Abstract
Atrazine is a potent endocrine disruptor that both chemically castrates and feminizes male amphibians. It depletes androgens in adult frogs and reduces androgen-dependent growth of the larynx in developing male larvae. It also disrupts normal gonadal development and feminizes the gonads of developing males. Gonadal malformations induced by atrazine include hermaphrodites and males with multiple testes [single sex polygonadism (SSP)], and effects occur at concentrations as low as 0.1 ppb (μg/L). Here, we describe the frequencies at which these malformations occur and compare them with morphologies induced by the estrogen, 17β-estradiol (E2), and the antiandrogen cyproterone acetate, as a first step in testing the hypothesis that the effects of atrazine are a combination of demasculinization and feminization. The various forms of hermaphroditism did not occur in controls. Nonpigmented ovaries, which occurred at relatively high frequencies in atrazine-treated larvae, were found in four individuals out of more than 400 controls examined (1%). Further, we show that several types of gonadal malformations (SSP and three forms of hermaphroditism) are produced by E2 exposure during gonadal differentiation, whereas a final morphology (nonpigmented ovaries) appears to be the result of chemical castration (disruption of androgen synthesis and/or activity) by atrazine. These experimental findings suggest that atrazine-induced gonadal malformations result from the depletion of androgens and production of estrogens, perhaps subsequent to the induction of aromatase by atrazine, a mechanism established in fish, amphibians, reptiles, and mammals (rodents and humans).
Keywords: amphibians, atrazine, chemical castration, endocrine disruption, feminization, hermaphroditism
The herbicide atrazine is probably the most widely used pesticide in the world (Capel and Larson 2001; Miller et al. 2000; Müller et al. 1997; Solomon et al. 1996). As a result, it is the most common contaminant of groundwater and surface water (Capel et al. 2001; Fenelon and Moore 1998; Fischer et al. 1995; Frank and Logan 1988; Frank and Sirons 1979; Frank et al. 1987a, 1987b, 1991; Insensee et al. 1990; Kolpin et al. 1998; Kucklick et al. 1994a, 1994b; Müller et al. 1997; Oberdorster et al. 2001; Pennington et al. 2001; Rudolf and Goss 1993; Scribner et al. 2000; Thurman and Cromwell 2000; Thurman et al. 1992). Atrazine contamination can spread well beyond areas where it is applied (Du Preez et al., 2005; Hayes et al. 2002a; Miller et al. 2000; Müller et al. 1997; Nations and Hallberg 1992; Thurman and Cromwell 2000; Van Dijk and Guicherit 1999), traveling up to 1,000 km (Van Dijk and Guicherit 1999), and can persist for decades after its use is halted (Hayes et al. 2002a; Hennion et al. 2004). Atrazine is also a potent endocrine disruptor that both chemically castrates and feminizes exposed male amphibians at ecologically relevant concentrations (as low as 0.1 ppb).
The adverse effects of atrazine on amphibian gonadal development were first demonstrated by Tavera-Mendoza et al. (2002a), who showed that atrazine decreased testicular volume (p = 0.004), decreased the frequency of nursing cells (p < 0.0001), and decreased the number of primary spermatogonial nests (p < 0.0001) in African clawed frogs (Xenopus laevis) after only 48 hr of exposure. Parshley (2000) reported that atrazine demasculinized exposed male larvae, causing a significant reduction in laryngeal size in males (p = 0.045) in the same species (X. laevis). Our laboratory has shown that atrazine disrupted male gonadal development (p < 0.05) at concentrations as low as 0.1 ppb in X. laevis larvae (Hayes et al. 2002a) and confirmed findings reported by Parshley (2000). Carr et al. (2003) further confirmed these laboratory’s findings, showing similar gonadal abnormalities (multiple testes, p = 0.0003; hermaphroditism, p = 0.0042) at similar frequencies and over similar atrazine concentrations (Hayes 2004). The comparisons between the two studies and the graphic presentation were described by Hayes (2004). We then showed that atrazine feminized leopard frogs (Rana pipiens) in the laboratory (p < 0.001), inducing testicular oogenesis (Hayes et al. 2002b, 2002c).
The laboratory findings described above were supported by a field study reported in Reeder et al. (1998), which suggested that atrazine was associated with gonadal malformations (testicular oocytes) in cricket frogs (Acris crepitans) (p = 0.07). This work was followed by our field study in leopard frogs (R. pipiens) (Hayes et al. 2002b, 2002c) showing a similar association between atrazine contamination and feminized frogs in the wild (p < 0.05). Further, Du Preez et al. (2005) showed that feminized X. laevis males were associated with atrazine contamination (≥ 0.1 ppb) in the wild in South Africa in both corn-growing and non-corn-growing regions (although no noncontaminated, atrazine-free areas were examined for reference). Given the adverse effects of atrazine at such low concentrations, the ubiquity of atrazine contamination, and its persistence in the environment, the impact on amphibian populations can be quite significant. The present study is a first step in understanding atrazine’s mechanism of action in amphibians.
Estrogens feminize exposed male amphibians in multiple species (Chang and Witschi, 1955; Hayes 1998; Richards and Nace 1978; Villapando and Merchant-Larios 1990), including the species feminized by atrazine in the laboratory studies described above (X. laevis and R. pipiens). Although atrazine has a negligible affinity for the estrogen receptor (Roberge et al. 2004), many studies have shown that atrazine exposure elevates estrogens in every species (and class of vertebrate) examined (see “Discussion”). Therefore, we hypothesized that atrazine may feminize amphibians via inappropriate estrogen synthesis in males after an increase in expression and/or activity of aromatase, the enzyme that converts androgens to estrogens (Figure 1) (Hayes et al. 2002a). Alternatively, some malformations may result from a decrease in androgens (as a substrate for aromatase) or through one of the several other mechanisms by which atrazine decreases androgen levels and/or activity (Babic-Gojmerac 1989; Kniewald et al. 1979, 1980, 1995, 2000; Šimic et al. 1991). As a first step to testing these hypotheses, we describe here the types of gonadal malformations observed with atrazine exposure in X. laevis and compare the types of malformations with morphologies produced by exposure to the antiandrogen cyproterone acetate (CPA) and exogenous estrogen [17β-estradiol (E2)].
Figure 1.
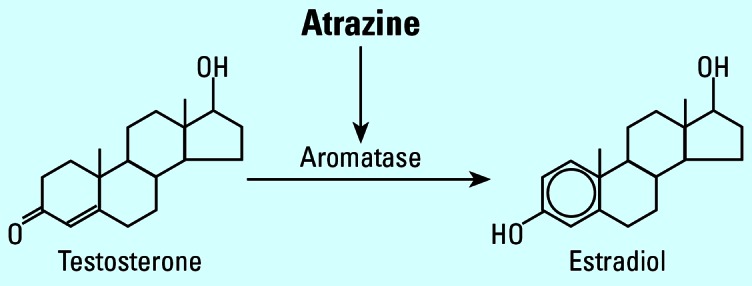
Proposed mechanism of atrazine action on amphibians. The induction of aromatase results in a decrease in androgens (aromatase substrate) and a subsequent increase in estrogens (aromatase product). Atrazine thus demasculinizes (chemically castrates) and feminizes amphibians. Molecular and endocrine support for the proposed mechanism has been demonstrated in amphibians as well as all other vertebrate classes examined (see “Discussion”).
Materials and Methods
Materials
Atrazine (2-chloro-4-ethylamino-6-isopropylamine-1,3,5-triazine) was obtained from Chemservice Inc. (Chester, PA). Cyproterone acetate (6-chloro-1β,2β-dihydro-17-hydroxy-3′H-cyclopropa[1,2]-pregna-1,4,6-triene-3,20-dione acetate) and 17β-estradiol (1,3,5-estratrien-3,17-β-diol), human choriogonadotropin, and benzocaine (ethyl-p-amino benzoate) were purchased from Sigma Chemical Co. (St. Louis, MO). All other reagents and histology supplies were obtained from Fisher Scientific (Fairlawn, NJ) except where indicated.
Animal breeding and larval care
Adult frogs for breeding were obtained from a long-term captive colony maintained at the University of California, Berkeley. For each experiment, three males and three females were injected with human choriogonadotropin (1,000 IU) 6 hr before harvesting gametes. Eggs were manually stripped from the females and fertilized in vitro in 0.3× modified mammalian Ringer’s solution by using sperm obtained from the dissected testes of the three males. Embryos were allowed to hatch and after 4 days apportioned into experimental tanks, five at a time, until all tanks contained 30 larvae. Larvae were raised and experiments conducted in covered plastic mouse boxes in 4 L of 10% (0.1×) aerated Holtfreter’s solution (Holtfreter 1931). All experiments were conducted at 22°C (± 1) and rooms were maintained on a 12/12-hr light/dark cycle (lights on at 0600 hr). Double distilled, ultraviolet-treated, carbon-filtered, deionized water used to make Holtfreter’s solution was certified atrazine-free by three laboratories (detection limit at least 0.1 ppb): one university laboratory (Iowa State Hygienics Laboratory, University of Iowa, Iowa City, IA), one government laboratory (U.S. Geological Survey, Denver, CO; detection limit, 0.02 ppb), and one private laboratory (PTRL West, Richmond, CA) as described previously (Hayes et al. 2002a, 2002b, 2002c). Water was also analyzed by the U.S. Geological Survey and found to be free (< 0.025 ppb) of 21 other triazine herbicides or metabolites, as well as free of alachlor, acetochlor, metolachlor, glyphosate, nicosulfuron, cyhalothrin, cyfluthrin, metalaxyl, propiconizole, and diazinon. Larvae were fed a solution of homogenized Purina rabbit chow (Purina Mills LLC, St. Louis, MO) daily, and the amount was adjusted as larvae grew to ensure that animals were fed ad libitum. All treatments (described below) were replicated three times (three 4-L tanks of 30 larvae each for a total of 90 larvae per treatment per experiment), and all tanks were color coded so that personnel delivering treatments and caring for animals were not aware of which groups were controls and which were experimental groups. Tanks were cleaned and solutions renewed every 3 days except during the E2 experiments (described below), when tanks were changed and solutions renewed daily. At each solution change, tanks were rotated around the rack to prevent position effects. Larvae were monitored daily, and animals at the end of metamorphosis [Niewkoop and Faber (NF) stage 66; Niewkoop and Faber 1994] were removed daily, euthanized in a 0.2% benzocaine solution, weighed, measured, fixed in Bouin’s fixative for 48 hr, and then preserved and stored in 70% ethanol until analysis, as described previously (Hayes et al. 2002a). Upon preservation, each specimen was given a coded specimen number so that personnel conducting final analyses were not aware of the color or treatment type for each individual.
Experimental design
We compared gonadal malformations induced by 0.1, 0.4, 0.8, 1, and 25 ppb atrazine [as reported by Hayes et al. (2002a)] for morphologies resulting from steroid or steroid antagonist exposure. Two alternative but not mutually exclusive hypotheses were tested: a) one or more of the types of gonadal malformations induced by atrazine represented “demasculinization” or loss of androgens, and/or b) one or more of the types of gonadal malformations represented feminization as a result of inappropriate estrogen production. We conducted two experiments to distinguish between these possibilities.
Experiment 1: effects of exposure to the antiandrogen CPA
Here, we tested the possibility that one or more of the atrazine-induced malformations represented demasculinization of the gonads as a result of androgen depletion. Larvae were treated with the androgen receptor antagonist CPA (5 mg/mL) throughout development (NF stages 50–66) to examine the effect of demasculinization on the developing gonads. At NF stage 66, animals were euthanized, fixed, and preserved. Sex was determined and gonads analyzed as described below. CPA was predissolved in methanol, and controls for this experiment were treated with an equal amount of methanol. Compounds were added to 16.5 L of 0.1× Holtfreter’s solution (final methanol concentration, 0.004% for all treatments), and the solution was divided among the three replicates. We also maintained a three-replicate set of untreated larvae (no solvent) for comparison with solvent-treated controls.
Experiment 2: effects of short-term E2 exposure
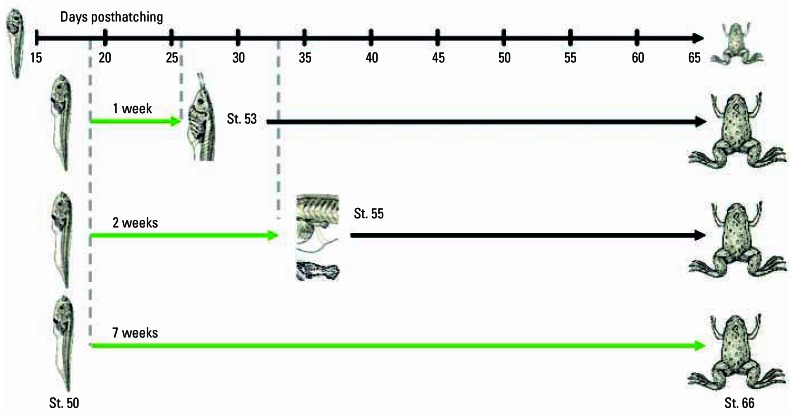
We tested the possibility that one or more of the atrazine-induced malformations represented partial feminization of the developing gonad. Although it is well known that estrogens induce 100% females in X. laevis (Chang and Witschi 1955; Gallien 1953; Hayes 1998), the concentration and timing of exposure are important for complete sex reversal of all exposed individuals. We treated larvae with E2 (100 μg/L) for 7 days (NF stages 50–53), 14 days (NF stages 50–55), or 49 days (NF stages 50–66) (Figure 2). Holtfreter’s solution was changed daily (as opposed to every 3 days) because previous studies showed that tadpoles rapidly metabolize exogenous estrogens (Hayes and Licht 1993). To deliver E2, the steroid was predissolved in 100% ethanol (final ethanol concentration, 0.004%), and the solution was divided among the nine replicates (three replicates each, representing each treatment regime described above). Controls were treated with an equal amount of ethanol for comparison with E2. We also maintained a three-replicate set of untreated larvae (no solvent) for comparison with solvent-treated controls. At NF stage 66, animals were euthanized, fixed in Bouin’s, and preserved in 70% ethanol. Sex was determined by gross morphology and histology as described below.
Figure 2.
. Experimental design for testing the effects of partial E2 exposure on sex differentiation in X. laevis larvae. The larval period from hatching [NF stage (St.) 48] to the completion of metamorphosis (complete tail reabsorption, NF stage 66) lasts 65 days on average under standard conditions in our laboratory. Sex differentiation occurs between NF stages 52 and 54 (~ 25 days posthatching). Animals were treated with E2 (100 μg/L) for 7 days (beginning at NF stage 50, day 19 posthatching, and ending at NF stage 53, day 26), for 14 days (beginning at NF stage 50, day 19 posthatching, and ending at NF stage 55, day 33), or 49 days (until complete tail reabsorption, NF stage 66). Treatment periods are shown by the solid green horizontal arrows, and termination of treatments, by the vertical dashed lines. In the final experimental group, treatment ended for each individual as they metamorphosed. Black arrows indicate that the animals were reared without E2 exposure after the treatments were completed. Larval pictures adapted from Niewkwoop and Faber (1994).
Gonadal analysis
Gonadal type (ovaries or testes) was scored using a Nikon SMZ 10A dissecting scope, fitted with a 0.5× lens (Technical Instruments, Burlingame, CA). A subset of animals (n = 150) was prepared for histologic analysis following procedures described by Hayes et al. (2002a). Initially, we identified and characterized gonadal malformations (morphologies that occurred in atrazine-treated animals but not in controls) by identifying anomalies in 30 animals treated with 25 ppb atrazine. An atlas of these malformations (see “Results”) was used to score and determine the frequency of malformations in controls and in animals treated with a range of atrazine concentrations.
Statistical analysis
Effects on sex ratios and frequency of gonadal malformations were analyzed using the G-test as described by Sokal and Rohlf (1981). First, we determined whether the sex ratio in untreated controls differed from the expected ratio of 50:50:0, male:female:malformed, after conducting a test of heterogeneity (GH) to determine whether there were any tank effects. Next, we tested each set of controls (from the three experiments) to determine if there were significant differences between the sex ratios of ethanol or methanol-treated larvae and untreated controls. Then, we examined each experiment separately (atrazine, CPA, and E2 treatments). We first conducted a test for heterogeneity, and if significant effects were found (p < 0.05), we then examined each experimental group using a test of heterogeneity to determine if there were any tank effects within each treatment. Finally, we examined each treatment group for deviation from the expected sex ratios, using results from the solvent-only controls to generate the expected ratios. Statistical significance was accepted at p < 0.05.
Results
Normal gonadal differentiation
Under the described conditions in our laboratory, gonadal differentiation occurs between NF stages 52 and 54, as determined by histologic analysis. At metamorphosis (complete tail reabsorption, NF stage 66), males and females can be distinguished by gross morphology of the gonads under the dissecting scope. Testes are shorter than ovaries (approximately one-third the length of the kidney) and lack pigment (Figure 3A). Ovaries extend the entire length of the kidney and are lobed and interspersed with melanin (Figure 3B). Histologically, testes are characterized by medullary development and distinguishable testicular lobules (Figure 3C). Ovaries are characterized by cortical development with a central ovarian cavity surrounded by a ring of connective tissue (Figure 3D). Oocytes are not observed in females even at NF stage 66 under the conditions used in our laboratory. In addition to the animals examined here, this characterization is based on more than 10,000 observations over 10 years of study in our laboratory and consistent with multiple reports from the literature dating back to 1953 (Gallien 1953; Hayes 1998, 2005b). In the present study, analysis of untreated controls and all solvent-only controls revealed that none differed from the expected sex ratio 50:50:0, male:female:malformed gonads (p > 0.5 in all cases). Analysis of heterogeneity revealed that there were no tank effects among controls, neither solvent (ethanol or methanol) affected the sex ratio, and control groups from across the three experiments were homogeneous (GH = 8.45, df = 22, p > 0.995).
Figure 3.
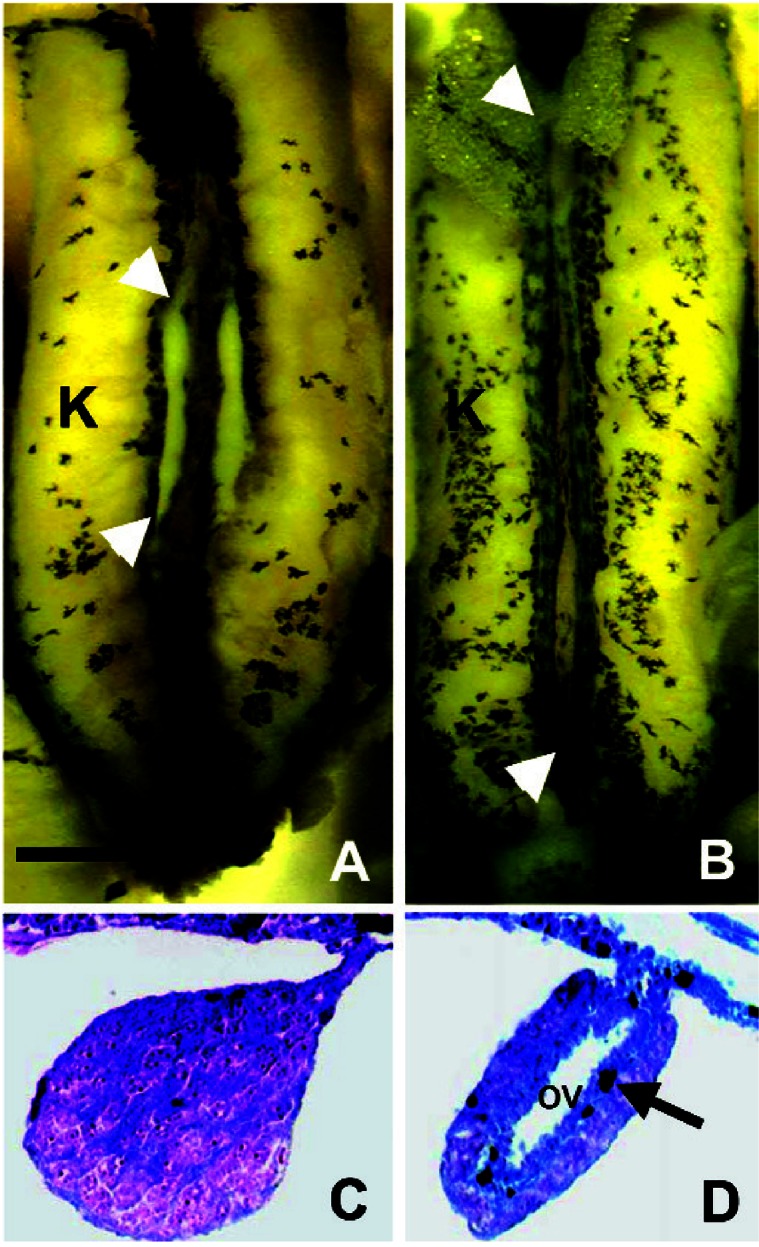
Gonads of a control postmetamorphic (NF stage 66) male (A,C) and female (B,D) African clawed frog (X. laevis). Abbreviations: OV, ovarian vesicle; K, kidney. (A,B) The entire kidney–interrenal–gonadal complex. The yellow color is the result of Bouin’s fixative. Arrowheads show the rostral and caudal ends of the animal’s right gonad. (C,D) Transverse cross-sections (8 μm) through the geometric center of each animal’s right gonad. Sections were stained with Mallory’s trichrome stain. Arrow indicates melanophore in the ovary. Scale bar: (A,B) 0.1 mm; (C,D) 10 μm. Figure adapted from Hayes et al. (2002a).
Types and frequencies of gonadal malformations induced by atrazine
By definition, “gonadal malformations” were defined initially as morphologies observed in atrazine-exposed larvae but not in controls. Gonadal malformations observed in atrazine-treated animals included two types of polygonadism (maximum six gonads): single-sex polygonadism (SSP; previously described as broken, lobed, or discontinuous testes; Carr et al. 2003; Hayes et al. 2002a, 2002b), where animals contained more than two distinct testes (Figure 4); and hermaphroditism, where individuals developed multiple combinations of testes and ovaries. Three morphologic types of hermaphrodites were observed. Morphologies included mixed hermaphrodites (Figure 5) in which animals contained multiple testes and ovaries, lateral hermaphrodites (Figure 6) in which animals contained only one pair of gonads with a complete testis on one side and an ovary on the other (no noted bias in which side was male and which was female), and rostral-caudal hermaphrodites (Figure 7) in which animals had two pair of gonads: one sex anterior and the other posterior. In a final type of malformation, animals had a single pair of gonads that appeared to be ovaries but lacked pigment (Figure 8). In some cases, the nonpigmented gonads were lobed like an ovary, whereas in others, the gonads had only shallow lobes or no lobes at all. Nonpigmented ovaries were the most common abnormality in atrazine-exposed animals. The second most common malformation was SSP. The frequency of all malformations varied between concentrations and the highest number of total malformations occurred at 1 ppb, although nonpigmented ovaries occurred at the highest frequency at 0.4 ppb, whereas hermaphrodites and SSP occurred at the highest frequency at 1 ppb (Figure 9). Atrazine caused a significant increase in the number of malformations in all cases (p < 0.001; Table 1). Although malformations were initially defined as morphologies found in atrazine-exposed animals but absent from controls, three methanol-treated controls from one replicate and a single ethanol-exposed animal (1%) with nonpigmented ovaries were later identified.
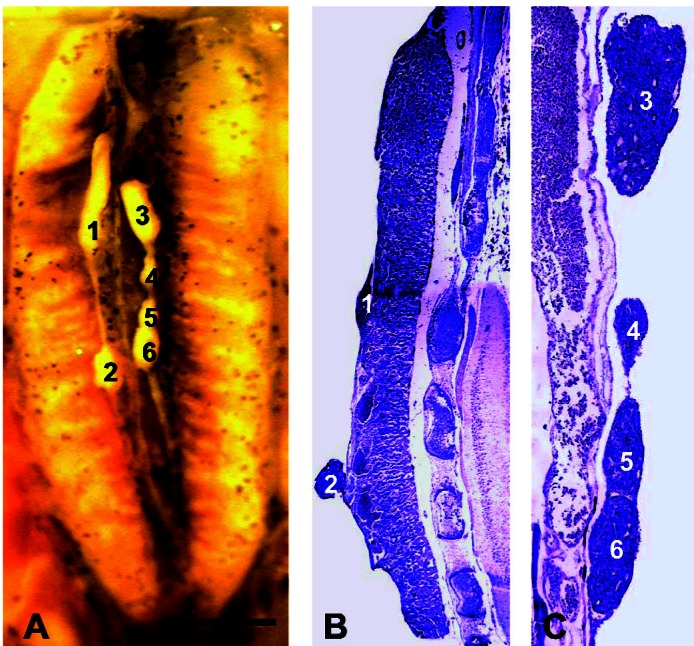
Figure 4.
Gonads of an NF stage 66 male X. laevis showing SSP. The individual shown was exposed to 0.1 ppb atrazine. (A) The entire dissected Bouin’s-fixed kidney–interrenal–gonadal complex. Each of the six testes is numbered. (B,C) Sagittal sections (stained in Mallory’s trichrome stain) through the animal’s right and left gonad, respectively. The six testes are numbered corresponding to numbers in A. Each is a distinct organ based on review of the entire series of sagittal sections. Scale bar: (A,B) 0.1 mm; (C) 40 μm.
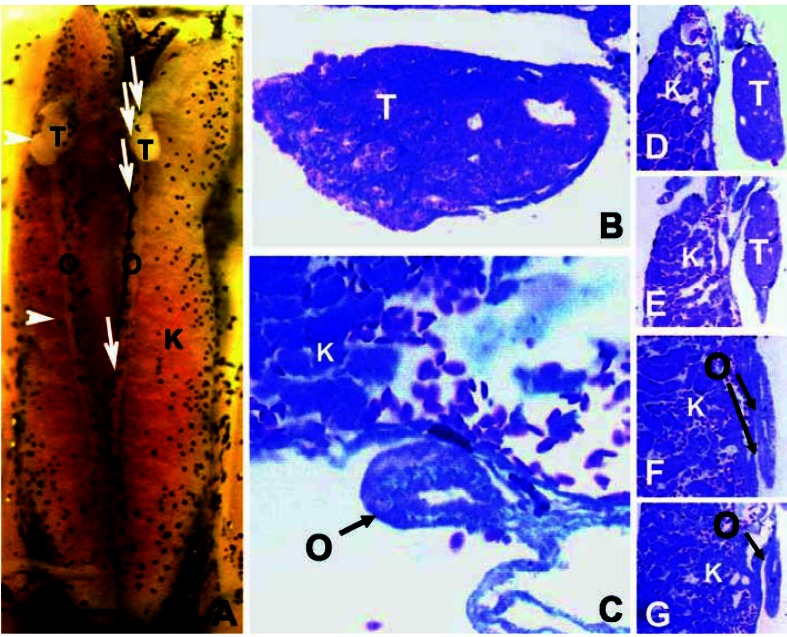
Figure 5.
A mixed hermaphrodite at NF stage 66, treated with 0.1 ppb atrazine. Abbreviations: FB, fatbody; K, kidney; O, ovary; T, testis. (A) The entire dissected, Bouin’s-fixed kidney–interrenal–gonadal complex. (B–E) Transverse cross-sections (8 μm) stained in Mallory’s trichrome stain. Sections were taken through areas indicated by the black lines. Note the absence of pigment in the ovaries, a conditional typical in hermaphrodites. Scale bar: (A) 0.1 mm; (B–E) 25 μm.
Figure 6.
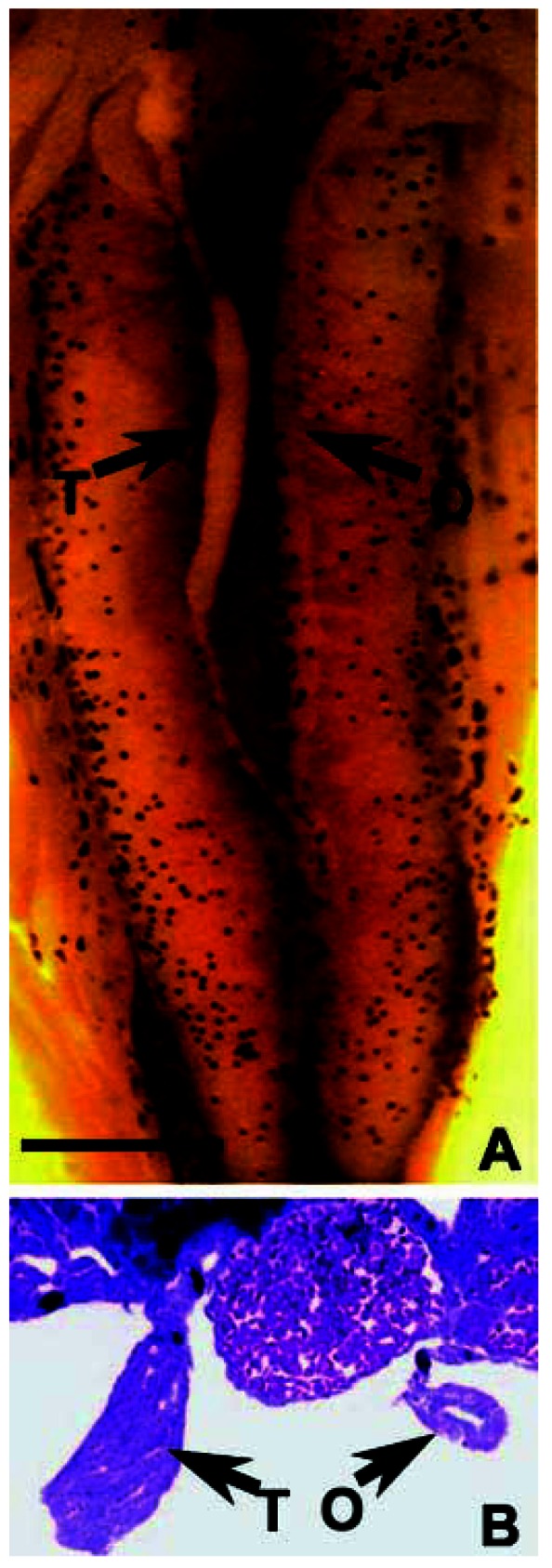
A lateral hermaphrodite at NF stage 66, treated with 0.1 ppb atrazine. Abbreviations: O, ovary, T, testis. (A) The entire dissected, Bouin’s-fixed kidney–interrenal–gonadal complex. (B) A transverse cross-section (8 μm) stained in Mallory’s trichrome stain. The section was taken from the center of the gonad indicated by the arrow in A. Scale bar: (A) 0.1 mm; B) 25 μm.
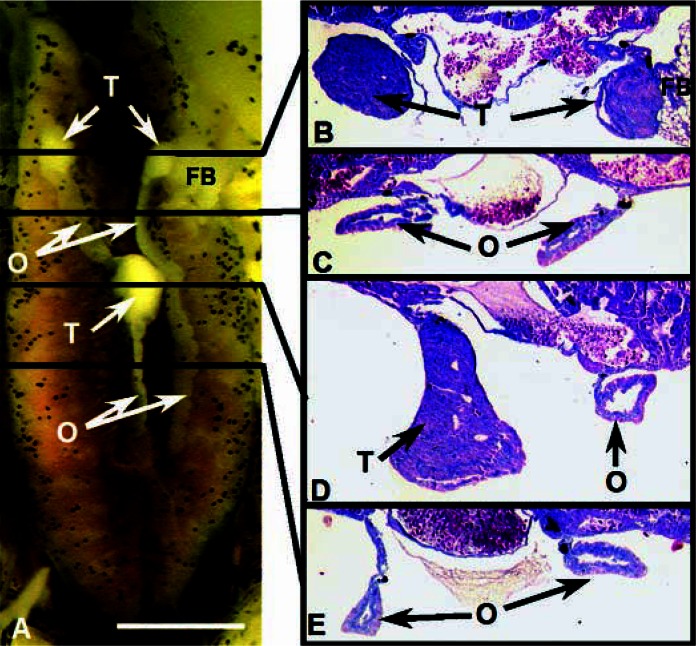
Figure 7.
A rostral-caudal hermaphrodite at NF stage 66, treated with 0.1 ppb atrazine. Abbreviations: K, kidney, O, ovary, T, testis. (A) The entire dissected, Bouin’s-fixed kidney–interrenal–gonadal complex. (B,C) Transverse cross-sections (8 μm) of the animal’s right gonad stained in Mallory’s trichrome stain. The sections were taken from the areas shown by the white arrowheads: right rostral testis in cross-section (B) and right caudal ovary (C). (D–G) Sagittal sections (8 μm) taken from the animal’s left gonad from the areas indicated by the white arrows: sagittal sections of the left rostral testis (D,E) and through the caudal ovary ( F,G). Note absence of pigment in the ovary. Scale bar: (A) 0.1 mm; (B–C) 10 μm; (D–G) 50 μm.
Figure 8.

An animal with nonpigmented ovaries. The individual shown was exposed to 0.1 ppb atrazine. Abbreviations: K, kidney; O, ovary; UO, unpigmented ovaries. (A) The entire dissected, Bouin’s-fixed kidney–interrenal–gonadal complex. Although the individual shown has distinct lobes, some animals with this morphology had only very shallow indications of lobes. (B) A transverse cross-section (8 μm) through the geometric center of the animal’s kidney and gonads. The section is stained in Mallory’s trichrome stain. This histology revealed that the animals were in fact females, even though their gonads lacked pigment, typical of normal ovaries. Scale bar: (A) 0.1 mm; (B) 40 μm.
Figure 9.
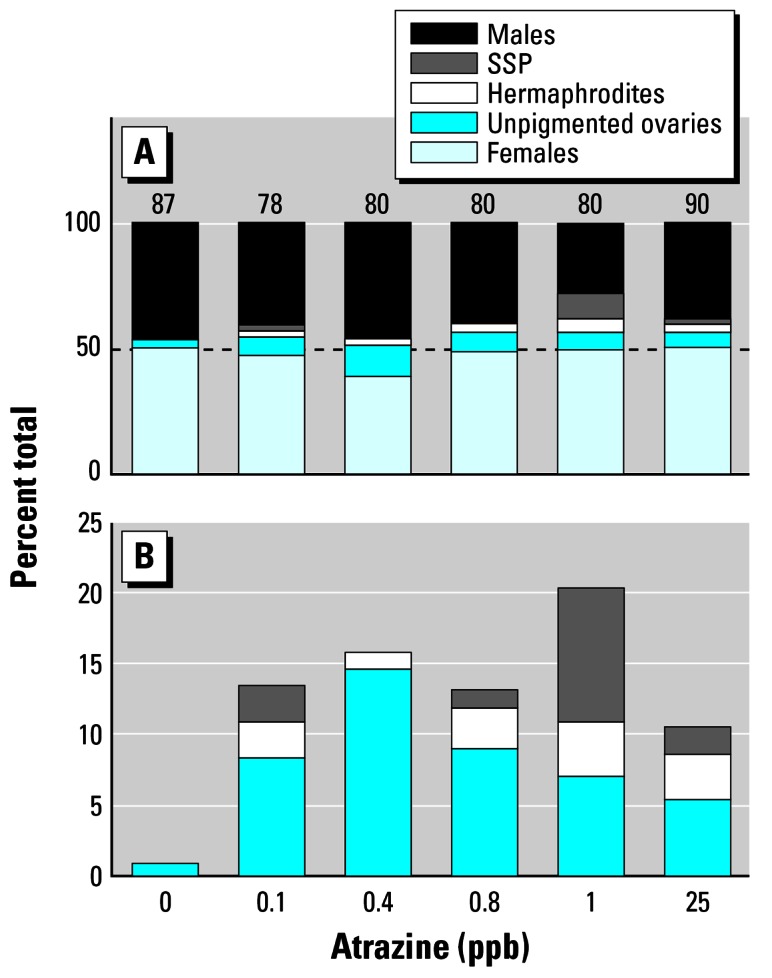
(A) Frequency of males, females, and specimens with gonadal malformations in controls and atrazine-treated animals (0.1–25 ppb). Numbers above bars are samples sizes and represent the number surviving to metamorphosis (of 90). Dashed line indicates 50%. (B) Frequency of gonadal malformations only. X-axis is categorical.
Table 1.
Statistics for effects of atrazine on frequency of gonadal malformations.
| Atrazine (ppb) | G | df | p-Value |
|---|---|---|---|
| 0 | −1.1 × 10−13 | 2 | > 0.995 |
| 0.1 | 24.94 | 2 | < 0.001 |
| 0.4 | 36.92 | 2 | < 0.001 |
| 0.8 | 29.21 | 2 | < 0.001 |
| 1.0 | 58.13 | 2 | < 0.001 |
| 25 | 25.70 | 2 | < 0.001 |
Comparison of atrazine-induced malformations with effects of E2 and CPA
Experiment 1
Exposure to the androgen receptor antagonist (CPA) did not result in hermaphroditism or SSP but produced a high percentage of animals (36%) with nonpigmented ovaries (G = 72.41, df = 2, p < 0.001), similar to the morphology produced by atrazine (Figures 10, 11).
Figure 10.
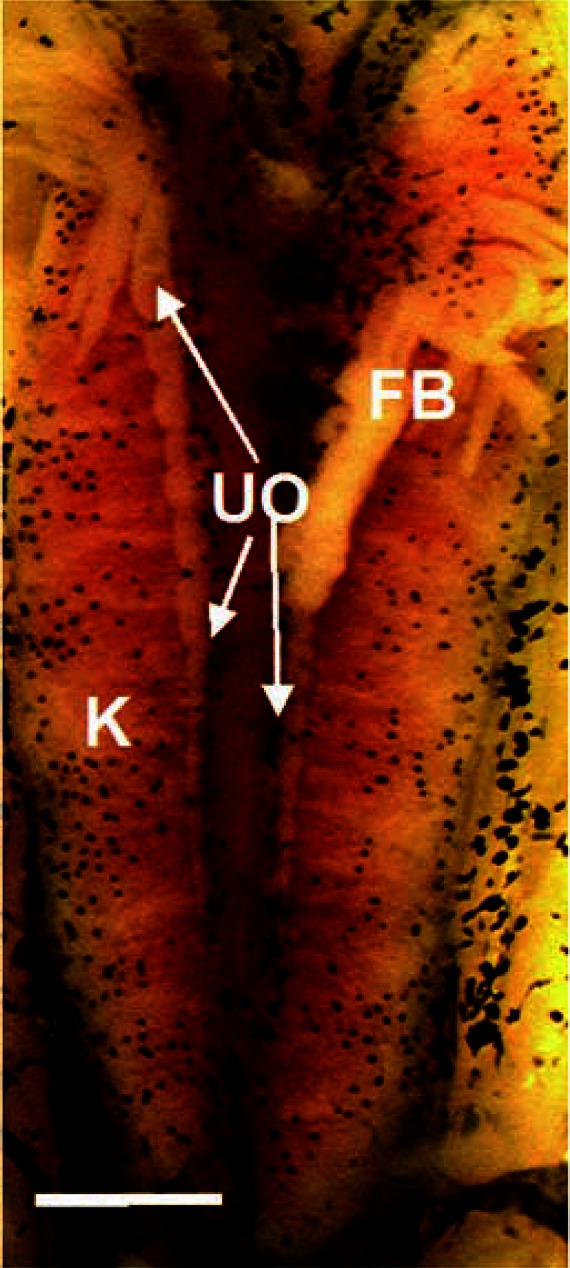
Unpigmented ovaries induced by CPA exposure. Abbreviations: FB, fat body; K, kidneys; UO, unpigmented ovary. Scale bar = 0.1 mm.
Figure 11.
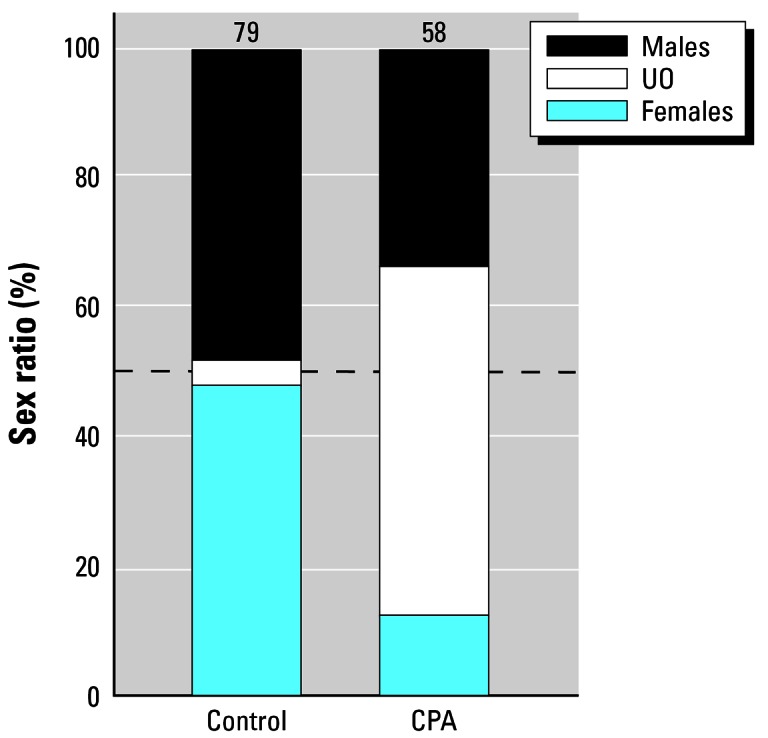
Frequency of males, females, and animals with nonpigmented ovaries in animals exposed to CPA compared with solvent (methanol)-treated controls. Numbers above bars are samples sizes and represent the number surviving to metamorphosis (of 90). Dashed line indicates 50%.
Experiment 2
Exposure to E2 for the entire larval period resulted in 100% females as determined by gross gonadal morphology and confirmed by histologic analysis. Larvae treated for 7 days (NF stages 50–53) or 14 days (NF stages 50–55) showed incomplete sex reversal, resulting in SSP and hermaphroditism (G = 1052.30, df = 2, p < 0.001 and G = 417.01, df = 2, p < 0.001, respectively) similar to morphologies observed with atrazine exposure (Figure 12). The highest frequency of malformations occurred in the 7-day treatment (Figure 13). Nonpigmented ovaries were not observed with complete or partial exposure to E2.
Figure 12.
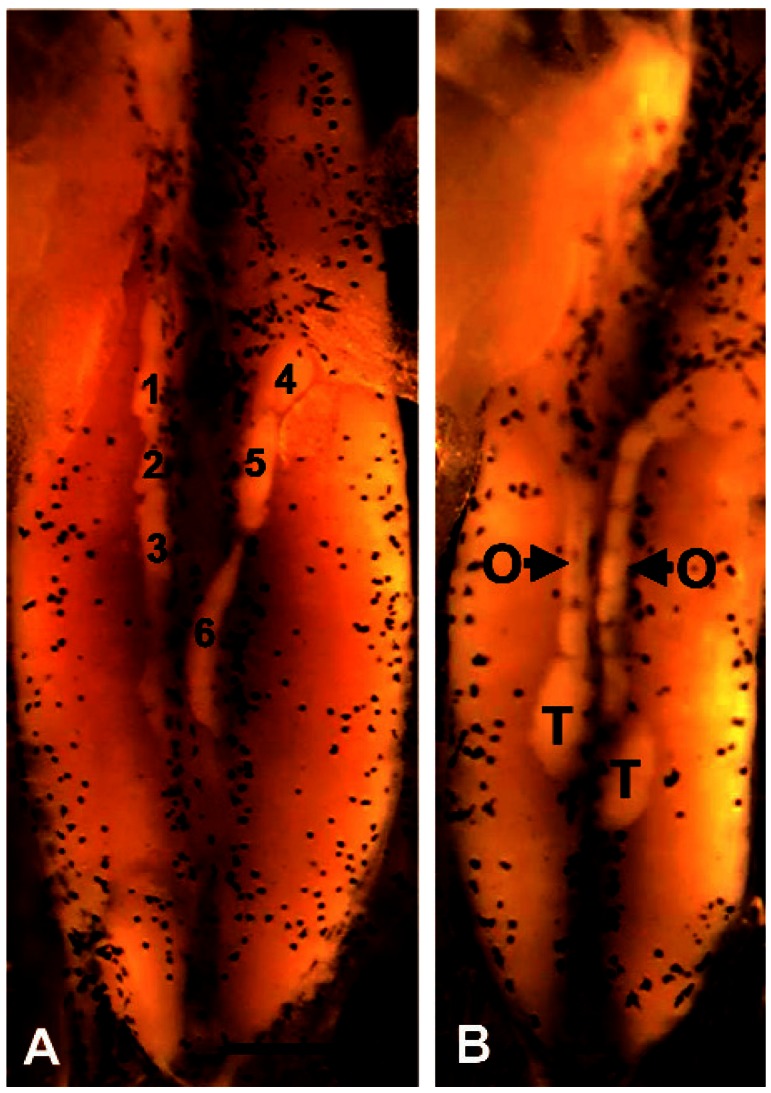
SSP (A) and hermaphroditism (B) in animals treated for 7 days (NF stage 50–53) with 100 μg/L E2. Six testes (three on each side) are numbered in A. Abbreviations: O, ovary; T, testis. Scale bar = 0.1 mm for A and B.
Figure 13.
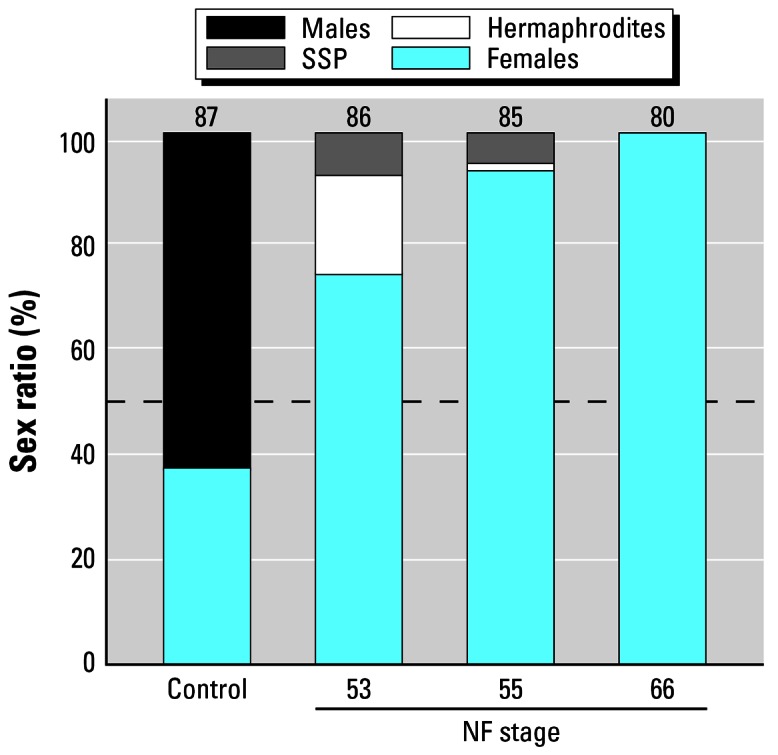
Frequency of males, females, animals with SSP, and hermaphrodites in animals treated for s7, 14, or 49 days with 100 μg/L E2. X-axis is categorical. Control bar shows the control sex ratio. NF stages below the bars indicate the stage at which E2 exposure was terminated. All E2 exposures began at NF stage 50 (Figure 2). Numbers above bars are samples sizes and represent the number surviving to metamorphosis (of 90). Dashed line indicates 50%.
Discussion
The gonadal malformations described in the present study were grouped into two categories: SSP (multiple sex organs of one type) and hermaphroditism (multiple sex organs with a mixture of testes and ovaries). As described previously (Hayes 1998), “primary sex differentiation” is the development of the undifferentiated (sometimes called “indifferent” or “bipotential”) gonads into testes (male) or ovaries (female). Although used to mean many different things in common language, a true hermaphrodite (after the Greek “Hermaphroditus,” the child of the mythical Hermes and Aphrodite who bore both male and female sex organs) is a single organism bearing both testicular and ovarian tissue. The organization of these tissues is not prescribed: there can be multiple organs or multiple tissue types within one organ. Historically, the term “intersex” has been used interchangeably with the term “hermaphrodite,” and the two should be considered synonyms here. In the case of SSP, the terms “broken testes” and “lobed testes” have been used synonymously in the literature (Carr et al. 2003; Hayes et al. 2002a, 2002b).
The gonadal morphologies associated with atrazine exposure described in the present study are consistent with effects described by Carr et al. (2003), who showed similar malformations (SSP and hermaphroditism) at similar frequencies induced by similar atrazine concentrations in X. laevis (Hayes 2004). Other effects of atrazine in X. laevis (e.g., gamete reduction/gonadal dysgenesis; Tavera-Mendoza et al. 2002a, 2002b) were not reported in our work, but treatments and husbandry differed greatly, and animals were not examined for this type of malformation in our studies. Also, reports in a recent study (Jooste et al. 2005) suggested that as many as 57% of atrazine-treated and control male X. laevis have testicular oocytes at metamorphosis. Testicular oocytes (although observed in other species) were not observed in any of our analyses in X. laevis (treated or control) and, in fact, have not been reported in any other study in X. laevis dating back to the 1950s (Carr et al. 2003; Chang and Witschi 1955; Coady et al. 2005; Gallien 1953; Hayes 2005b; Merchant-Larios and Villapando 1981; Villapando and Merchant-Larios 1990). The single report that described these effects was based on larvae reared at near-lethal feminizing temperatures (10°C) during critical stages of gonadal development, which likely explains this isolated anomalous finding (Hayes 2005b).
Like most anurans, X. laevis does not have morphologically distinguishable sex chromosomes, so it is not possible to determine the genetic sex of the hermaphrodites by karyotype (are they genetic females with testes or genetic males with ovaries?). Exposure to estrogens throughout the larval period results in 100% females in X. laevis (produces ovaries in genetic males) (Chang and Witschi 1955), but female sex differentiation is not altered by exogenous sex steroids in X. laevis: exogenous androgens induce testicular development in genetic females of other anuran species (Hayes 1998), but androgens do not affect primary sex differentiation in genetic female X. laevis larvae (Hayes 1998). Given the immutability of ovarian differentiation in female X. laevis larvae, we suggest that the hermaphrodites represent males that have been partially feminized rather than females that have been masculinized. The observation that SSP occurs in males only (females with multiple ovaries are not observed) further suggests that only males are affected. In addition, in other species, female secondary sex characteristics, including oocytes (Hayes 2005a; Hayes et al. 2002b, 2002c; Reeder et al. 1998), vitellogenin (Miyahara et al. 2003), and skin coloration (McCoy et al. 2002), are observed in male amphibians exposed to atrazine, but females with masculine secondary sex features have not been observed.
Further supporting our suggestion that the atrazine only affects male gonads, Carr et al. (2003), who reported significant induction of similar gonadal malformations, observed that the frequency of animals with gonadal malformations was matched by a paucity of males in their studies and suggested that only males are affected by atrazine:
The effects seem to be restricted to males, since the percentage reduction in males was proportional to the increase in intersex animals and atrazine did not affect the percentage of females, which remained at 50%.
Our findings with atrazine are in agreement with those of Carr et al. (2003), and we similarly suggest that the hermaphrodites represent demasculinized/feminized males, as opposed to masculinized/defeminized females.
Given that only males are affected, the true frequency of affected X. laevis in our previous report was 32–40% of the exposed males (hermaphrodites + SSP + unpigmented ovaries) as opposed to 16–20% of the total exposed population reported in Hayes et al. (2002a). Further, in the Carr et al. study (2003), the frequency of gonadal malformations (SSP and hermaphrodites only) ranged from 10 to 26% of the males [compared with 10–28% (SSP and hermaphrodites only) in our studies] in a concentration-dependent manner. The frequency reported by Carr et al. (2003) did not include nonpigmented ovaries; thus, the frequency of gonadal malformations (already 3-fold higher at some concentrations when only hermaphrodites and SSP are considered; Hayes 2004) was significantly higher than frequencies observed in our studies.
The malformations induced by atrazine in X. laevis (Carr et al. 2003; Hayes et al. 2002a) were reproduced in the present study by exposure to an antiandrogen (nonpigmented ovaries) or exposure to exogenous estrogen (hermaphroditism and SSP). The ability of antiandrogens and estrogens to produce the same types of malformations as atrazine provides support for the aromatase hypothesis: atrazine-exposed larvae suffer from a combination of androgen reduction and increased estrogen production. The highly significant induction of unpigmented ovaries by the antiandrogen CPA suggests that this malformation is the result of androgen depletion in atrazine-treated larvae, potentially as a result of the induction of aromatase (which uses androgen as a substrate for estrogen production), although atrazine reduces androgens through other mechanisms (Babic-Gojmerac et al. 1989; Kniewald et al. 1979, 1980, 1995, 2000; Šimic et al. 1991). It is also notable that the frequency of females was low in the CPA-treated group, however. The high mortality in the CPA treatment (42%; Figure 11) may explain the sex ratio skew (differential mortality with females more susceptible), but this hypothesis needs to be further tested.
Miyahara et al. (2003) showed that atrazine affects aromatase in X. laevis, although they were not able to track aromatase induction with malformations in males consistently (Tooi O, personal communication). Another research group was unable to show a significant induction of aromatase in captive X. laevis adults, but as reported by the authors, the study was not robust enough to identify such differences: “Based on the relatively low power to determine significant differences between plasma hormone concentrations in atrazine treated and control frogs it cannot be ruled out entirely that there have been some minor influences of atrazine treatment on plasma E2 or T [testosterone]” (Hecker et al. 2005). Consistent with our findings and hypotheses presented in the present study, this same research group detected negative relationships between plasma testosterone levels and atrazine in X. laevis in the wild. Although no relationship between atrazine and aromatase activity was reported, Hecker et al. (2005) concluded that “effects of atrazine or co-applied pesticides on sex steroid homeostasis cannot be excluded at this point.” It should also be pointed out that localized induction of aromatase and subsequent developmental effects of the resulting estrogens do not require (and often are not associated with) elevated plasma hormone levels. For example, in breast cancer, uterine cancer, endometriosis (Bulun et al. 2005), and prostate cancer (Ellem et al. 2004) or in normal processes such as sex differentiation of the brain in fish (Goto-Kazeto et al. 2004), birds (Ball and Balthazart 2004), and mammals (Ikeda et al. 2005), local induction of aromatase has permanent effects on cell and tissue differentiation through paracrine, autocrine, and even intracrine actions but does not result in changes in plasma sex steroid hormones.
Most significant, the proposed mechanism of action for atrazine (aromatase induction) has been observed in all vertebrate classes examined: aromatase gene expression (cytochrome P450-19 hydroxylase) is increased by atrazine in X. laevis metamorphs (Miyahara et al. 2003), human cells (Sanderson et al. 2000, 2001), and human tissues (Foster et al. 2002; Holloway et al. 2003). Atrazine induces aromatase activity and/or expression in fish (Moore and Waring 1998; Spano et al. 2004; Yukinori et al. 2002), amphibians (Miyahara et al. 2003), and two orders of reptiles (alligators, Crain et al. 1997; turtles, Keller and McClellan-Green 2004). Atrazine increases circulating estrogen levels in rodents (Eldridge and Wetzel 1999; Eldridge et al. 1994; Stevens et al. 1994; Wetzel et al. 1994) and is associated with estrogen-like activities and estrogen-dependent effects in fish (Moore and Waring 1998; Spano et al. 2004), amphibians (Carr et al. 2003; Carr and Solomon 2003; Du Preez et al., 2005; Gross et al. 2003; Hayes 2004, 2005a; Hayes et al. 2002a, 2002b, 2002c; Reeder et al. 1998; Tavera-Mendoza et al. 2002a, 2002b), reptiles (Crain et al. 1997; Keller and McClellan-Green 2004), and birds (Matsushita 2003; Matsushita S, personal communication). Atrazine also increases estrogen-sensitive reproductive cancers in rodents (Eldridge and Wetzel 1999; Eldridge et al. 1994; Stevens et al. 1994; Wetzel et al. 1994) and is associated with similar estrogen-sensitive reproductive cancers in humans (Kettles et al. 1997; Maclennan et al. 2002). Likewise, the demasculinization is consistent with the chemical castration (testosterone depletion) and decreased milt induced by atrazine in fish (Moore and Waring 1998) and adult X. laevis (Hayes et al. 2002a), androgen depletion and decrease in sperm in rodents (Babic-Gojmerac and Kniewald 1989; Friedmann 2002; Kniewald et al. 1995; Trentacoste et al. 2001), and the association between poor semen quality and atrazine exposure in human males (Swan et al. 2003). Thus, the combined demasculinization (depletion of androgens) and feminization (elevation of estrogens) has been demonstrated in fish, amphibians, reptiles, and mammals (including humans). With a single study showing similar effects in birds (Matsushita 2003; Matsushita S, personal communication), this mechanism has been demonstrated now in all vertebrate classes with the exception of Chondrichthyes (e.g., sharks and rays) and agnathid (e.g., lampreys), which have not been examined. These data collected independently across all vertebrate classes examined support the warning of Sanderson et al. (2000), who first reported atrazine’s mechanism of action in a human cell line:
A logical concern would be that exposure to triazine herbicides, which are produced and used in large quantities, and are ubiquitous environmental contaminants, may similarly contribute to estrogen-mediated toxicities and inappropriate sexual differentiation.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
We thank Novartis, Syngenta Crop Protection, Ecorisk Inc., and members of the Atrazine Endocrine Ecological Risk Assessment Panel of Ecorisk Inc. for comments, criticisms, and encouragement.
All work was conducted in compliance with animal use protocol R209-0402BCR to T.B.H. We thank H.H. Wheeler and Park Water Company for funding parts of the work. This work was also funded by a grant from the National Science Foundation (IBN-9513362) and by the Biology Faculty Award, University of California–Berkeley to T.B.H. N.N. was a Presidential Fellow (University of California, Berkeley) when the work was conducted. D.K., R.L., and G.J. were funded by the Howard Hughes Biology Scholar’s Program.
References
- Babic-Gojmerac T, Kniewald Z, Kniewald J. Testosterone metabolism in neuroendocrine organs in male rats under atrazine and deethylatrazine influence. Steroid Biochem. 1989;33:141–146. doi: 10.1016/0022-4731(89)90369-5. [DOI] [PubMed] [Google Scholar]
- Ball G, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Bulun S, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- Capel P, Larson S. Effect of scale on the behavior of atrazine in surface waters. Environ Sci Technol. 2001;35:648–657. doi: 10.1021/es001220f. [DOI] [PubMed] [Google Scholar]
- Carr J, Gentles A, Smith E, Goleman W, Urquidi L, Thuett K, et al. Response of larval Xenopus laevis to atrazine: assessment of growth, metamorphosis, and gonadal and laryngeal morphology. Environ Toxicol Chem. 2003;22:396–405. [PubMed] [Google Scholar]
- Carr JA, Solomon KR. Is atrazine causing frog deformities at very low doses? SETAC Globe. 2003;4:30–32. [Google Scholar]
- Chang C, Witschi E. Genic control and hormonal reversal of sex differentiation in Xenopus. Proc Soc Exp Biol Med. 1955;93:140–144. doi: 10.3181/00379727-93-22688. [DOI] [PubMed] [Google Scholar]
- Coady KK, Murphy J, Villeneuve DL, Hecker MJ, Carr J, Solomon K, et al. Effects of atrazine on metamorphosis, growth, laryngeal and gonadal development, aromatase activity, and plasma sex steroid concentrations in Xenopus laevis. Ecotoxicol Environ Saf. 2005;62(2):160–173. doi: 10.1016/j.ecoenv.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Crain D, Guillette LJ, Rooney AA, Pickford D. Alterations in steroidogenesis in alligators (Alligator mississippiensis) exposed naturally and experimentally to environmental contaminants. Environ Health Perspect. 1997;105:528–533. doi: 10.1289/ehp.97105528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Preez LH, Solomon K, Carr J, Giesy J, Gross C, Kendall RJ, et al. Population structure characterization of the clawed frog (Xenopus laevis) in corn-growing versus non-corn-growing areas in South Africa. Afr J Herpetol. 2005;54:61–68. [Google Scholar]
- Du Preez L, Solomon K, Jooste A, Jansen G, van Rensburg P, Smith E, et al. 2002. Exposure characterization and responses to field exposures of Xenopus laevis to atrazine and related triazines in South African corn growing regions [Abstract]. In: 23rd Annual Meeting in North America, Soc Environ Toxicol Chem, Salt Lake City, UT. Available: http://abstracts.co.allenpress.com/pweb/setac2002 [accessed 25 March 2005].
- Eldridge J, Fleenore-Heyser D, Extron P, Wetzel L, Breckenridge C, Gillis J, et al. Short-term effects of chlorotriazines on estrus in female Sprague-Dawley and Fischer 344 rats. J Toxicol Environ Health. 1994;43:155–167. doi: 10.1080/15287399409531912. [DOI] [PubMed] [Google Scholar]
- Eldridge J, Wetzel L, Trey L. Estrous cycle patterns of Sprague-Dawley rats during acute and chronic atrazine administration. Reprod Toxicol. 1999;13:491–499. doi: 10.1016/s0890-6238(99)00056-8. [DOI] [PubMed] [Google Scholar]
- Ellem S, Schmitt J, Pedersen J, Frydenberg M, Risbridger G. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab. 2004;89:2434–2441. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- Fenelon J, Moore R. Transport of agrichemicals to ground and surface waters in a small central Indiana watershed. J Environ Qual. 1998;27:884–894. [Google Scholar]
- Fischer J, Apedaile B, Vanclief L. Seasonal loadings of atrazine and metolachlor to a southeastern Ontario river from surface runoff and groundwater discharge. Water Qual Res J Canada. 1995;30:533–553. [Google Scholar]
- Foster W, Daya S, Holloway A. 2002. Inappropriate estrogen production induced by environmental toxicants [Abstract]. In: 48th Annual Meeting, Canadian Fertility, Andrology Society, 25–28 September 2002, Charvevoix, Quebec.
- Frank R, Clegg B, Ripley B, Braun H. Investigations of pesticide contaminants in rural wells, 1979–1984, Ontario Canada. Environ Contam Toxicol. 1987a;16:9–22. [Google Scholar]
- Frank R, Logan L. Pesticide and industrial chemical residues at the mouth of the Grand, Saugeen and Thames rivers, Ontario, Canada, 1981–85. Arch Environ Contam Toxicol. 1988;17:741–754. doi: 10.1007/BF01183882. [DOI] [PubMed] [Google Scholar]
- Frank R, Logan L, Clegg B. Pesticide and polychlorinated biphenyl residues in waters at the mouth of the Grand, Saugeen and Thames rivers, Ontario, Canada, 1986–1990. Arch Environ Contam Toxicol. 1991;21:585–595. doi: 10.1007/BF01183882. [DOI] [PubMed] [Google Scholar]
- Frank R, Ripley B, Braun H, Clegg B, Johnson R. Survey of farm wells for pesticides, Ontario, Canada. Arch Environ Contam Toxicol. 1987b;16:1–8. [Google Scholar]
- Frank R, Sirons G. Atrazine: its use in corn production and its loss to stream waters in southern Ontario. Sci Total Environ. 1979;12:223–239. [Google Scholar]
- Friedmann A. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod Toxicol. 2002;16:275–279. doi: 10.1016/s0890-6238(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Gallien L. Inversion totale du sexe chez Xenopus laevis Daud. À la suite d’un traitment gynogène par le benzoate of oestradiol, administré pendant la vie larvaire. C R Acad Sci. 1953;237:1565–1566. [PubMed] [Google Scholar]
- Goto-Kazeto R, Kight K, Zohar Y, Place A, Trant J. Localization and expression of aromatase rnRNA in adult zebrafish. Gen Comp Endocrinol. 2004;139:72–84. doi: 10.1016/j.ygcen.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Gross TS, Smith EE, Wiebe E, Sepulveda MS, Carr J, Du Preez LH, et al. 2003. An evaluation of gonadal anomalies and agrichemical exposures for the cane toad (Bufo marinus) in south Florida. In: 24th Annual Meeting in North America, Soc Environ Toxicol Chem, Austin, Texas. Available: http://abstracts.co.allenpress.com/pweb/setac2003 [accessed 25 March 2005].
- Hayes TB. Sex determination and primary sex differentiation in amphibians. J Exp Zool. 1998;281:373–399. [PubMed] [Google Scholar]
- Hayes TB. There is no denying this: defusing the confusion about atrazine. Bioscience. 2004;54:1138–1149. [Google Scholar]
- Hayes TB. Comment on “Gonadal development of larval male Xenopus laevis exposed to atrazine in outdoor microcosms. Environ Sci Technol. 2005a;39(19):7757–7758. doi: 10.1021/es051099i. [DOI] [PubMed] [Google Scholar]
- Hayes TB. Welcome to the revolution: integrative biology and assessing the impact of endocrine disruptors on environmental and public health. J Integr Comp Biol. 2005b;45:321–329. doi: 10.1093/icb/45.2.321. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, et al. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci USA. 2002a;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environ Health Perspect. 2002b;111:568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Feminization of male frogs in the wild. Nature. 2002c;419:895–896. doi: 10.1038/419895a. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Licht P. Metabolism of exogenous steroids by anuran larvae. Gen Comp Endocrinol. 1993;91:250–258. doi: 10.1006/gcen.1993.1124. [DOI] [PubMed] [Google Scholar]
- Hecker MJ, Giesy JP, Jones P, Jooste AM, Carr J, Solomon KR, et al. Plasma sex steroid concentrations and gonadal aromatase activities in African clawed frogs (Xenopus laevis) from South Africa. Environ Toxicol Chem. 2004;23:1996–2007. doi: 10.1897/03-450. [DOI] [PubMed] [Google Scholar]
- Hecker M, Kim W, Park J-W, Murphy M, Villeneuve D, Coady K, et al. Plasma concentrations of estradiol and testosterone, gonadal aromatase activity and ultrastructure of the testis in Xenopus laevis exposed to estradiol or atrazine. Aquat Toxicol (Amsterdam) 2005;72:383–396. doi: 10.1016/j.aquatox.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Hennion M, Pichon V, Legeay P, Cohen M. 2004. A ten-year survey of ground water: high persistence of some pesticide metabolites [Abstract]. In: The Ninth Symposium on Chemistry and Fate of Modern Pesticides, 16–19 August 2004 Vail, CO.
- Holloway A, Beecroft M, Sinasac S, Younglai E, Daya S, Edmunds K, et al. Environmental toxicant induced changes in aromatase activity in estrogen sensitive target tissues [Abstract]. In: Society for the Study of Reproduction, 36th Annual Meeting, 19–22 July 2003, Cincinnati, OH. Biol Reprod. 2003;68(suppl 1):128. [Google Scholar]
- Holtfreter J. Uber die Aufzucht isolierter Teile des Amphibian Keimes II. Arch F Ent Mech. 1931;124:404–465. doi: 10.1007/BF00652482. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Mitsui T, Setani K, Tamura M, Kakeyama M, Sone H, et al. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats disrupts brain sexual differentiation. Toxicol Appl Pharmacol. 2005;205:98–105. doi: 10.1016/j.taap.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Insensee A, Nash R, Helling C. Effect of conventional vs. no-tillage on pesticide leaching to shallow groundwater. J Environ Qual. 1990;19:434–440. [Google Scholar]
- Jooste AM, Du Preez LH, Carr J, Giesy JP, Gross C, Kendall RJ, et al. Gonadal development of larval male Xenopus laevis exposed to atrazine in outdoor microcosms. Environ Sci Technol. 2005;39:5255–5261. doi: 10.1021/es048134q. [DOI] [PubMed] [Google Scholar]
- Keller J, McClellan-Green P. Effects of organochlorine compounds on cytochrome P450 aromatase activity in an immortal sea turtle cell line. Mar Environ Res. 2004;58:347–351. doi: 10.1016/j.marenvres.2004.03.080. [DOI] [PubMed] [Google Scholar]
- Kettles MA, Browning SR, Prince TS, Hostman SW. Triazine exposure and breast cancer incidence: an ecologic study of Kentucky counties. Environ Health Perspect. 1997;105:1222–1227. doi: 10.1289/ehp.971051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniewald J, Jakominic M, Tomljenovic A, Šimic B, Romac P, Vranes ic D, et al. Disorders of male rat reproductive tract under the influence of atrazine. J Appl Toxicol. 2000;20:61–68. [PubMed] [Google Scholar]
- Kniewald J, Mildner P, Kniewald Z. Effects of s-triazine herbicides on 5 α-dihydrotestosterone receptor complex formation, 5 α-reductase and 3 β-hydroxysteroid dehydrogenase activity at the anterior pituitary level. J Steroid Biochem. 1979;11:833–838. doi: 10.1016/0022-4731(79)90018-9. [DOI] [PubMed] [Google Scholar]
- Kniewald J, Mildner P, Kniewald Z. 1980. Effects of s-triazine herbicides on 5 α-dihydrotestosterone receptor complex formation in the hypothalamus and ventral prostate. In: Pharmacological Modulation of Steroid Action (Genazzani E, DiCarlo F, Mainwaring WIP, eds). New York:Raven Press, 159–169.
- Kniewald J, Osredecki V, Gojmerac T, Zechner V, Kniewald Z. Effect of s-triazine compounds on testosterone metabolism in the rat prostate. J Appl Toxicol. 1995;15:215–218. doi: 10.1002/jat.2550150312. [DOI] [PubMed] [Google Scholar]
- Kolpin D, Barbash J, Gilliom R. Occurence of pesticides in shallow groundwater of the United States: initial results from the National Water-Quality Assessment Program. Environ Sci Technol. 1998;32:558–566. [Google Scholar]
- Kucklick J, Bidleman T. Organic contaminants in Winyah Bay South Carolina. I: Pesticides and polycyclic aromatic hydrocarbons in subsurface and microlayer waters. Mar Environ Res. 1994a;37:63–78. [Google Scholar]
- Kucklick J, Bidleman T. Organic contaminants in Winyah Bay, South Carolina II: using natural fluorescence to follow atrazine levels and river mixing. Mar Environ Res. 1994b;37:79–91. [Google Scholar]
- Maclennan P, Delzell E, Sathiakumar N, Myers S, Cheng H, Grizzle W, et al. Cancer incidence among triazine herbicide manufacturing workers. J Occup Environ Med. 2002;44:1048–1058. doi: 10.1097/00043764-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Matsushita S. 2003. Atrazine effect on the reproductive tract in the chicken [Abstract]. In: 6th Annual EDC Meeting, Sendai, Japan, 2003.
- McCoy KA, Sepulveda MS, Gross T, S. 2002. Atrazine exposure and reproductive system abnormalities in field collected Bufo marinus [abstract]. In: Society of Environmental Toxicology and Chemistry, 23rd Annual Meeting in North America, 16–20 November 2002, Salt Lake City, UT. Lawrence, KS:Allen Press. Available: http://abstracts.co.allenpress.com/pweb/setac2002 [accessed 25 March 2005].
- Merchant-Larios H, Villapando I. Ultrastructural events during early gonadal development in Rana pipiens and Xenopus laevis. Anat Rec. 1981;199:349–360. doi: 10.1002/ar.1091990305. [DOI] [PubMed] [Google Scholar]
- Miller S, Sweet C, Depinto J, Hornbuckle K. Atrazine and nutrients in precipitation: results from the Lake Michigan mass balance study. Environ Sci Technol. 2000;34:55–61. doi: 10.1021/es991463b. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Oka T, Mitsui N, Sagoe C, Kashiwagi A, Shinkai T, et al. 2003. Evaluation of atrazine on Xenopus laevis in a partial life test [Abstract]. In: 6th Annual Meeting of Japan Society of Endocrine Disruptor Research, 2–3 December 2003, Sendai, Japan, 259.
- Moore A, Waring C. Mechanistic effects of a triazine pesticide on reproductive endocrine function in mature male Atlantic salmon (Salmo salar L.) parr. Pest Biochem Physiol. 1998;62:41–50. [Google Scholar]
- Müller S, Berg M, Ulrich M, Schwarzenbach RP. Atrazine and its primary metabolites in Swiss lakes: input characteristics and long-term behavior in the water column. Environ Sci Technol. 1997;31:2104–2113. [Google Scholar]
- Nations B, Hallberg G. Pesticides in Iowa precipitation. J Environ Qual. 1992;21:486–492. [Google Scholar]
- Niewkoop P, Faber J. 1994. Normal Table of Xenopus laevis (Daudin). Amsterdam:North Holland Publishing.
- Oberdorster E, Clay M, Cottam D, Wilmot F, McLachlan J, Milner M. Common phytochemicals are ecdysteroid agonists and antagonists: a possible evolutionary link between vertebrate and invertebrate steroid hormones. J Steroid Biochem Mol Biol. 2001;77:229–238. doi: 10.1016/s0960-0760(01)00067-x. [DOI] [PubMed] [Google Scholar]
- Parshley T. 2000. Report of an Alleged Adverse Effect from Atrazine: Atrazine Technical. EPA Reg. no. 100–529. Washington, DC: U.S. Environmental Protection Agency.
- Pennington P, Daugomah J, Colbert A, Fulton M, Key P, Thompson B, et al. Analysis of pesticide runoff from mid-Texas estuaries and risk assessment implication for marine phytoplankton. J Environ Sci Health B. 2001;36:1–14. doi: 10.1081/pfc-100000912. [DOI] [PubMed] [Google Scholar]
- Reeder A, Foley G, Nichols D, Hansen L, Wikoff B, Faeh S, et al. Forms and prevalence of intersexuality and effects of environmental contaminants on sexuality in cricket frogs (Acris crepitans) Environ Health Perspect. 1998;106:261–266. doi: 10.1289/ehp.98106261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C, Nace G. Gynogenetic and hormonal sex reversal used in tests of the XX-XY hypothesis of sex determination in Rana pipiens. Growth. 1978;42:319–331. [Google Scholar]
- Roberge M, Hakk H, Larsen G. Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicol Lett. 2004;154:61–68. doi: 10.1016/j.toxlet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Rudolf D, Goss MJ. 1993. Ontario Farm Groundwater Quality Survey—Summer 1992. Guelph, Ontario, CN:Agriculture Canada, Agri-Food Development Branch.
- Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, van den Berg M. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect. 2001;109:1027–1031. doi: 10.1289/ehp.011091027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JT, Seinen W, Giesy JP, van den Berg M. 2-Chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- Scribner E, Battaglin W, Goolsby D, Thurman E. Changes in herbicide concentrations in midwestern streams in relation to changes in use, 1989–1998. Sci Total Environ. 2000;248:255–263. doi: 10.1016/s0048-9697(99)00547-1. [DOI] [PubMed] [Google Scholar]
- Šimic B, Kniewald Z, Davies J, Kniewald J. Reversibility of inhibitory effect of atrazine and lindane on 5 α-dihydrotestosterone receptor complex formation in rat prostate. Bull Environ Contam Toxicol. 1991;46:92–99. doi: 10.1007/BF01688260. [DOI] [PubMed] [Google Scholar]
- Sokal R, Rohlf F. 1981. Biometry: The Principles and Practice of Statistics in Biological Research. New York:W.H. Freeman.
- Solomon K, Baker D, Richards R, Dixon K, Klaine S, LaPoint T, et al. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 1996;15:31–76. doi: 10.1002/etc.2050. [DOI] [PubMed] [Google Scholar]
- Spano L, Tyler C, van Aerle R, Devos P, Mandiki S, Silvestre F, et al. Effects of atrazine on sex steroid dynamics, plasma vitellogenin concentration and gonad development in adult goldfish (Carassius auratus) Aquat Toxicol (Amsterdam) 2004;66:369–379. doi: 10.1016/j.aquatox.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Stevens JT, Breckenridge CB, Wetzel LT, Gillis JH, Luempert LG, III, Eldridge JC. Hypothesis for mammary tumorigenesis in Sprague-Dawley rats exposed to certain triazine herbicides. J Toxicol Environ Health. 1994;43:139–154. doi: 10.1080/15287399409531911. [DOI] [PubMed] [Google Scholar]
- Swan S, Kruse R, Liu F, Barr D, Drobnis E, Redmon J, et al. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111:1478–1484. doi: 10.1289/ehp.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavera-Mendoza L, Ruby S, Brousseau P, Fournier M, Cyr D, Marcogliese D. Response of the amphibian tadpole (Xenopus laevis) to atrazine during sexual differentiation of the testis. Environ Toxicol Chem. 2002a;21:527–531. doi: 10.1897/1551-5028(2002)021<0527:rotatx>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tavera-Mendoza L, Ruby S, Brousseau P, Fournier M, Cyr D, Marcogliese D. Response of the amphibian tadpole Xenopus laevis to atrazine during sexual differentiation of the ovary. Environ Toxicol Chem. 2002b;21:1264–1267. [PubMed] [Google Scholar]
- Thurman E, Cromwell A. Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in pristine areas at Isle Royale National Park. Environ Sci Technol. 2000;34:3079–3085. [Google Scholar]
- Thurman E, Goolsby D, Meyer M, Mills M, Pomes M, Kolpin D. A reconnaissance study of herbicides and their metabolites in surface water of the midwestern United States using immunoassay and gas chromatography/mass spectrometry. Environ Sci Technol. 1992;26:2440–2447. [Google Scholar]
- Trentacoste S, Friedmann A, Youker R, Breckenridge C, Zirkin B. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Androl. 2001;22:142–148. [PubMed] [Google Scholar]
- Van Dijk H, Guicherit R. Atmospheric dispersion of current-use pesticides: a review of the evidence from monitoring studies. Water Air Soil Pollut. 1999;115:21–70. [Google Scholar]
- Villapando I, Merchant-Larios H. Determination of the sensitive stages for gonadal sex-reversal in Xenopus laevis tadpoles. Int J Dev Biol. 1990;34:281–285. [PubMed] [Google Scholar]
- Wetzel LT, Luempert LG, III, Breckenridge CB, Tisdel MO, Stevens JT, Thakur AK, et al. Chronic effects of atrazine on estrus and mammary gland formation in female Sprague-Dawley and Fischer-344 rats. J Toxicol Environ Health. 1994;43:169–182. doi: 10.1080/15287399409531913. [DOI] [PubMed] [Google Scholar]
- Yukinori K, Place A, Trant J. Possible roles of endocrine disrupting chemical on the expression of CYP19 genes in zebrafish [Abstract]. In: 35th Annual Meeting of the Society for the Study of Reproduction, 28–31 July 2002, Baltimore MD. Biol Reprod. 2002;66(suppl 1):203. [Google Scholar]