-CAM, and ICAM-1 (not shown) but not LFA-3 (Fig. 9F) on newly infected pDMVEC.
FIG. 9.
Downregulation of immunoregulatory proteins on pDMVEC cells following coculture infection with KSHV. (A and B) Flow cytometric analysis of KSHV-infected (green line) and mock-infected (pink line) pDMVEC cells stained for surface protein expression with the indicated antibodies 48 h after infection. Mock-infected pDMVEC cells were cocultured with BCBL-1 cells induced in the presence of phosphonoformic acid. (C and D) Simultaneous downregulation of PE-CAM and ICAM-1, respectively, with MHC class I on the surface of pDMVEC cells after coculture infection. Quadrants were set as in Fig. 8. (E and F) Expression of MHC class I but not of LFA-3 decreased on cells displaying the highest levels of LANA staining. Quadrants were set as in Fig. 7. Note that the levels of KSHV infection per cell and, as a result, the number of cells exhibiting downregulation was slightly lower in this experiment than in most others.
We next characterized the relative levels of viral lytic gene expression within the newly infected pDMVEC as we had for the T4 TIME cells. Sakurada and colleagues reported that coculture infection of HUVEC results in a predominantly latent system of infection and that detection of lytic expression by immunofluorescence assay was minimal (58). To confirm whether KSHV infection within pDMVEC displayed a similar latent predisposition after coculture, we assayed for KSHV lytic protein expression by the immunofluorescence assay with the same set of antibodies to immediate-early and late lytic proteins we had assayed in the T4 TIME cell system (see above). We found that coculture infection of pDMVEC cells led to a predominantly latent infection, with immunofluorescent evidence of immediate-early expression of the lytic proteins RTA and MIR-2 confined to less than 1% of infected cells (Fig. 10A, third column). The remaining 99% of the infected cells demonstrated staining for those two proteins at levels no greater than with secondary antibody alone (Fig. 10A, third column), despite characteristic LANA staining (Fig. 10A, first column). Immunofluorescence assays with antibodies to the ORF 45, SCAF, and MCP proteins showed a similarly low prevalence in the pDMVEC cultures (not shown). These findings paralleled the results we obtained after direct cell-free KSHV infection of T4 TIME cells and indicate that de novo infection of either primary or telomerase-immortalized endothelial cells leads primarily to viral latency, with only rare immunofluorescence assay evidence of lytic gene expression within the population.
FIG. 10.
KSHV-infected pDMVEC cells stained for latent and lytic protein expression with the indicated antibodies either 2 (A) or 23 (B) days after coculture infection. The first, second, and third columns represent immunofluorescence assays of a single field for LANA, DAPI, and the indicated lytic protein, respectively. Note that the background reactivity of KSHV-infected pDMVEC cells to the indicated lytic antibodies was indistinguishable from that with secondary antibody alone (not shown for day 23).
Finally, to assess the duration of KSHV-induced downregulation in primary cells, we examined changes in cell surface protein expression in pDMVEC cells 3 weeks postinfection. To facilitate this, we infected pDMVEC with a lower initial ratio of induced BCBL-1 to pDMVEC than we used for short-term cultures (1:1 versus 10:1, respectively) and let the virus spread slowly through the culture. A similar approach has led to long-term KSHV infection with small initial amounts of purified virus to infect immortalized endothelial cells (13, 46). By 3 weeks postinfection, approximately 95% of cells became LANA positive in the immunofluorescence assay (Fig. 10B, first column) and exhibited morphological changes consistent with infection by KSHV (not shown). Long-term-infected pDMVEC also continued to display a primarily latent repertoire of KSHV gene expression, with immunofluorescence assay evidence of immediate-early lytic proteins such as RTA and MIR-2 confined to an extremely small (<1%) proportion of the infected cells (Fig. 10B, third column).
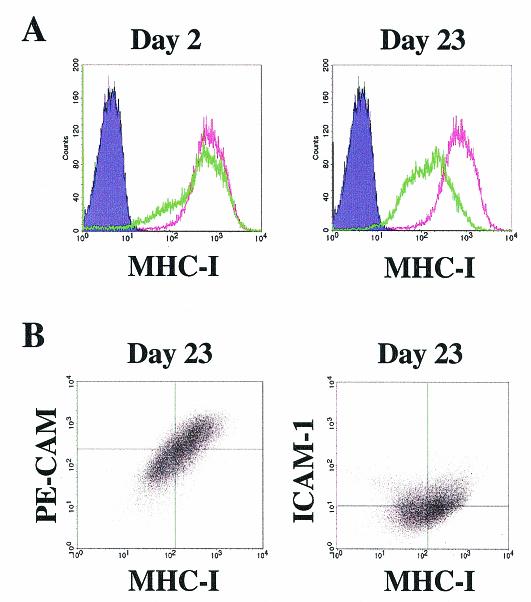
Along with the nearly 100% LANA reactivity in these long-term cultures, virtually all of the cells continued to display marked downregulation of MHC class I compared with a smaller fraction at day 2 postinfection, when LANA reactivity was confined to less than 10% of the cells (Fig. 11A). PE-CAM and ICAM-1 were also downregulated by KSHV following long-term coculture infection, and their downregulation paralleled the reduction in MHC class I (Fig. 11B, lower left quadrants). These findings suggest that downregulation of immunoregulatory molecules by KSHV is not a transient process but rather a continuous phenomenon associated with infection.
FIG. 11.
Continued downregulation of MHC class I, PE-CAM, and ICAM-1 on pDMVEC cells after long-term KSHV infection. (A) pDMVEC cells stained for MHC class I expression by flow cytometry at days 2 and 23 postcoculture infection. (B) Simultaneous downregulation of PE-CAM and ICAM-1 with MHC class I on the surface of KSHV-infected pDMVEC cells 23 days after coculture infection. Quadrants were set as in Fig. 8.
DISCUSSION
Here we describe the surface downregulation of immunoregulatory proteins by KSHV in newly infected endothelial cells in culture. Using flow cytometry, we observed clear downregulation of MHC class I, PE-CAM, and ICAM-1 on both immortalized (Fig. 3) and primary (Fig. 9) endothelial cells following de novo infection with KSHV. This downregulation appears to be a specific phenomenon rather than a result of a global downregulatory mechanism, since levels of other surface markers, including Fas (not shown) and LFA-3 (Fig. 3 and 9), remain unchanged or slightly elevated after infection. Furthermore, the downregulation that we observed is not a transient mechanism associated only with the early events of viral infection. Instead, downregulation of immunoregulatory proteins persists in KSHV-infected cells even after 3 weeks in culture (Fig. 11). However, we cannot exclude the possibility that long-term infection with KSHV requires continual reinfection of naïve cells rather than the long-lived growth and expansion of KSHV-infected cells from earlier time points postinfection.
Importantly, work by others has demonstrated that KSHV infection of similarly derived TIME cells results in a predominantly latent profile of viral gene expression (4, 38). The present work is the first demonstration of efficient KSHV infection of pDMVEC by coculture, although infection of HUVEC cells by this method also results in a primarily latent viral gene expression profile (58). Similarly, our analysis of both immediate-early and late lytic protein expression by immunofluorescence also indicates a mainly latent infection not only for the T4 TIME cells but also for pDMVEC. In both systems, less than 1% of KSHV-infected (LANA-positive) cells expressed detectable lytic proteins after KSHV infection (Fig. 2 and 10).
The number of cells exhibiting surface molecule downregulation varied proportionally with the amount of input virus and correlated roughly with the extent of KSHV infection within each infected culture. This suggests that direct infection with KSHV may lead to immunoregulatory protein downregulation and led us to investigate downregulation on an individual-cell basis. To this end, we created a flow cytometric assay targeting the LANA protein of KSHV to identify newly infected cells in culture. This assay exhibits both high sensitivity and specificity when tested on mixtures of KSHV-infected PEL cells and uninfected B cells (Fig. 6A) and also distinguishes newly infected T4 TIME cells from uninfected cells in the same culture (Fig. 6B). T4 TIME cells, however, are less well separated because they have lower overall levels of LANA expression than BCBL-1 cells (Fig. 6A and B). Nevertheless, it is apparent that, when used together with staining of immunoregulatory molecules on the surface of cells, this approach confirms that downregulation of MHC class I, PE-CAM, and ICAM-1 but not LFA-3 occurs primarily on KSHV-infected (LANA-positive) cells (Fig. 8, fourth column).
These results are supported by paracrine experiments showing that virus-free medium from KSHV-infected cells fails to induce downregulation of the same markers on naïve cells (Fig. 5). However, we also found that a small fraction (approximately 15%) of LANA-negative cells exhibited immunoregulatory protein downregulation. While it is possible that the poor LANA expressers (the R2 gate in Fig. 6D) contain a subset of infected cells with LANA levels that fall below the detection limit of our assay, they may also represent an essentially uninfected group of cells. Therefore, we cannot exclude the possibility that infected cells may exert a downregulatory effect on some of their uninfected neighbors. Similarly, a small but consistent minority (approximately 25%) of KSHV-infected (LANA-positive cells) failed to undergo immunoregulatory protein downregulation. This may be explained by a threshold effect of downregulation where measurable immunoregulatory protein downregulation requires a critical level of viral gene expression.
In agreement with this hypothesis, our recent findings indicate that the degree of downregulation best parallels the percentage of infected cells in the culture exhibiting 25 or more discrete punctate LANA dots per nucleus in the immunofluorescence assay (Tomescu and Kedes, unpublished observations). In contrast, infection of T4 TIME cells or pDMVEC with smaller amounts of virus leads to fewer dots per nucleus and a correspondingly lower degree of immunoregulatory protein downregulation. These analyses indicate that downregulation of immunomodulatory proteins following de novo infection occurs preferentially on KSHV-infected cells and that manifestation of the effect, and perhaps its magnitude, may depend on initially delivering a sufficient number of viral genomes into each cell or, in longer-term infections, generating sufficient copies of the viral episome.
The incubation of naïve T4 TIME cells with conditioned medium from KSHV-infected T4 TIME cells leads to a modest upregulation of PE-CAM and ICAM-1, likely reflecting the inflammatory nature of KSHV infection (Fig. 5). Likewise, incubation of naïve T4 TIME cells with either UV- or heat-inactivated KSHV leads to similar upregulation during mock infection (data not shown). Finally, flow cytometry indicates that even within KSHV-infected cultures, a fraction of cells demonstrates an increased level of PE-CAM and ICAM-1 cell surface expression compared to cells in parallel cultures not exposed to virus (data not shown). The size of this population of cells is proportional to the percentage of cells judged to be uninfected (LANA negative) in each experiment by parallel immunofluorescence assay (not shown). The remainder of the cells within these KSHV-infected culture has levels of PE-CAM and ICAM-1 that are at or below the basal levels of these proteins on naïve T4 TIME cells.
One explanation for these results is that that the presence of KSHV induces a strong inflammatory reaction in T4 TIME cells but that infection of these cells with KSHV is sufficient to prevent this upregulation. This may have important implications for immune evasion since PE-CAM and ICAM-1 signaling by endothelial cells is required to induce the full activation and killing capacity of CTLs (12, 73, 74). Furthermore, these findings emphasize the strength of this immunomodulatory effect and highlight the ability of KSHV to induce downregulation in the presence of an inflammatory environment normally aimed at upregulating these molecules. In vivo, immunohistochemical studies indicate that while PE-CAM and ICAM-1 expression is detectable in Kaposi's sarcoma lesions, their expression within tumor spindle cells themselves is often patchy or absent (18, 31, 51, 56). Therefore, downregulation of PE-CAM and ICAM-1 expression by KSHV may constitute a continual protective strategy used by the virus within the inflammatory environment of the lesion (60, 69).
The downregulation of MHC class I and/or costimulatory molecules is a common immune evasion tactic used by many viruses, including several members of the herpesvirus family (2, 29, 59, 68). For herpesviruses, these strategies help to establish and maintain infection in the host despite the continuous presence of a virus-specific cellular immune response. The importance of such defensive mechanisms in herpesviruses is particularly well exemplified by human cytomegalovirus, which encodes four separate proteins to block MHC class I presentation at three fundamentally different steps (68). Recently, several groups have shown that KSHV encodes two immediate-early lytic proteins that serve an analogous function. These proteins, MIR-1 and MIR-2, are capable of downregulating MHC class I proteins as well as T-cell costimulatory molecules when overexpressed in heterologous cells in vitro (14, 15, 26, 28). At least in these single-gene experiments, the resultant downregulation is sufficient to provide cells with resistance to immune cells in culture (27) and therefore may protect KSHV-infected cells undergoing lytic replication in vivo.
The contribution of MIR-1 and MIR-2 to immune evasion by KSHV during latency, however, is less clear. Analysis of viral mRNA expression both in vivo within Kaposi's sarcoma lesions and in vitro in infected PEL cell lines indicates that, in most cells, KSHV gene expression is restricted to a small subset of latent genes (19, 33, 49, 62, 65, 67, 77). In addition, our immunofluorescence assay of lytic protein expression in the culture systems described in this report also argues that only a small proportion (approximately 1%) of all infected cells undergoes spontaneous lytic reactivation, with the remainder of cells harboring KSHV in the latent form. A priori, an effective overall immune evasion strategy employed by KSHV would protect not only this small number of lytically infected cells during their relatively short life span, but also the more predominant population of latently infected cells that may persist within the host for prolonged periods.
Since the majority of KSHV-infected cells adopt a latent phenotype, it follows that downregulation of immunoregulatory proteins both in vivo and in the two models of infection that we describe here may depend on a latent gene or combination of latent genes. Nevertheless, we cannot rule out the distinct possibility that extremely low levels of lytic gene products, including MIR-1 and/or MIR-2, remain below the detection levels of our assays yet are responsible, at least in part, for the immunoregulatory protein downregulation that we observed following de novo infection with KSHV. Recent studies have shown that MIR-1 and MIR-2 function as type 3 ubiquitin ligases, targeting surface molecules for internalization and subsequent destruction through the ubiquitin-proteosome pathway (16, 41). Consequently, their enzymatic nature allows the theoretical possibility that low levels of these proteins (potentially undetectable by the immunofluorescence assay) may be sufficient to induce surface molecule downregulation. In addition, a recent study has shown that MIR-2 may represent a unique KSHV immediate-early lytic protein whose expression may be independent of the main lytic transactivator of KSHV, RTA (48). Therefore, determining the importance of latent and/or lytic genes in biologically meaningful modes of immune evasion during the different stages of KSHV infection and tumorigenesis will depend on not only determining the genes that are capable of inducing these effects but also assessing the nature of the balance between the latent and lytic state within individually infected cells.
Acknowledgments
We thank David Lukac, Yan Yuan, and Gary Hayward for the kind gifts of polyclonal antisera for this project. We also thank Robert Weinberg for providing us with the pBABE vector encoding hTERT and Christian Brander for sharing data prior to publication and for helpful discussions.
This work was supported by the Doris Duke Charitable Foundation (20000355), NIH (CA88768-01), and the Pew Memorial Trust (97003260-000).
REFERENCES
- 1.Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. L. Ploegh, P. A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the human cytomegalovirus glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, K., T. H. Meyer, S. Uebel, P. Sempe, H. Djaballah, Y. Yang, P. A. Peterson, K. Fruh, and R. Tampe. 1996. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 15:3247-3255. [PMC free article] [PubMed] [Google Scholar]
- 3.Andre, S., O. Schatz, J. R. Bogner, H. Zeichhardt, M. Stoffler-Meilicke, H. U. Jahn, R. Ullrich, A. K. Sonntag, R. Kehm, and J. Haas. 1997. Detection of antibodies against viral capsid proteins of human herpesvirus 8 in AIDS-associated Kaposi's sarcoma. J. Mol. Med. 75:145-152. [DOI] [PubMed] [Google Scholar]
- 4.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beersma, M. F., M. J. Bijlmakers, and H. L. Ploegh. 1993. Hum. cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J. Immunol. 151:4455-4464. [PubMed] [Google Scholar]
- 6.Brander, C., P. O'Connor, T. Suscovich, N. G. Jones, Y. Lee, D. Kedes, D. Ganem, J. Martin, D. Osmond, S. Southwood, A. Sette, B. D. Walker, and D. T. Scadden. 2001. Definition of an optimal cytotoxic T lymphocyte epitope in the latently expressed Kaposi's sarcoma-associated herpesvirus kaposin protein. J. Infect. Dis. 184:119-126. [DOI] [PubMed] [Google Scholar]
- 7.Brander, C., T. Suscovich, Y. Lee, P. T. Nguyen, P. O'Connor, J. Seebach, N. G. Jones, M. van Gorder, B. D. Walker, and D. T. Scadden. 2000. Impaired CTL recognition of cells latently infected with Kaposi's sarcoma-associated herpes virus. J. Immunol. 165:2077-2083. [DOI] [PubMed] [Google Scholar]
- 8.Burysek, L., W. S. Yeow, and P. M. Pitha. 1999. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2). J. Hum. Virol. 2:19-32. [PubMed] [Google Scholar]
- 9.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 10.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 11.Chatterjee, M., J. Osborne, G. Bestetti, Y. Chang, and P. S. Moore. 2002. Viral IL-6-induced cell proliferation and immune evasion of interferon activity. Science 298:1432-1435. [DOI] [PubMed] [Google Scholar]
- 12.Chen, Y., P. G. Schlegel, N. Tran, D. Thompson, J. L. Zehnder, and N. J. Chao. 1997. Administration of a CD31-derived peptide delays the onset and significantly increases survival from lethal graft-versus-host disease. Blood 89:1452-1459. [PubMed] [Google Scholar]
- 13.Ciufo, D. M., J. S. Cannon, L. J. Poole, F. Y. Wu, P. Murray, R. F. Ambinder, and G. S. Hayward. 2001. Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures. J. Virol. 75:5614-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T-cell costimulation. J. Clin. Investig. 107:1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coscoy, L., D. J. Sanchez, and D. Ganem. 2001. A novel class of herpesvirus-encoded membrane-bound E3 ubiquitin ligases regulates endocytosis of proteins involved in immune recognition. J. Cell Biol. 155:1265-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Waal Malefyt, R., J. Haanen, H. Spits, M. G. Roncarolo, A. te Velde, C. Figdor, K. Johnson, R. Kastelein, H. Yssel, and J. E. de Vries. 1991. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T-cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 174:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeYoung, B. R., P. E. Swanson, Z. B. Argenyi, J. H. Ritter, J. F. Fitzgibbon, D. J. Stahl, W. Hoover, and M. R. Wick. 1995. CD31 immunoreactivity in mesenchymal neoplasms of the skin and subcutis: report of 145 cases and review of putative immunohistologic markers of endothelial differentiation. J. Cutan. Pathol. 22:215-222. [DOI] [PubMed] [Google Scholar]
- 19.Dittmer, D., M. Lagunoff, R. Renne, K. Staskus, A. Haase, and D. Ganem. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Djerbi, M., V. Screpanti, A. I. Catrina, B. Bogen, P. Biberfeld, and A. Grandien. 1999. The inhibitor of death receptor signaling, FLICE-inhibitory protein defines a new class of tumor progression factors. J. Exp. Med. 190:1025-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupin, N., T. L. Diss, P. Kellam, M. Tulliez, M. Q. Du, D. Sicard, R. A. Weiss, P. G. Isaacson, and C. Boshoff. 2000. HHV-8 is associated with a plasmablastic variant of Castleman disease that is linked to HHV-8-positive plasmablastic lymphoma. Blood 95:1406-1412. [PubMed] [Google Scholar]
- 22.Flore, O., S. Rafii, S. Ely, J. J. O'Leary, E. M. Hyjek, and E. Cesarman. 1998. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature 394:588-592. [DOI] [PubMed] [Google Scholar]
- 23.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 24.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith, K., W. Chen, D. C. Johnson, and R. L. Hendricks. 1998. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T-cell response. J. Exp. Med. 187:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haque, M., K. Ueda, K. Nakano, Y. Hirata, C. Parravicini, M. Corbellino, and K. Yamanishi. 2001. Major histocompatibility complex class I molecules are down-regulated at the cell surface by the K5 protein encoded by Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8. J. Gen. Virol. 82:1175-1180. [DOI] [PubMed] [Google Scholar]
- 27.Ishido, S., J. K. Choi, B. S. Lee, C. Wang, M. DeMaria, R. P. Johnson, G. B. Cohen, and J. U. Jung. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13:365-374. [DOI] [PubMed] [Google Scholar]
- 28.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janeway, C. A., P. Travers, M. Walport, and J. D. Capra. 1999. Immunobiology, 4th ed. Elsevier Science Ltd./Garland Publishing, New York, N.Y.
- 30.Jun, Y., E. Kim, M. Jin, H. C. Sung, H. Han, D. E. Geraghty, and K. Ahn. 2000. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 164:805-811. [DOI] [PubMed] [Google Scholar]
- 31.Kaaya, E. E., E. Castanos-Velez, H. Amir, L. Lema, J. Luande, J. Kitinya, M. Patarroyo, and P. Biberfeld. 1996. Expression of adhesion molecules in endemic and epidemic Kaposi's sarcoma. Histopathology 29:337-346. [DOI] [PubMed] [Google Scholar]
- 32.Katano, H., T. Sata, T. Suda, T. Nakamura, N. Tachikawa, H. Nishizumi, S. Sakurada, Y. Hayashi, M. Koike, A. Iwamoto, T. Kurata, and S. Mori. 1999. Expression and antigenicity of human herpesvirus 8 encoded ORF59 protein in AIDS-associated Kaposi's sarcoma. J. Med. Virol. 59:346-355. [DOI] [PubMed] [Google Scholar]
- 33.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 1999. High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi's sarcoma. Am. J. Pathol. 155:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedes, D. H., M. Lagunoff, R. Renne, and D. Ganem. 1997. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi's sarcoma-associated herpesvirus. J. Clin. Investig. 100:2606-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in Kaposi's sarcoma risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 36.Kellam, P., C. Boshoff, D. Whitby, S. Matthews, R. A. Weiss, and S. J. Talbot. 1997. Identification of a major latent nuclear antigen, LNA-1, in the human herpesvirus 8 genome. J. Hum. Virol. 1:19-29. [PubMed] [Google Scholar]
- 37.Kellam, P., D. Bourboulia, N. Dupin, C. Shotton, C. Fisher, S. Talbot, C. Boshoff, and R. A. Weiss. 1999. Characterization of monoclonal antibodies raised against the latent nuclear antigen of human herpesvirus 8. J. Virol. 73:5149-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, B. S., X. Alvarez, S. Ishido, A. A. Lackner, and J. U. Jung. 2000. Inhibition of intracellular transport of B cell antigen receptor complexes by Kaposi's sarcoma-associated herpesvirus K1. J. Exp. Med. 192:11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, J. J., Y. Q. Huang, C. J. Cockerell, and A. E. Friedman-Kien. 1996. Localization of human herpes-like virus type 8 in vascular endothelial cells and perivascular spindle-shaped cells of Kaposi's sarcoma lesions by in situ hybridization. Am. J. Pathol. 148:1741-1748. [PMC free article] [PubMed] [Google Scholar]
- 41.Lorenzo, M. E., J. U. Jung, and H. L. Ploegh. 2002. Kaposi's sarcoma-associated herpesvirus K3 utilizes the ubiquitin-proteasome system in routing class major histocompatibility complexes to late endocytic compartments. J. Virol. 76:5522-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma, W., and J. S. Pober. 1998. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J. Immunol. 161:2158-2167. [PubMed] [Google Scholar]
- 44.Micheletti, F., P. Monini, C. Fortini, P. Rimessi, M. Bazzaro, M. Andreoni, M. Giuliani, S. Traniello, B. Ensoli, and R. Gavioli. 2002. Identification of cytotoxic T lymphocyte epitopes of human herpesvirus 8. Immunology 106:395-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 46.Moses, A. V., K. N. Fish, R. Ruhl, P. P. Smith, J. G. Strussenberg, L. Zhu, B. Chandran, and J. A. Nelson. 1999. Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J. Virol. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nealon, K., W. W. Newcomb, T. R. Pray, C. S. Craik, J. C. Brown, and D. H. Kedes. 2001. Lytic replication of Kaposi's sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J. Virol. 75:2866-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okuno, T., Y. B. Jiang, K. Ueda, K. Nishimura, T. Tamura, and K. Yamanishi. 2002. Activation of human herpesvirus 8 open reading frame K5 independent of ORF50 expression. Virus Res. 90:77-89. [DOI] [PubMed] [Google Scholar]
- 49.Parravicini, C., B. Chandran, M. Corbellino, E. Berti, M. Paulli, P. S. Moore, and Y. Chang. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rainbow, L., G. M. Platt, G. R. Simpson, R. Sarid, S. J. Gao, H. Stoiber, C. S. Herrington, P. S. Moore, and T. F. Schulz. 1997. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by ORF73 and is a component of the latency-associated nuclear antigen. J. Virol. 71:5915-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Regezi, J. A., L. A. MacPhail, T. E. Daniels, Y. G. DeSouza, J. S. Greenspan, and D. Greenspan. 1993. Hum. immunodeficiency virus-associated oral Kaposi's sarcoma. A heterogeneous cell population dominated by spindle-shaped endothelial cells. Am. J. Pathol. 143:240-249. [PMC free article] [PubMed] [Google Scholar]
- 52.Rehm, A., A. Engelsberg, D. Tortorella, I. J. Korner, I. Lehmann, H. L. Ploegh, and U. E. Hopken. 2002. Human cytomegalovirus gene products US2 and US11 differ in their ability to attack major histocompatibility class I heavy chains in dendritic cells. J. Virol. 76:5043-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 55.Rivas, C., A. E. Thlick, C. Parravicini, P. S. Moore, and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus LANA2 is a B-cell-specific latent viral protein that inhibits p53. J. Virol. 75:429-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Russell Jones, R., G. Orchard, B. Zelger, and E. Wilson Jones. 1995. Immunostaining for CD31 and CD34 in Kaposi sarcoma. J. Clin. Pathol. 48:1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutgers, J. L., R. Wieczorek, F. Bonetti, K. L. Kaplan, D. N. Posnett, A. E. Friedman-Kien, and D. M. Knowles 2nd. 1986. The expression of endothelial cell surface antigens by AIDS-associated Kaposi's sarcoma. Evidence for a vascular endothelial cell origin. Am. J. Pathol. 122:493-499. [PMC free article] [PubMed] [Google Scholar]
- 58.Sakurada, S., H. Katano, T. Sata, H. Ohkuni, T. Watanabe, and S. Mori. 2001. Effective human herpesvirus 8 infection of human umbilical vein endothelial cells by cell-mediated transmission. J. Virol. 75:7717-7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salek-Ardakani, S., J. R. Arrand, and M. Mackett. 2002. Epstein-Barr virus encoded interleukin-10 inhibits HLA-class I, ICAM-1, and B7 expression on human monocytes: implications for immune evasion by Epstein-Barr virus. Virology 304:342-351. [DOI] [PubMed] [Google Scholar]
- 60.Samaniego, F., P. D. Markham, R. C. Gallo, and B. Ensoli. 1995. Inflammatory cytokines induce AIDS-Kaposi's sarcoma-derived spindle cells to produce and release basic fibroblast growth factor and enhance Kaposi's sarcoma-like lesion formation in nude mice. J. Immunol. 154:3582-3592. [PubMed] [Google Scholar]
- 61.Sanchez, D. J., L. Coscoy, and D. Ganem. 2002. Functional organization of MIR2, a novel viral regulator of selective endocytosis. J. Biol. Chem. 277:6124-6130. [DOI] [PubMed] [Google Scholar]
- 62.Sarid, R., O. Flore, R. A. Bohenzky, Y. Chang, and P. S. Moore. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarid, R., T. Sato, R. A. Bohenzky, J. J. Russo, and Y. Chang. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat. Med. 3:293-298. [DOI] [PubMed] [Google Scholar]
- 64.Spiller, O. B., M. Robinson, E. O'Donnell, S. Milligan, B. P. Morgan, A. J. Davison, and D. J. Blackbourn. 2003. Complement regulation by Kaposi's sarcoma-associated herpesvirus ORF4 protein. J. Virol. 77:592-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swerlick, R. A., E. Garcia-Gonzalez, Y. Kubota, Y. L. Xu, and T. J. Lawley. 1991. Studies of the modulation of MHC antigen and cell adhesion molecule expression on human dermal microvascular endothelial cells. J. Investig. Dermatol. 97:190-196. [DOI] [PubMed] [Google Scholar]
- 67.Talbot, S. J., R. A. Weiss, P. Kellam, and C. Boshoff. 1999. Transcriptional analysis of human herpesvirus-8 open reading frames 71, 72, 73, K14, and 74 in a primary effusion lymphoma cell line. Virology 257:84-94. [DOI] [PubMed] [Google Scholar]
- 68.Tortorella, D., B. Gewurz, D. Schust, M. Furman, and H. Ploegh. 2000. Down-regulation of MHC class I antigen presentation by human cytomegalovirus: lessons for tumor immunology. Immunol. Investig. 29:97-100. [DOI] [PubMed] [Google Scholar]
- 69.Walter, P., E. Philippe, T. Khalil, C. Nguemby-Mbina, and A. Chamlian. 1984. Kaposi's sarcoma. A vascular neoplasm of presumably viral origin. Histologic and ultrastructural characteristics. Ann. Pathol. 4:19-25. [PubMed] [Google Scholar]
- 70.Wang, H. W., T. V. Sharp, A. Koumi, G. Koentges, and C. Boshoff. 2002. Characterization of an anti-apoptotic glycoprotein encoded by Kaposi's sarcoma-associated herpesvirus which resembles a spliced variant of human survivin. EMBO J. 21:2602-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang, Q. J., F. J. Jenkins, L. P. Jacobson, L. A. Kingsley, R. D. Day, Z. W. Zhang, Y. X. Meng, P. E. Pellett, K. G. Kousoulas, A. Baghian, C. R. Rinaldo, Jr., and P. E. Pellet. 2001. Primary human herpesvirus 8 infection generates a broadly specific CD8(+) T-cell response to viral lytic cycle proteins. Blood 97:2366-2373. [DOI] [PubMed] [Google Scholar]
- 72.Weber, K. S., H. J. Grone, M. Rocken, C. Klier, S. Gu, R. Wank, A. E. Proudfoot, P. J. Nelson, and C. Weber. 2001. Selective recruitment of Th2-type cells and evasion from a cytotoxic immune response mediated by viral macrophage inhibitory protein-II. Eur. J. Immunol. 31:2458-2466. [DOI] [PubMed] [Google Scholar]
- 73.Westphal, J. R., H. W. Willems, D. J. Ruiter, and R. M. De Waal. 1993. Involvement of LFA-1/ICAM and CD2/LFA-3 in human endothelial cell accessory function. Behring Inst. Mitt. 92:51-62. [PubMed]
- 74.Westphal, J. R., H. W. Willems, W. J. Tax, R. A. Koene, D. J. Ruiter, and R. M. De Waal. 1993. Endothelial cells promote anti-CD3-induced T-cell proliferation via cell-cell contact mediated by LFA-1 and CD2. Scand. J. Immunol. 38:435-444. [DOI] [PubMed] [Google Scholar]
- 75.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]
- 76.Zeidler, R., G. Eissner, P. Meissner, S. Uebel, R. Tampe, S. Lazis, and W. Hammerschmidt. 1997. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood 90:2390-2397. [PubMed] [Google Scholar]
- 77.Zhong, W., H. Wang, B. Herndier, and D. Ganem. 1996. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc. Natl. Acad. Sci. USA 93:6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]