Abstract
Steroidal estrogens, originating principally from human excretion, are likely to play a major role in causing widespread endocrine disruption in wild populations of the roach (Rutilus rutilus), a common cyprinid fish, in rivers contaminated by treated sewage effluents. Given the extent of this problem, risk assessment models are needed to predict the location and severity of endocrine disruption in river catchments and to identify areas where regulation of sewage discharges to remove these contaminants is necessary. In this study we attempted to correlate the extent of endocrine disruption in roach in British rivers, with their predicted exposure to steroid estrogens derived from the human population. The predictions of steroid estrogen exposure at each river site were determined by combining the modeled concentrations of the individual steroid estrogens [17β -estradiol (E2), estrone (E1), and 17α -ethinylestradiol (EE2)] in each sewage effluent with their predicted dilution in the immediate receiving water. This model was applied to 45 sites on 39 rivers throughout the United Kingdom. Each site studied was then categorized as either high, medium, or low “risk” on the basis of the assumed additive potency of the three steroid estrogens calculated from data derived from published studies in various cyprinid fish species. We sampled 1,438 wild roach from the predicted high-, medium-, and low-risk river sites and examined them for evidence and severity of endocrine disruption. Both the incidence and the severity of intersex in wild roach were significantly correlated with the predicted concentrations of the natural estrogens (E1 and E2) and the synthetic contraceptive pill estrogen (EE2) present. Predicted steroid estrogen exposure was, however, less well correlated with the plasma vitellogenin concentration measured in the same fish. Moreover, we found no correlation between any of the end points measured in the roach and the proportion of industrial effluents entering the rivers we studied. Overall, our results provide further and substantive evidence to support the hypothesis that steroidal estrogens play a major role in causing intersex in wild freshwater fish in rivers in the United Kingdom and clearly show that the location and severity of these endocrine-disrupting effects can be predicted.
Keywords: disruption, endocrine, estrogen, estrone, estradiol, ethinylestradiol, intersex, roach, vitellogenin
Increasing evidence shows that wild populations of both estuarine and freshwater fish are being exposed to endocrine-disrupting chemicals (EDCs) in concentrations sufficient to cause disruption of their reproductive physiology [reviewed by Jobling and Tyler (2003)]. In particular, the occurrence of intersex fish in a number of countries has been associated with the proximity of these fish to point source sewage effluent discharges (Allen et al. 1999a, 1999b; Aravindakshan et al. 2004; Folmar et al. 1996, 2001; Gercken and Sordyl 2002; Harshbarger et al. 2000; Hassanin et al. 2002; Hecker et al. 2002; Jobling et al. 1998; Kavanagh et al. 2004; Minier et al. 2000; van Aerle et al. 2001, Vethaak et al. 2002; Vigano et al. 2001). Intersex fish can have feminized reproductive ducts and/or developing oocytes within their testes (Nolan et al. 2001). They can also have abnormal concentrations of sex steroid hormones (Jobling et al. 2002a) and (often) elevated concentrations of the estrogen-dependent blood protein vitellogenin (VTG) in their blood (Jobling et al. 1998). In severely feminized fish, fertility is reduced (Jobling et al. 2002b); hence, the contribution of these fish to the growth rate of the population is likely to be reduced. Studies published to date strongly suggest that the concentration of sewage effluent in a river is a major cause of intersexuality in wild roach (Jobling et al. 1998). Furthermore, the association between the degree of intersexuality in fish and their plasma VTG concentration suggests that the two effects have a common cause and, therefore, the estrogenic constituents of sewage effluents are likely to be responsible for the occurrence of intersexuality in wild fish populations (Jobling et al. 1998).
Toxicity, identification, and evaluation procedures employing in vitro estrogen screening assays have identified the steroid estrogens as being the most significant estrogenic compounds present in most sewage effluents (e.g. Desbrow et al. 1998; Korner et al. 2001; Matsui et al. 2000;). Because of their human origin, steroid estrogens are regularly detected in domestic sewage effluents throughout Europe, Japan, and the United States, with concentrations ranging from < 1 to 48 ng/L for 17β -estradiol (E2), from 1 to 76 ng/L for estrone (E1), and from < 1 to 7 ng/L for 17α -ethinylestradiol (EE2) (e.g., Desbrow et al. 1998; Johnson et al. 2000; Komori et al. 2004; Niven et al. 2001). The concentrations of these estrogens in river water and their impact on fish are dictated in the first instance by the available dilution in the receiving water. Over time (and with increasing distance from the discharge point), biodegradation and sorption (Jurgens et al. 2002; Williams et al. 1999) further lower these concentrations, as has been illustrated clearly in the river Nene in the United Kingdom (Williams et al. 2003). Although the concentrations of these estrogenic substances in effluents are extremely low (in the tens of nanograms per liter range), when replicated in laboratory experiments, they are high enough to induce VTG synthesis and intersex in some fish species (e.g., Balch et al. 2004; Lange et al. 2001; Metcalfe et al. 2001; Nash et al. 2004; Orn et al. 2003; Palace et al. 2002; Thorpe et al. 2003a; Van den Belt et al. 2004). Furthermore, laboratory studies have shown that the combined effects of steroid estrogens can be additive (Brian et al. 2005; Silva et al. 2002; Thorpe et al. 2003b). Hence, it is now an acknowledged possibility that even where the water concentration of one of these steroid estrogens is below the lowest observable effect concentration (LOEC), the combined mixture of steroid estrogens present could still cause an effect.
Association does not always imply causation, however, and there is still no overwhelming evidence that steroidal estrogens are the only cause of endocrine disruption in wild freshwater fish. Xenobiotic endocrine disruptors with estrogenic properties, such as 4-t-nonylphenol, are also present in some effluents at high enough concentrations alone to cause significant effects on reproductive development and function (Blackburn and Waldock 1995; Harries et al. 1996; Sheahan et al. 2002; Sole et al. 2000). The parent alkylphenol polyethoxylates may be discharged into the sewers from both domestic cleaning products (Bennie 1999) and from more specialized industrial users.
Undoubtedly, the most important issue from a regulatory perspective is to determine whether steroidal estrogens cause the majority of the disruptive reproductive effects seen in wild fish populations. One approach to testing this hypothesis is to search for relationships between the spatial distribution and severity of endocrine disruption, the size of the local human population, and the available dilution in the river water. The human excretion of steroid estrogens is predictable (Johnson and Williams 2004; Johnson et al. 2000) and should be linked to the human population (at least outside significant animal husbandry areas). Conversely, the discharge of different xenobiotic EDCs is likely to be far more random (Blackburn and Waldock 1995).
If a convincing correlation were to be found between sexual disruption in wild fish and the predicted concentrations of steroid estrogens within the river water in which they reside, it would provide confidence to regulators for using such steroid estrogen models to predict the location and severity of endocrine disruption in river catchments, and consequently, where regulatory controls might need to be implemented.
Materials and Methods
Risk assessment.
Estimation of effluent loads
We estimated effluent discharge concentrations of steroidal estrogens using the method of Johnson and Williams (2004); therefore, only a brief overview of this approach will be given here. E1 and E2 are natural steroid estrogens excreted by humans in varying amounts, depending on their age, sex, and whether the females are pregnant. EE2 is used in the contraceptive pill and in hormone replacement therapy drugs and is excreted by women taking these products. The load of these steroids arriving at a sewage treatment works (STW) will be a linear function of the population served by that works. Johnson and Williams (2004) reviewed the literature on excretion values of these steroid estrogens by various cohorts of the human populations. They calculated that on average each person excreted 0.89 μg/day of EE2, 3.3 μg/day of E2, and 13.8 μg/day of E1 (either as free hormone or in forms likely to be transformed into the free hormones). Thus, simply multiplying the domestic population served by an STW gives the load of these chemicals in the influent to the sewage treatment works.
The sewage treatment process removes a significant proportion of the steroid estrogens. From a review of the published data, Johnson and Williams (2004) suggested that removal efficiencies of 85, 65, and 82% for EE2, E1, and E2, respectively, would be expected from activated sludge treatment works (commonly used for large conurbations). Written mathematically, the average daily load of an individual steroid delivered to the receiving water, Le, is given by
 |
where re is the fraction of the steroid removed by the STW, P is the domestic population served by the STW, and α e is the per capita steroid load excreted. The latter value varies for each steroid estrogen and reflects the amount excreted by males and females at various times in their lives. It is important to note that while the model assumes that the efficiency of all STW is the same and constant, there is evidence that the efficiency of similar or individual STW can vary, even on a daily basis (Baronti et al. 2000; Williams et al. 2003.
Population equivalent and nonindustrial component
STW are routinely described with respect to the population equivalent (PE) that they handle. The value is used by water companies as a measure of the organic strength of the wastewater (based on a biological oxygen demand of the water of 65 g/day generated by a human in European Union countries). PE reflects the net waste of the connected human population and industrial concerns. For any steroid estrogen prediction, it is therefore vital to obtain the true human PE associated with the works. Both the human and industrial PEs for each of the STW were obtained by contacting the water utilities responsible for their operation.
STW flows
The flow of sewage effluent leaving each sewage works was measured (wherever possible) on two occasions a few weeks apart during periods when there had not been significant rainfall to represent dry weather flows. This measurement provided effluent flow derived from human discharges and for “typical” summer conditions. Overall, we observed the following relationship:
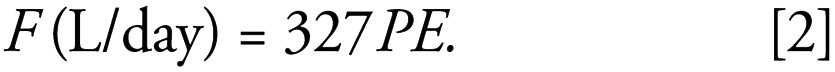 |
Because in some cases it was not possible to obtain flow values for all the STW in the survey, the above flow relationship was assumed and applied to all STW.
Estimation of river flows at fish capture sites
STW effluents are likely to have maximum impact under low river flow conditions. Therefore, to give a reasonable worst-case risk assessment, a flow rate of the receiving river that corresponds to typical summer levels was used. The 95th percentile river flow (the flow that is exceeded 95% of the time) was selected. The 95th percentile river flow was estimated for the fish capture sites by scaling the 95th percentile flow value from the nearest (generally downstream) gauging station by area. Thus we derived the following expression:
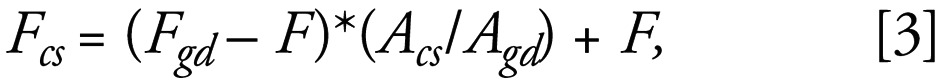 |
where A is the area; subscripts cs and gd refer to the capture site and the downstream gauging site, respectively, and F is the flow of the STW. The nearest gauging station, flow, and area for each site were taken from Marsh and Lees (2003). The catchment area draining to the capture site was estimated using the Flood Estimation Handbook CD-Rom software (Centre for Ecology and Hydrology 1999). The flow at a (fish capture) site can be predicted when only flow data from a downstream gauging site is available by comparing the areas draining to the two sites. The flow at a fish capture site can be predicted by comparing the flow data of a downstream gauging site with the flow data of the catchment area to the capture site. Thus, if the larger catchment area associated with the downstream site generates so much flow per unit area, then the flow for the upstream site, with its smaller catchment, can be calculated. But this area-based calculation can be obtained only by first subtracting the flow of the STW within the catchment. Once this deduction has beenmade, the flow of the STW upstream of the fish capture site must be added to complete the calculation. When the only appropriate flow gauging site was upstream of the capture site, the flow at the fish capture site was estimated as the sum of the area-adjusted flow plus the dry weather flow from the STW.
Estimation of steroid concentrations in rivers at fish capture sites
The concentration of each steroid in each of the rivers, Cr, was then given by
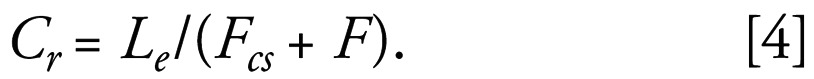 |
Note that Cr = Le is derived from Equation 1; Fcs is derived from Equation 3; and F is derived from Equation 2. Fcs is the 95th percentile river flow at the fish capture point, and F is the effluent flow from the STW. This calculation assumes that there are no steroid estrogens in the river upstream of the discharge point. Equation 4 was used to estimate the concentration of the three steroid estrogens E2, E1, and EE2 in each of the rivers sampled.
Setting of the risk categories for each biological end point
Estimation of the relative potencies of E1, E2, and EE2
Steroid estrogens cause a wide range of feminizing effects in fish, including the induction of intersex and the synthesis of VTG in male fish. Moreover, it is increasingly acknowledged that the combined effects of steroid estrogens on any of these end points are likely to be additive (Brian et al. 2005; Silva et al. 2002; Thorpe et al. 2003b). Hence, they are better predicted using a toxic equivalency approach.
The relative potencies of each steroid also depend on the end points measured, the fish species used, and the timing and duration of the exposure. With these factors in mind, we estimated predicted equivalent concentrations of E2 separately for induction of VTG and for induction of intersex, using data from studies on cyprinid fish only. We used this procedure to try to avoid false positives or false negatives caused by the possible differences in sensitivity to the three estrogens among fish species. Wherever possible, data to assess relative potencies were used only from studies in which the actual concentrations of estrogen dosed had been analytically verified.
The E2 equivalent concentrations calculated using the previously described methods were compared with the no observed effect concentrations (NOECs) and LOECs of E2 required to induce feminization (VTG induction or intersex) in cyprinid fish. We then placed each sample site into high-risk (> LOEC), medium-risk (between the NOEC and the LOEC), or low-risk (< NOEC) categories for each end point separately, using the values discussed below as boundaries.
Vitellogenin induction
We found three studies in the literature on the relative potencies of the steroid estrogens in inducing VTG in cyprinid fish in vivo. Van den Belt et al. (2004) reported relative potencies of 1E2:0.8E1:30.6EE2 in adult female zebrafish (Danio rerio) in a 21-day study, while Brian et al. (2005) reported 1E2:40EE2 (E1 was not tested) in adult male fathead minnow (Pimephales promelas) during an identical exposure period. Thorpe et al. (2001) reported 1E2:0.36E1:25EE2 in female fathead minnow after 14 days of exposure. Studies on the potency of E1 in male cyprinid fish (Panter et al. 1998; Routledge et al. 1998) indicate that its potency is about 1.5 times lower than that of E2 (Brion et al. 2004). On the basis of this information, we used the relative potencies of 1E2:0.75E1:40EE2 to calculate the equivalent concentrations of E2 for the VTG end point.
The LOECs and NOECs selected for setting of the high-, medium-, and low-risk categories were from in vivo studies on adult male cyprinid fish over a 3-week exposure period, and were thus:
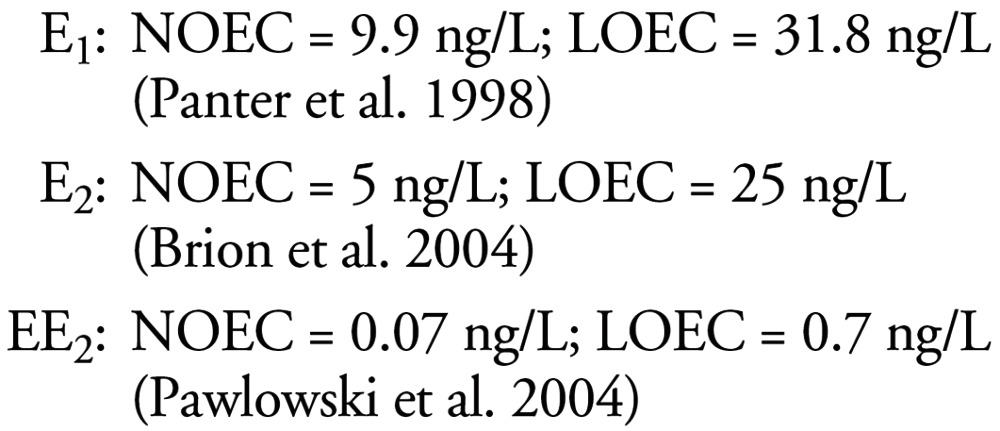 |
Intersex induction
We found no articles that adequately assessed the relative effects of steroid estrogens on the induction of intersex. An article by Metcalfe et al. (2001) attempted to compare the effects of E1, E2, and EE2 on the intersex end point in the Japanese medaka. The results regarding the potency of EE2, however, were inconclusive. Although the authors suggest a LOEC for EE2 of 0.1 ng/L, 10 ng/L did not cause an effect; hence, a clear dose–response curve was not apparent. More recent studies (Balch et al. 2004) suggested that the LOEC for EE2 on the intersex end point may be closer to 2 ng EE2/L, which indicates that the relative potencies of E2, EE2, and E1 for inducing intersex are probably around 1:5:1 for this end point.
The LOECs and NOECs selected for setting the risk categories for the induction of intersex were also taken from in vivo studies on medaka (Oryzias latipes), as they are the most conclusive studies for the suite of steroid estogens of interest in which the exposure concentrations of the three steroids were measured (Balch et al. 2004; Metcalfe et al. 2001). The data used were from long-term studies in which the fish were exposed to the test chemicals from hatch to sexual maturity (approximately 4 months). These data led us to the following relationships:
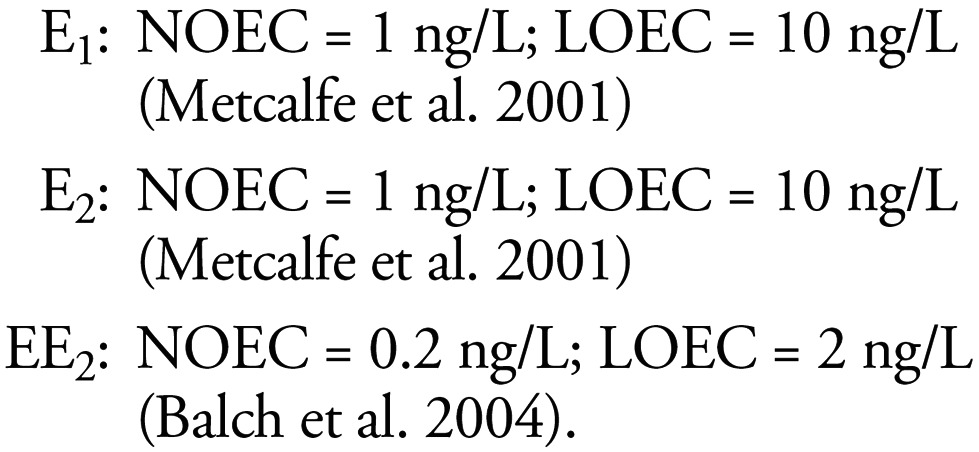 |
Fish sampling site selection
Forty-five sites were selected representing a range of high-, medium-, and low-risk categories, as defined by the predictive model. Other necessary criteria for site selection included an abundance of roach and the existence of data on the characteristics of sewage discharges that entered the rivers.
The sites were defined as 2-km river sections around the point of discharge from the named STW (i.e., 1 km upstream and 1 km downstream). If an insufficient number of fish were caught within the 2-km stretch, the site was extended up to a farther 9 km downstream. Fish sampled from these sites were in many cases (but not all) still subject to exposure to sewage effluents from smaller works located even farther upstream.
Descriptions of each capture site regarding the characteristics of the STW directly upstream of the capture point and the predicted E1, E2, EE2, and E2 equivalent concentrations at the capture point are given in Table 1.
Table 1.
Descriptions of each capture site in the United Kingdom regarding the characteristics of the STW directly upstream of the capture point and the predicted E1, E2, EE2, and E2 equivalent concentrations at the capture point.
| Population equivalent (no.)
|
E2 equivalent (ng/L)
|
No. of discharge licenses upstream of this STW
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| STW name | Receiving river | Estimated STW flow (m3/sec) | Estimated river flow (m3/sec) | Domestic | Total | VTGa | Intersex 1b | Industrial indexc | > 10,000 PE | < 10,000 PE |
| Flitwick | Flit | 0.11 | 0.28 | 26,370 | 27,317 | 10.43 | 6.72 | 1.24 | 1 | 0 |
| Hitchin | Hiz | 0.13 | 0.49 | 31,737 | 32,353 | 7.19 | 4.63 | 0.39 | 0 | 0 |
| Clifton | Ivel | 0.05 | 0.47 | 13,604 | 13,623 | 3.22 | 2.07 | 0.03 | 2 | 0 |
| Leek | Churnet | 0.19 | 0.35 | 20,650 | 48,639 | 6.42 | 4.13 | 16.21 | 0 | 0 |
| Ilkeston | Erewash | 0.19 | 0.36 | 44,500 | 49,000 | 13.67 | 8.80 | 2.56 | 5 | 3 |
| Melton Mowbray | Eye | 0.23 | 0.27 | 27,732 | 58,337 | 11.17 | 7.19 | 19.17 | 0 | 14 |
| Blackburn Meadows | Don | 2.24 | 3.86 | 458,791 | 573,489 | 13.12 | 8.45 | 0.52 | 1 | 0 |
| Worsborough | Trib of Dearne | 0.08 | 0.13 | 18,810 | 19,441 | 16.08 | 10.35 | 2.52 | 0 | 0 |
| Walbutt | Foss | 0.07 | 0.37 | 18,768 | 18,780 | 5.63 | 3.62 | 0.02 | 0 | 6 |
| Wisley | Wey | 0.09 | 1.93 | 22,240 | 22,320 | 1.27 | 0.82 | 0.02 | 5 | 15 |
| Harpenden | Stanford | 0.12 | 0.13 | 31,600 | 31,600 | 26.00 | 16.74 | 0.00 | 0 | 14 |
| Swindon | Ray | 0.77 | 0.77 | 177,530 | 197,867 | 25.36 | 16.33 | 1.33 | 0 | 0 |
| Reading | Foudry Brook | 1.09 | 1.30 | 180,000 | 280,000 | 15.32 | 9.87 | 2.76 | 0 | 0 |
| Lincoln | Sincil Dyke | 0.41 | 1.39 | 97,348 | 105,494 | 7.75 | 4.99 | 0.56 | 2 | 25 |
| North Hykeham | Witham | 0.07 | 0.71 | 18,737 | 18,811 | 2.91 | 1.87 | 0.06 | 0 | 27 |
| Louth | Louth Canal | 0.06 | 0.28 | 14,332 | ND | 6.98 | 4.49 | ND | 0 | 0 |
| Broadholme | Nene | 0.74 | 0.96 | 173,574 | 188,838 | 19.92 | 12.82 | 0.84 | 2 | 43 |
| Braintree | Brain | 0.07 | 0.12 | 17,901 | 18,633 | 16.51 | 10.63 | 3.29 | 0 | 7 |
| Bocking | Blackwater | 0.07 | 0.15 | 17,770 | 17,769 | 12.65 | 8.14 | 0.00 | 0 | 6 |
| Halstead | Colne | 0.00 | 0.09 | 107 | 107 | 0.14 | 0.09 | 0.00 | 0 | 6 |
| Stowmarket | Gipping | 0.07 | 0.08 | 17,220 | 18,075 | 23.81 | 15.33 | 5.93 | 0 | 5 |
| Thetford | Little Ouse | 0.16 | 1.18 | 20,000 | 40,000 | 1.86 | 1.20 | 4.22 | 0 | 18 |
| Cambridge | R. Cam | 0.59 | 0.89 | 140,166 | 151,444 | 17.43 | 11.22 | 0.84 | 1 | 17 |
| Whittlesey | Whittlesey Dyke | 0.06 | ND | 14,897 | 14,921 | ND | ND | 0.27 | ND | ND |
| Nuneaton | Anker | 0.35 | 0.44 | 79,341 | 88,841 | 20.01 | 12.88 | 2.45 | 1 | 13 |
| Atherstone | Anker | 0.05 | 0.27 | 11,014 | 12,911 | 4.53 | 2.92 | 5.48 | 2 | 13 |
| Pye Bridge | Erewash | 0.03 | 0.11 | 7,883 | 8,303 | 7.90 | 5.08 | 4.60 | 2 | 15 |
| Rugby | Avon | 0.35 | 0.43 | 72,944 | 89,531 | 18.52 | 11.92 | 4.27 | 0 | 28 |
| Pershore | Bow | 0.04 | 0.15 | 11,027 | 11,058 | 8.36 | 5.38 | 0.20 | 1 | 34 |
| Brighouse | Calder | 0.22 | 2.25 | 48,706 | 55,769 | 2.38 | 1.54 | 0.56 | 6 | 2 |
| Mitchell Laithes | Calder | 1.11 | 4.27 | 156,376 | 285,463 | 4.04 | 2.60 | 1.06 | 8 | 3 |
| Maple Lodge | Grand Union Canal | 1.82 | ND | 455,586 | 467,167 | ND | ND | 0.14 | ND | |
| Ashford | Great Stour | 0.58 | 0.58 | 82,203 | 149,829 | 15.51 | 9.99 | 7.72 | 0 | ND |
| Canterbury | Great Stour | 0.27 | 1.50 | 67,254 | 68,502 | 4.93 | 3.18 | 0.12 | 1 | ND |
| Lingfield | Ray Brook | 0.04 | 0.07 | 10,982 | 10,996 | 17.34 | 11.16 | 0.18 | 0 | ND |
| Hatfield | Trib of Lee | 0.06 | 0.41 | 14,884 | 14,913 | 4.00 | 2.58 | 0.05 | 1 | 5 |
| Camberley | Blackwater | 0.56 | 0.65 | 141,412 | 143,708 | 24.10 | 15.52 | 0.25 | 2 | 1 |
| Wargrave | Loddon | 0.44 | 4.70 | 111,600 | 112,407 | 2.62 | 1.69 | 0.02 | 7 | 30 |
| Horley | Mole | 0.13 | 0.39 | 32,113 | 32,162 | 9.13 | 5.88 | 0.04 | 1 | 1 |
| Chertsey | Bourne | 0.29 | 0.29 | 71,774 | 75,285 | 26.95 | 17.35 | 1.59 | 1 | 0 |
| Bicester | Langford Brook | 0.16 | 0.95 | 36,488 | 39,900 | 4.22 | 2.72 | 0.90 | 0 | 0 |
| Newbury | Kennet | 0.43 | 3.15 | 110,051 | ND | 3.97 | 2.56 | ND | 1 | 20 |
| Wateringberry | Medway | 0.04 | 1.51 | 9,622 | 9,622 | 0.70 | 0.45 | 0.00 | 0 | ND |
| Letchworth | Hiz | 0.16 | 0.18 | 39,397 | 42,257 | 23.95 | 15.42 | 3.73 | 1 | 0 |
| Tewksbury | Severn | 0.09 | 19.42 | 18,758 | 22,290 | 0.11 | 0.07 | 0.08 | ||
| Farnham | Wey | 0.14 | 0.72 | 36,762 | 36,960 | 7.98 | 5.14 | 6.12 | 1 | 2 |
| Eastleigh Chickenhal | Itchen | 0.38 | 2.92 | 80,624 | 98,469 | 5.59 | 3.60 | 0.07 | ND | ND |
ND, no data available.
VTG: E2 equivalent (ng/L) = [E2] + 0.75 [E1] + 40.0 [EE2].
Intersex 1: E2 equivalent (ng/L) = [E2] + [E1] + 5.0 [EE2].
Industrial index calculated as fraction of industrial PE multiplied by 10 and divided by the river flow.
Biological samples
Between 10 and 60 adult male and female roach were collected from each location between May 2002 and October 2003. All fish were taken to the Fish Health Laboratory in Brampton, UK, for necropsy. Fish were killed with a lethal dose of anesthetic [MS-222 or benzocaine, according to Home Office regulations (Home Office 2006)]. Blood was collected via the caudal sinus into 1-mL heparinized syringes containing aprotinin (2 trypsin inhibitor units/mL). After centrifugation, the plasma was frozen on dry ice for transportation and stored at –70º before VTG analysis. Total length, total weight, and gonadal weight were determined for each fish. Both gonads were removed and preserved in Bouins fixative for 6 hr before they were removed to 70% alcohol in preparation for histological processing.
Histological analysis
We divided gonads from each fish into three equal portions. Representative transverse sections 3–5 mm thick were taken from the center of each portion to provide a total of 6 sections per fishone section each from the anterior, mid, and posterior regions of each gonad. The sections were then processed histologically, embedded in paraffin, and sectioned at 3 μm. All sections were stained with Mayer’s hemotoxylin–eosin, mounted, and examined by light microscopy. We determined the gonadal status of the fish as described in Nolan et al. (2001) and noted any abnormalities in sexual development (e.g., abnormal reproductive ducts or germ cells).
We described the number of oocytes in the testes of intersex fish and, thus, the severity of this condition, using a numerical scale that ranged from 0 (all testis tissue) to 7 (all ovarian tissue) (Table 2). We assigned testes sections to one of eight categories on the basis of the number of oocytes present. The arithmetic mean of the scores (measured on six sections per fish) for each intersex fish was used to derive an average intersex index (or severity score) for each sample.
Table 2.
The intersex index scoring system.
| Score 0 = Normal male testis. |
| Score 1 = Multifocal ovotestis with 1–5 oocytes (usually singly) scattered among the testicular tissue. |
| Score 2 = Multifocal ovotestis, 6–20 oocytes often in small clusters scattered among the testicular tissue. |
| Score 3 = Multifocal ovotestis, 21–50 oocytes in clusters. |
| Score 4 = > 50 < 100 oocytes. Section is usually multifocal and has the appearance of a mosaic of testicular and ovarian tissue. |
| Score 5 = > 100 oocytes, usually multifocal but could also be focal with clearly identifiable zones of ovarian and testicular tissue separated from the testicular tissue. |
| Score 6 = > 50% of the gonadal tissue on the section is ovarian and is clearly separated from the testicular tissue by epithelial cells and phagocytic tissues. |
| Score 7 = 100% of gonadal tissue on the section is ovarian. |
Quantification of plasma VTG concentrations
Quantification of VTG in the plasma samples was achieved using an established homologous carp enzyme-linked immunosorbent assay (Tyler et al. 1999) that has been validated for use with a wide variety of cyprinid fish (Tyler et al. 1996).
Statistical analyses
We performed the statistical analyses using the STATVIEW statistical program (Cherwell Scientific, Oxford, UK). Before analysis, the data obtained were log transformed where necessary to improve normality and homogeneity of variance.
Plasma VTG concentrations measured in male, female, or intersex fish from sites within each risk category were categorized according to the age of the fish and the month in which the fish were sampled. Analyses of variance were then carried out to determine the effect of risk category, sex, age, and time of sampling on this variable.
The proportions of male, female, and intersex fish at each site and within each risk category were also determined. The incidence of intersexuality in “perceived male fish” of different ages was calculated by determination of the percentage of “males” that were found to be intersex, with feminized reproductive ducts, multiple ducts, and/or ovotestes after histological examination. Chi-squared analyses were then used to determine a) deviations from the expected sex ratio in fish sampled from the different risk categories, and b) differences in the proportions of “male” fish with oocytes within their testes, feminized ducts, and/or multiple sperm ducts between different sampling sites and between different risk categories.
Differences in the intersex index were analyzed using a two-way analysis of variance (ANOVA) with site, risk category, and age as between factors. This analysis was followed by Fisher’s protected least-squares difference (to examine all pairwise comparisons). The relationship between the age of the “male” fish within each risk category and the incidence and severity of intersex or VTG concentration was examined using regression analyses.
To examine the possible relationship between predicted exposure to steroid estrogens and estrogenic effects in roach, we used the following approaches: In addition to the predicted concentration of steroid estrogens in the receiving water, each sample site was also given an industrial impact number. Because industrial PEs can be 0–100% of the total PE, we gave each STW a score of 0–10 industrial units based on the proportion of the total PE from industrial sources. We adjusted these industrial unit numbers, using the dilution calculation in the model, to give industrial impact scores for each sample site (Table 2). We then performed regression analyses on fish of different ages to examine the relationships between the plasma VTG/intersex index and the predicted concentrations of steroid estrogens (using the human population equivalent values) and between the plasma VTG/intersex index and the industrial impact number.
Results and Discussion
Overview
Predicted individual concentrations of E1, E2, and EE2 in the rivers where roach were captured ranged from 0.07 to 13.78 ng/L for E1, from 0.01 to 1.7 ng/L for E2, and from zero to 0.37 ng/L for EE2. Individually, with the exception of estrone, these concentrations generally were not thought to be sufficient to cause feminizing effects in fish. However, when we combined all three steroids using E2 equivalent concentrations, many more locations were predicted to cause endocrine-disrupting effects in fish (based on data from studies on small laboratory cyprinid fish species). When we calculated equivalent concentrations from E2equiv/intersex = 1E2 + 5EE2 + 1E1, they ranged from 0.060 to 17.34 ng/L. When we calculated equivalent concentrations using VTG as the end point, however (using E2equiv/VTG =1E2 + 40EE2 + 0.75E1), they ranged from 0.48 ng E2/L to 26.85 ng E2/L. Both methods for calculating the E2 equivalent concentrations yielded concentrations of E2 that were above the chosen NOECs for intersex (1 ng/L) and VTG (5 ng/L) for all sites. At some sites, the predicted E2 equivalent concentrations were higher than the chosen LOECs (25 ng/L for VTG and 10 ng/L for intersex). It should be noted that to ensure comparability, the LOECs and NOECs for VTG were from the limited data available on the induction of this end point in short-term studies. Very recent long-term (lifetime) studies with medaka indicate that exposure to estradiol can induce VTG at concentrations ≥ 8.66 ng E2/L (Seki et al. 2005), thus lowering the threshold for the induction of this response under these conditions.
The results of the biological analyses revealed that intersex fish were present at many of the sites sampled (Figure 1A). When fish collected from all of the sites in each of the high-, medium-, and low-risk categories were pooled, clear differences could be discerned between predicted high-, medium-, and low-risk groups for intersex incidence and severity (Figures 1B, 2, 3, and 4). The pooled VTG analyses, however, revealed no clear dose-dependent relationship between VTG concentration and E2 equivalent in the male and intersex fish (r2 = 0.003, p = 0.184; Figure 5). This result was thought to be because the fish were sampled at different times throughout the year. Hence, seasonal variations in endogenous and/or exogenous estrogen (and, therefore, plasma VTG) concentrations in female, male, and intersex fish may have complicated the interpretation of the data (Figure 5). It is also possible that long-term (lifelong) exposure to steroid estrogens might attenuate the expected response and clarify the relationships between exposure and effect that are difficult to discern. Nash et al. (2004) reported a strong down-regulation of the VTG response in the F1 generation of zebrafish after multigenerational long term exposure of zebrafish to EE2. Seki et al. (2005) also reported this phenomenon in F1 medaka. Therefore, it is likely that some of the variation in VTG concentrations in exposed male and female fish in the wild is caused by differences in the timing and duration of their exposure to estrogenic compounds.
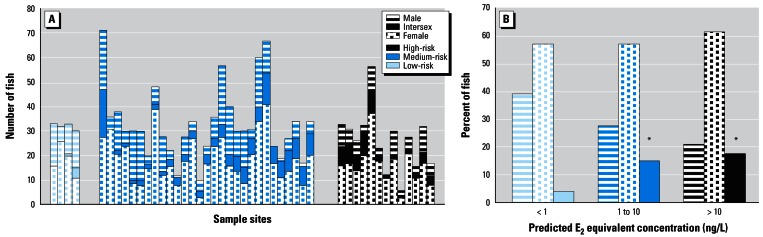
Figure 1.
Sex ratios of roach sampled. (A) The proportions of each sex at each study site. (B) Sex ratios of roach sampled (pooled data). Significant difference from the expected sex ratio (50:50): *p = 0.05.
Figure 2.
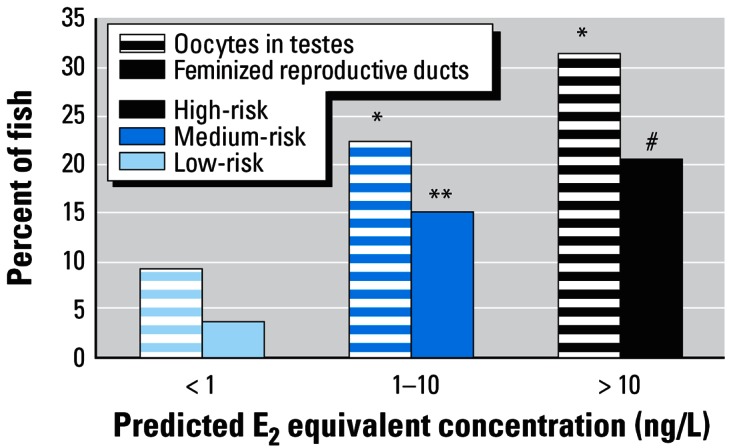
The proportion of “male” roach with oocytes in their testes or feminized reproductive ducts from pooled high- (> 10 ng E2 equivalent/L), medium- (1–10 ng/L), and low-risk (< 1 ng/L E2 equivalent) sites.
Significant differences from the expected sex ratio at the following significance levels: *p = 0.05, **p = 0.01, #p = 0.001.
Figure 3.
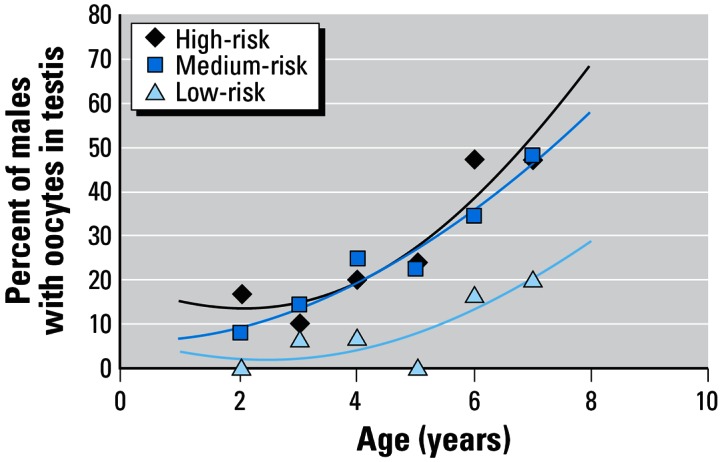
Relationship between the incidence of oocytes in the testes of roach caught from high-, medium-, and low-risk categories and age of roach at capture. Each point represents an individual site within each category.
Figure 4.
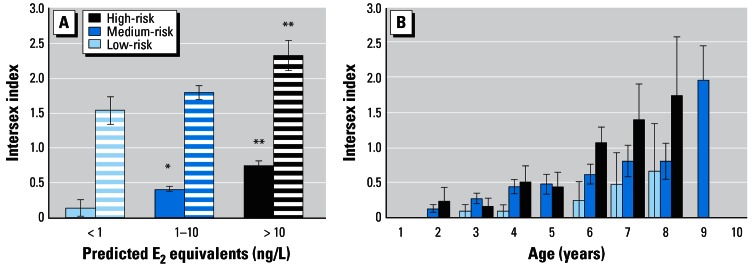
(A) Mean intersex index values in pooled samples of roach collected from predicted high-, medium-, and low-risk sites. The number of oocytes within the testes was assessed using the intersex index (0–7). The mean of the scores (six sections per fish) for all intersex fish was used to derive the intersex index for each group of fish. Both the absolute index (fish with ovotestes only, striped bars) and the combined index (including the males and intersex fish with no oocytes in testes, solid bars) are shown. (B) Average intersex index for each age group of fish and for each category (high-, medium-, and low-risk). Error bars indicate SD. Significant differences from the low-risk category: *p = 0.05, **p = 0.01.
Figure 5.
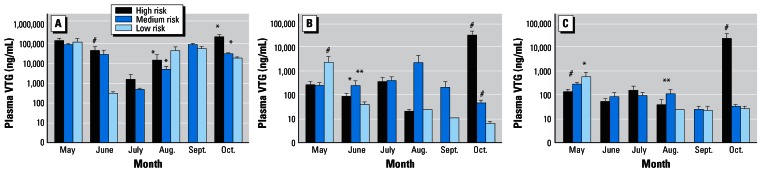
Monthly variation in VTG concentrations in female (A), intersex (B), and male (C) roach within each of the predicted risk categories: high-risk , > 10 ng E2 equivalent/L; medium-risk, 1–10 ng/L; low-risk, < 1 ng/L. The month in which the fish were sampled is also shown. All sites were within 9 km of a sewage effluent input. Error bars indicate SD. Significant difference from the low-risk category: *p = 0.05, **p = 0.01, #p = 0.001.
Despite the obvious differences in biological effects between fish sampled from pooled high-, medium-, and low-risk sites, the biological results also revealed large variations in the various effects between the fish sampled from the different sites as well as between fish from the same site. Consequently, on some occasions, when a single low-risk site was compared with a single high-risk site, the expected differences in effects between the fish were not as clear as when pooled data were used. This finding is not surprising when one considers the range of possible confounding variables such as the number of fish captured, their proximity to the sewage discharge, the unknown extent of the true territorial nature of the fish, the age of the captured fish (which may influence the incidence and severity of intersex), inaccuracies in the estimation of sewage and water flow volumes, the true efficiency of the STW relative to one another, and the presence of steroids from upstream STW.
Feminization
The proportions of female roach sampled from each of the sites within each risk category are summarized in Figure 1. A total of 1,438 fish were analyzed. It was not possible to compare statistically the sex ratios for all of the individual sites because insufficient fish were captured from some of the sites. Departures from the expected sex ratio of 50:50 male:female were found at several of the sites, but these sites were not found to be associated with the risk category in which they were placed. At some study sites, more males than females were found, but in most cases more females than males or intersex fish were found. When all data were pooled, however, we found no statistically significant difference between the proportions of females in each of the risk categories (61.8% high, 57.4% medium, 57.0% low, p > 0.05; Figure 1B). These findings would be predicted by the model described above; feminization of the sex ratio, caused by exposure to steroids during early life, would be expected to occur only where the concentration of E2 (or its equivalent) approached 100 ng E2/L. This concentration of “total steroid estrogen” was not predicted to be present at any of the sites we studied.
Incidence of intersex
Our histological examination of the testes from male roach revealed that a significant proportion of these fish were intersex, as characterized by the presence of developing eggs (oocytes) within the testes and/or an ovarian cavity (female reproductive duct) in addition to the sperm duct. Moreover, a small proportion of the fish had two sperm ducts seen on either side of the ovarian cavity (in transverse section).
In general agreement with the model, intersex fish were found at all high- and medium-risk sites and in a much more limited frequency at the low-risk sites (Figure 1A). When the sites were pooled and placed into risk categories (Figure 1B), we found the overall prevalence of intersex fish to be influenced strongly by the predicted exposure of the fish to steroid estrogens. Analysis of the pooled data (from all year classes) within each risk category (Figure 2) indicated that the incidence of intersex in “male” fish was significantly greater at the high-risk (31.25% with oocytes in testes and 20.31% with ovarian cavities, n = 128) and medium-risk (22.24% with oocytes in testes and 14.74% with ovarian cavities, n = 409) sites compared to the incidence of intersex in “male” fish at the low-risk sites (9.1% of the males with oocytes and 3.57% with ovarian cavities, n = 55; chi square = 11.066, p = 0.0040 for oocytes in testes and chi square =11.078, p = 0.0257 for ovarian cavities). There were only four sites where the E2equiv/intersex was predicted to be below the NOEC for intersex induction in medaka. As predicted by the model, few intersex fish were found at these sites (Figure 1A).
The proportion of fish with oocytes in their testes was also shown to be strongly influenced by their age (r2 = 0.7, p < 0.0001 overall; Figure 3), but a similar association was not seen between the age of the fish and the incidence of oviducts (r2 = 297, p = 0.175; data not shown). Feminization of the reproductive ducts is known to occur during early life (Rodgers-Gray et al. 2001), whereas the incidence of ovotestes appears to be a progressive condition (Jobling S, Tyler CR, Brighty GC, Sumpter JP, unpublished data). Consequently, on the basis of the available knowledge of intersex in roach, these results were as we expected. The fact that ovotestis is a progressive condition that appears to arise as a consequence of long-term exposure to estrogens could also be the reason why so many intersex roach were found at the medium-risk sites (where E2equiv/intersex was greater than the NOEC but less than the LOEC for intersex in medaka). Long-term exposure of adult roach to estrogenic effluents may play a role in the manifestation of these effects, as it is well known that the threshold concentration of effluent required to induce VTG synthesis decreases with increased duration of exposure (Rodgers-Gray et al. 2000; van Aerle et al. 2002). It is also possible, however, that ovotestis occurs as a delayed reaction to larval exposure to endocrine disruptors that does not manifest itself until the fish undergo puberty (at around 2 years of age). This possibility has not been explored experimentally.
Convincing plots that illustrate how the incidence of ovotestes increases with age and predicted exposure for each risk category (high: r2 = 0.8775, p = 0.0089; medium: r2 = 0.9473, p = 0.0011; low: r2 = 0.6965, p = 0.0512) are presented in Figure 3. The strong associations suggest that if one knows the age of a fish and its predicted exposure to steroid estrogens, the incidence of ovotestes can be estimated using one of these three curves.
Severity of the intersex condition
As established in previous studies of intersexuality in roach in U.K. rivers (Jobling et al. 1998), in the present study, we found a large variation in the severity of feminization of the intersex fish. Intersexuality in the fish ranged from those with a few oocytes within the testicular tissue (index = 1–2) to those fish with predominantly female tissue scattered within small patches of testicular tissue (index 5–6).
Comparisons of the intersex index between different fish indicated that the mean intersex index varied considerably both within and between sites. ANOVA showed that the mean intersex index (measured in intersex fish only) also varied significantly between the three risk categories (p = 0.0119) and that it was higher in fish from the high risk category than in the medium-risk (p = 0.0047) category. The mean intersex index in the high-risk category was also higher than that in the low-risk category, although this distinction was not significant at the 95% level (p = 0.091) because of the low number of fish that were intersex in the low-risk category and the variation in their intersex indices (Figure 4A, solid bars). A combined intersex index was also calculated for each category (Figure 4A; striped bars) by including those fish without oocytes in their testes (scoring 0) in the calculation of the mean percentage of fish that were intersex. This process includes both males and intersex fish and gives rise to an index that considers both the incidence and severity of intersex at each of the sites sampled. Using this index, significant differences in the mean index were seen between the high- and medium-risk categories (p = 0.0005), the high- and low-risk categories (p < 0.0001), and the medium- and low-risk categories (p = 0.0488). As with the incidence of ovotestes, the severity of feminization (or intersex index) was related to the age of the fish when they were sampled. Figure 4B illustrates how the severity of the intersex condition can be estimated for fish in particular age classes for the high-, medium-, and low-risk categories.
VTG induction
Analysis of the VTG concentrations measured in the wild roach (Figure 5) provided strong evidence that some populations of fish were exposed to estrogenic contaminants. In contrast with the other end points, however, the predictions of the model for this end point did not correlate as well with the biological data. At many sites where VTG induction was not predicted, it was nonetheless found in the fish, and at some sites where VTG induction was expected, it was not found. It is well documented that long-term exposure results in a lowering of the threshold concentration of estrogen/effluent required to invoke a VTG response (Rodgers-Gray et al. 2000). Conversely, Nash et al. (2004) reported that lifelong (multigenerational) exposure to EE2 might result in a reduced vitellogenic response in males because of acclimation of the fish to their surroundings.
In addition to these factors, there was also a seasonal variation in the VTG concentrations, particularly in the females (as expected). However, rather unexpectedly, there also appeared to be a similar trend in the VTG concentrations found in the males and intersex fish (Figure 5). Further studies are necessary to determine whether the apparent seasonality in VTG induction seen in this study is a real phenomenon, as the limited data collected rely almost entirely on the strength of the low values seen in the samples collected in July. It should be noted that seasonality in VTG induction in wild male fish was also reported in marine flounder by Kleinkauf et al. (2004); Scott et al. (2006) found this induction was attributed to seasonal variations in exogenous rather than endogenous estrogen.
While the intersex condition appears to be a result of an accumulative or early life exposure to estrogens, VTG can be manufactured in the liver within a few days following exposure to estrogens, and it has a half-life in the plasma from 10 to 21 days (Schmid et al. 2002). Consequently, in contrast to the other indicators, VTG is not a stable biomarker of long-term exposure to estrogens, and it is much more likely than intersex to be sensitive to changes in short-term temporal factors, related either to fish movement or to changes in river flow. The risk class, on the other hand, is based on the average low-flow conditions and does not have any intra- or interannual variation built into its estimation. It is more suitable therefore, to make comparisons of the model’s predictions with end points indicative of long-term exposure. Predictions suitable for VTG end points would require exposure estimates and risk classification based on river flow and STW effluents flows and concentrations for the several weeks preceding fish capture.
Assessment of the relationship between intersex in wild roach and steroidal estrogens present in sewage effluents
We designed the present study to test whether the degree and prevalence of sexual disruption in wild roach at a range of river sites across the United Kingdom could be predicted using a simple combined estrogen and a hydrological model. The hypothesis that steroid estrogens are the major common cause of endocrine disruption in fish would, therefore, predict that endocrine disruption would not be generally related to the industrial component of sewage effluent. To address this question, we attempted to correlate the incidence and severity of intersex with the industrial index (industrial PE on a scale of 1–10 divided by river flow of effluent entering the rivers immediately above the points where the fish were sampled; Table 2).
Using this approach, we found no apparent correlation between the industrial index and the intersex index (r2 = 0.013, p = 0.2). This nonsignificant correlation can be compared with the relationship between predicted E2 equivalents and the intersex index, where a weak although highly statistically significant (r2 = 0.081, F = 11.765, p = 0.0008) relationship was seen. Similar regression analyses were performed for E1, E2, and EE2 individually, and for all three of these analyses there were highly significant relationships between each steroid and the intersex index (data not shown). These results suggest strongly that the concentration of steroidal estrogens in the river is one of the most likely contributing causes of intersex in wild fish populations, although it is interesting to note that this regression coefficient of only 0.08 indicates that the equivalent estrogen concentration explains only 8% of the variation in the intersex index. Indeed, the convincing correlations between the age of the fish and their intersex index (r2 = from 0.6965 to 0.94) indicate that the remaining variation in the intersex index in wild fish populations likely is caused by variations in the age of the intersex fish. Moreover, it is theoretically possible that another component of domestic effluent gives rise to risk categories similar to those for steroid estrogens and that this other component is instead responsible for the correlations that we have observed and attributed to estrogen exposure. Previous work on the fractionation of STW effluent, however, identified the steroid estrogens as the most potent estrogens, making this theoretical possibility highly unlikely.
From a practical point of view, this exercise has demonstrated that predictive hydrologically based exposure models can be used on their own to predict the severity of intersex (but not necessarily VTG induction) in roach in waters receiving treated sewage effluents. Intersex appears to be a very suitable indicator because, if, as it appears, it is an accumulating phenomenon, it integrates the many variables that may change widely on a day-to-day basis. Further efforts are necessary to improve the accuracy of the model. The model assumes that all STW work to an equivalent efficiency, which is clearly an erroneous assumption. This risk assessment methodology was based on the assumption that single STW exclude any upstream influences. Clearly, river catchmentwide assessment would be preferable despite the initial effort involved.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
We thank all the Environment Agency’s fisheries officers involved in the project for their sterling efforts in collecting the samples of roach. We also thank the staff at the National Fisheries Laboratory for receiving and processing the samples.
This project was funded by the Environment Agency. S.J. was funded by the Environment Agency and by European Chemical Industry Council Long Range Initiative project number BTBL-0409. R.vA. was sponsored by the European Union (EVK1-CT-2002-00129), and E.S. was funded by the Biotechnology and Biological Science Research Council (S15001).
References
- Allen Y, Matthiessen P, Scott AP, Haworth S, Feist S, Thain JE. The extent of oestrogenic contamination in the UK estuarine and marine environments: further surveys of flounder. Sci Tot Environ. 1999a;233(1–3):5–20. doi: 10.1016/s0048-9697(99)00175-8. [DOI] [PubMed] [Google Scholar]
- Allen Y, Scott AP, Matthiessen P, Haworth S, Thain JE, Feist S. Survey of estrogenic activity in United Kingdom estuarine and coastal waters and its effects on gonadal development of the flounder (Platichthys flesus) Environ Toxicol Chem. 1999b;18:1791–1800. [Google Scholar]
- Aravindakshan J, Paquet V, Gregory M, Dufresne J, Fournier M, Marcogliese DJ, et al. Consequences of xenoestrogen exposure on male reproductive function in spottail shiners (Nortropis hudsonius) Toxicol Sci. 2004;78(1):156–165. doi: 10.1093/toxsci/kfh042. [DOI] [PubMed] [Google Scholar]
- Balch GC, Mackenzie CA, Metcalfe CD. Alterations to gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17 alpha-ethinylestradiol. Environ Toxicol Chem. 2004;23(3):782–791. doi: 10.1897/02-539. [DOI] [PubMed] [Google Scholar]
- Baronti C, Curini R, D’ Assenzo G, Di Corcia A, Gentili A, Samperi R. Monitoring natural and synthetic estrogens in activated sludge sewage treatment plants and in recieving river water. Environ Sci Technol. 2000;24:5059–5065. [Google Scholar]
- Bennie DT. Review of the environmental occurrence of alkylphenols and alkylphenol ethoxylates. Water Qual Res J Can. 1999;34:79–122. [Google Scholar]
- Blackburn MA, Waldock MJ. Concentrations of alkylphenols in rivers and estuaries in England and Wales. Water Res. 1995;29(7):1623–1629. [Google Scholar]
- Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, et al. Prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect. 2005;113:721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion F, Tyler CR, Palazzi X, Laillet B, Porcher JM, Garric J, et al. Impacts of 17 beta-estradiol, including environmentally relevant concentrations, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio) Aquat Toxicol. 2004;68(3):193–217. doi: 10.1016/j.aquatox.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Centre for Ecology and Hydrology. 1999. Flood Estimation Handbook. CD-ROM, Version 1.0. Wallingford, Oxfordshire, UK.
- Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Tech. 1998;32(11):1549–1558. [Google Scholar]
- Folmar LC, Denslow ND, Kroll K, Orlando EF, Enblom J, Marcino J, et al. Altered serum sex steroids and vitellogenin induction in walleye (Stizostedion vitreum) collected near a metropolitan sewage treatment plant. Arch Environ Contam Toxicol. 2001;40(3):392–398. doi: 10.1007/s002440010188. [DOI] [PubMed] [Google Scholar]
- Folmar LC, Denslow ND, Rao V, Chow M, Crain DA, Enblom J, et al. Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environ Health Perspect. 1996;104:1096–1101. doi: 10.1289/ehp.961041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gercken J, Sordyl H. Intersex in feral marine and freshwater fish from northeastern Germany. Mar Environ Res. 2002;54(3–5):651–655. doi: 10.1016/s0141-1136(02)00156-3. [DOI] [PubMed] [Google Scholar]
- Harries JE, Sheahan DA, Jobling S, Matthiessen P, Neall P, Routledge EJ, et al. A survey of estrogenic activity in United Kingdom inland waters. Environ Toxicol Chem. 1996;15:1993–2002. [Google Scholar]
- Harshbarger JC, Coffey MJ, Young MY. Intersexes in Mississippi River shovelnose sturgeon sampled below Saint Louis, Missouri, USA. Mar Environ Res. 2000;50(1–5):247–250. doi: 10.1016/s0141-1136(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Hassanin A, Kuwahara S, Nurhidayat, Tsukamoto Y, Ogawa K, Hiramatsu K, et al. Gonadosomatic index and testis morphology of common carp (Cyprinus carpio) in rivers contaminated with estrogenic chemicals. J Vet Med Sci. 2002;64(10):921–926. doi: 10.1292/jvms.64.921. [DOI] [PubMed] [Google Scholar]
- Hecker M, Tyler CR, Hoffmann M, Maddix S, Karbe L. Plasma biomarkers in fish provide evidence for endocrine modulation in the Elbe River, Germany. Environ Sci Tech. 2002;36(11):2311–2321. doi: 10.1021/es010186h. [DOI] [PubMed] [Google Scholar]
- Home Office. 2006. Animals (Scientific Procedures) Act 1986. London:Home Office, Science Research & Statistics. Available: http://www.archive.official-documents.co.uk/document/hoc/321/321-xa.htm [accessed 16 April 2006].
- Jobling S, Beresford N, Nolan M, Rodgers-Gray T, Brighty GC, Sumpter JP, et al. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol Reprod. 2002a;66:272–281. doi: 10.1095/biolreprod66.2.272. [DOI] [PubMed] [Google Scholar]
- Jobling S, Coey S, Whitmore JG, Kime DE, Van Look KJW, McAllister BG, et al. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol Reprod. 2002b;67:515–524. doi: 10.1095/biolreprod67.2.515. [DOI] [PubMed] [Google Scholar]
- Jobling S, Nolan M, Tyler CR, Brighty GC, Sumpter JP. Widespread sexual disruption in wild fish. Environ Sci Tech. 1998;32(17):2498–2506. [Google Scholar]
- Jobling S, Tyler CR. Endocrine disruption in wild freshwater fish. Pure Appl Chem. 2003;75:2219–2234. doi: 10.1017/s0031182003003652. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Belfroid A, Di Corcia A. Estimating steroid oestrogen inputs into activated sludge treatment works and observations on their removal from the effluent. Sci Tot Environ. 2000;256(2–3):163–173. doi: 10.1016/s0048-9697(00)00481-2. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Williams RJ. A model to estimate influent and effluent concentrations of estradiol, estrone, and ethinylestradiol at sewage treatment works. Environ Sci Tech. 2004;38(13):3649–3658. doi: 10.1021/es035342u. [DOI] [PubMed] [Google Scholar]
- Jurgens MD, Holthaus KIE, Johnson AC, Smith JJL, Hetheridge M, Williams RJ. The potential for estradiol and ethinylestradiol degradation in English rivers. Environ Toxicol Chem. 2002;21(3):480–488. [PubMed] [Google Scholar]
- Kavanagh RJ, Balch GC, Kiparissis Y, Niimi AJ, Sherry J, Tinson C, et al. Endocrine disruption and altered gonadal development in white perch (Morone americana) from the lower Great Lakes region. Environ Health Perspect. 2004;112:898–902. doi: 10.1289/ehp.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkauf A, Scott AP, Stewart C, Simpson MG, Leah RT. Abnormally elevated VTG concentrations in flounder (Platichthys flesus) from the Mersey Estuary (UK)—continuing problem. Ecotox Environ Safety. 2004;58:356–364. doi: 10.1016/j.ecoenv.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Komori K, Tanaka H, Okayasu Y, Yasojima M, Sato C. Analysis and occurrence of estrogen in wastewater in Japan. Water Sci Tech. 2004;50:93–100. [PubMed] [Google Scholar]
- Korner W, Spengler P, Bolz U, Schuller W, Hanf V, Metzger JW. Substances with estrogenic activity in effluents of sewage treatment plants in southwestern Germany. 2. Biological analysis. Environ Toxicol Chem. 2001;20(10):2142–2151. [PubMed] [Google Scholar]
- Lange R, Hutchinson TH, Croudace CP, Siegmund F. Effects of the synthetic estrogen 17 alpha-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2001;20(6):1216–1227. doi: 10.1897/1551-5028(2001)020<1216:eotsee>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Marsh TJ, Lees ML, eds. 2003. Hydrological Data U.K.—Hydrometric Register and Statistics 1996–2000. Wallingford, UK:Centre for Ecology and Hydrology.
- Matsui S, Takigami H, Matsuda T, Taniguchi N, Adachi J, Kawami H, et al. Estrogen and estrogen mimics contamination in water and the role of sewage treatment. Water Sci Tech. 2000;42(12):173–179. [Google Scholar]
- Metcalfe CD, Metcalfe TL, Kiparissis Y, Koenig BG, Khan C, Hughes RJ, et al. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes) Environ Toxicol Chem. 2001;20(2):297–308. [PubMed] [Google Scholar]
- Minier C, Caltot G, Leboulanger F, Hill EM. An investigation of the incidence of intersex fish in Seine-Maritime and Sussex regions. Analusis. 2000;28:801–806. [Google Scholar]
- Nash JP, Kime DE, Van der Ven LT, Wester PW, Brion F, Maack G, et al. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ Health Perspect. 2004;112:1725–1733. doi: 10.1289/ehp.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven SJ, Snape J, Hetheridge M, Evans M, McEvoy J, Sutton PG, et al. Investigations of the origins of estrogenic Aring aromatic steroids in UK sewage treatment works effluents. Analyst. 2001;126(3):285–287. doi: 10.1039/b009033f. [DOI] [PubMed] [Google Scholar]
- Nolan M, Jobling S, Brighty GC, Sumpter JP, Tyler CR. A histological description of intersexuality in the roach. J Fish Biol. 2001;58(1):160–176. [Google Scholar]
- Orn S, Holbech H, Madsen TH, Norrgren L, Petersen GI. Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquat Toxicol. 2003;65(4):397–411. doi: 10.1016/s0166-445x(03)00177-2. [DOI] [PubMed] [Google Scholar]
- Palace VP, Evans RE, Wautier K, Baron C, Vandenbyllardt L, Vandersteen W, et al. Induction of vitellogenin and histological effects in wild fathead minnows from a lake experimentally treated with the synthetic estrogen, ethynylestradiol. Water Qual Res J Can. 2002;37(3):637–650. [Google Scholar]
- Panter GH, Thompson RS, Sumpter JP. Adverse reproductive effects in male fathead minnows (Pimephles promelas) expsed to environmentally relevant concentrations of the natural oestrogens, oestradiol and oestrone. Aquat Toxicol. 1998;42(4):243–253. [Google Scholar]
- Pawlowski S, van Aerle R, Tyler CR, Braunbeck T. Effects of 17α -ethynylestradiol in a fathead minnow (Pimephales promelas) gonadal recrudescence assay. Ecotox Environ Safe. 2004;57:330–345. doi: 10.1016/j.ecoenv.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Rodgers-Gray TP, Jobling S, Kelly C, Morris S, Brighty GC, Waldock MJ, et al. Exposure of juvenile roach (Rutilus rutilus) to treated sewage effluent induces dose-dependent and persistent disruption in gonadal duct development. Environ Sci Tech. 2001;35:426–470. doi: 10.1021/es001225c. [DOI] [PubMed] [Google Scholar]
- Rodgers-Gray TP, Jobling S, Morris S, Kelly C, Kirby S, Janbakhsh A, et al. Long-term temporal changes in the estrogenic composistion of treated sewage effluent and its biological effect on fish. Environ Sci Tech. 2000;34:1521–1528. [Google Scholar]
- Routledge EJ, Sheahan D, Desbrow GC, Brighty M, Waldock M, Sumpter JP. Identification of estrogenic chemicals in stw effluent. 2. In vivo responses in trout and roach. Environ Sci Tech. 1998;32:1559–1565. [Google Scholar]
- Schmid T, Gonzalez-Valero J, Rufli H. Determination of vitellogenin kinetics in male fathead minnows (Pimephales promelas) Toxicol Lett. 2002;131:65–74. doi: 10.1016/s0378-4274(02)00043-7. [DOI] [PubMed] [Google Scholar]
- Scott AP, Katsiadaki I, Kirby MF, Thain J. Relationship between sex steroid and vitellogenin concentrations in flounder (Platichthys flesus) sampled from an estuary contaminated with estrogenic endocrine-disrupting compounds. Environ Health Perspect. 2006;114(suppl 1):27–31. doi: 10.1289/ehp.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Yokota H, Maeda M. Fish full life-cycle testing for 17-β -estradiol on medaka (Oryzias latipes) Environ Toxicol Chem. 2005;24(5):1259–1266. doi: 10.1897/04-379r.1. [DOI] [PubMed] [Google Scholar]
- Sheahan DA, Brighty GC, Daniel M, Kirby SJ, Hurst MR, Kennedy J, et al. Estrogenic activity measured in a sewage treatment works treating industrial inputs containing high concentrations of alkylphenolic compounds—a case study. Environ Toxicol Chem. 2002;21(3):507–514. [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”—eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Tech. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Sole M, Lopez De Alda MJ, Castillo M, Porte C, Ladegaard-Pedersen K, et al. Estrogenicity determination in sewage treatment plants and surface waters from the Catalonian area (NE Spain) Environ Sci Tech. 2000;34:5076–5083. doi: 10.1021/es010799u. [DOI] [PubMed] [Google Scholar]
- Thorpe KL, Benstead R, Hutchinson TH, Cummings RI, Tyler CR. Reproductive effects of exposure to oestrone in the fathead minnow. Fish Physiol Biochem. 2003a;28(1–4):451–452. [Google Scholar]
- Thorpe KL, Cummings RI, Hutchinson TH, Scholze M, Brighty GC, Sumpter JP, et al. Relative potencies and combination effects of steroidal estrogens in fish. Environ Sci Tech. 2003b;37(6):1142–1149. doi: 10.1021/es0201348. [DOI] [PubMed] [Google Scholar]
- Thorpe KL, Hutchinson TH, Hetheridge MJ, Scholze M, Sumpter JP, Tyler CR. Assessing the biological potency of binary mixtures of environmental estrogens using vitellogenin induction in juvenile rainbow trout (Oncorhynchus mykiss) Environ Sci Tech. 2001;35(12):2476–2481. doi: 10.1021/es001767u. [DOI] [PubMed] [Google Scholar]
- Tyler CR, vandereerden B, Jobling S, Panter G, Sumpter JP. Measurement of vitellogenin, a biomarker for exposure to oestrogenic chemicals, in a wide variety of cyprinid fish. J Comp Physiol B. 1996;166(7):418–426. [Google Scholar]
- Tyler CR, van Aerle R, Hutchinson TH, Maddix S, Trip H. An in vivo testing system for endocrine disrupters in fish early life stages using induction of vitellogenin. Environ Toxicol Chem. 1999;18:337–347. [Google Scholar]
- van Aerle R, Nolan M, Jobling S, Christiansen LB, Sumpter JP, Tyler CR. Sexual disruption in a second species of wild cyprinid fish (the Gudgeon, gobio gobio) in United Kingdom freshwaters. Environ Toxicol Chem. 2001;20(12):2841–2847. [PubMed] [Google Scholar]
- van Aerle R, Pounds N, Hutchinson TH, Maddix S, Tyler CR. Window of sensitivity for the estrogenic effects of ethinylestradiol in early life-stages of fathead minnow, Pimephales promelas. Ecotoxicology. 2002;11(6):423–434. doi: 10.1023/a:1021053217513. [DOI] [PubMed] [Google Scholar]
- Van den Belt K, Berckmans P, Vangenechten C, Verheyen R, Witters H. Comparative study on the in vitro and in vivo estrogenic potencies of 17 beta-estradiol, estrone, 17 alpha-ethynylestradiol, and nonylphenol. Aquat Toxicol. 2004;66(2):183–195. doi: 10.1016/j.aquatox.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Vethaak AD, Lahr J, Kuiper RV, Grinwis GCM, Rankouhi TR, Giesy JP, et al. Estrogenic effects in fish in The Netherlands: some preliminary results. Toxicology. 2002;181:147–150. doi: 10.1016/s0300-483x(02)00271-8. [DOI] [PubMed] [Google Scholar]
- Vigano L, Arillo A, Bottero S, Massari A, Mandich A. First observation of intersex cyprinids in the Po River (Italy) Sci Total Environ. 2001;269(1–3):189–194. doi: 10.1016/s0048-9697(00)00821-4. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Johnson AC, Smith JJL, Kanda R. Steroid estrogens profiles along river stretches arising from sewage treatment works discharges. Environ Sci Tech. 2003;37(9):1744–1750. doi: 10.1021/es0202107. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Jurgens MD, Johnson AC. Initial predictions of the concentrations and distribution of 17 beta-oestradiol, oestrone, and ethinyl oestradiol in 3 English rivers. Water Res. 1999;33(7):1663–1671. [Google Scholar]