Abstract
Little is known concerning the potential ecological effects of hormonally active substances associated with discharges from animal feeding operations. Trenbolone acetate is a synthetic anabolic steroid that is widely used in the United States to promote growth of beef cattle. Metabolites of trenbolone acetate include the stereoisomers 17α- and 17β-trenbolone, both of which are stable in animal wastes and are relatively potent androgens in fish and mammals. Our purpose in this study was to evaluate the occurrence of 17α- and 17β-trenbolone in a beef cattle feedlot discharge and in river water upstream and downstream from the discharge. In conjunction with that effort, we measured in vitro androgenic activity of the discharge using CV-1 cells that had been transiently cotransfected with human androgen receptor and reporter gene constructs. Samples were collected on nine different occasions during 2002 and 2003. Whole-water samples from the discharge caused a significant androgenic response in the CV-1 cells and contained detectable concentrations of 17α- and 17β-trenbolone. Further work is needed to ascertain the degree to which synthetic androgens such as trenbolone contribute to androgenic activity of feedlot discharges.
Keywords: environmental androgen, feedlot runoff, trenbolone
Environmental contaminants that adversely affect fish and wildlife through disruption of the hypothalamic–pituitary–gonadal (HPG) axis or that have direct effects on the reproductive tract are currently of interest. Although most early work focused on changes in the HPG axis caused by chemicals that bind to and activate estrogen receptors, recent studies have highlighted the variety of contaminants that can affect HPG function through interactions with the androgen receptor. For example, investigations of pulp and paper mill effluents from different locations in North America as well as an examination of a beef feedlot discharge from one site (Nebraska), have associated morphological alterations in fish collected from the field with in vitro androgenic activity in water samples from the affected sites (Hewitt et al. 2003; Jenkins et al. 2001; Orlando et al. 2004; Parks et al. 2001). Partly because of sample complexity, chemicals responsible for androgenic activity in pulp and paper mill effluents have not been identified successfully (Durhan et al. 2002; Hewitt et al. 2003). Similarly, little is known concerning the identity of chemicals that might be responsible for androgenic activity in feedlot discharges; however, with these types of samples, insights may be gained through consideration of chemicals used for livestock production. Specifically, much of the beef production in the United States utilizes anabolic androgenic materials to promote production of muscle mass in the animals. One of the most commonly used chemicals for this purpose is the synthetic androgen precursor trenbolone acetate (Meyer 2001; Neumann 1976; Raloff 2002; Roche 1986). Two metabolites of that acetate, 17α- and 17β-trenbolone, are relatively stable in animal waste and in the environment (Schiffer et al. 2001). These metabolites also bind with high affinity to fish androgen receptor(s) (Ankley et al. 2003; Bauer et al. 2000; Pottier et al. 1981; Wilson et al. 2004). In vivo studies with both isomers have shown that they are highly potent in fish; they masculinize females and decrease fecundity at water concentrations in the low nanogams per liter range (Ankley et al. 2003; Jensen K, unpublished data).
The present study had two objectives. First, we sought to evaluate whether the observation of Orlando et al. (2004) concerning androgenic activity in beef cattle feedlot discharge was an isolated or a more generalized (widespread) phenomenon. To achieve this goal, we first collected samples from several sites adjacent to a beef cattle feedlot in Ohio over the course of more than a year. We then determined androgenic activity in the samples using a cell-based assay with a transiently transfected (human) androgen receptor and promoter reporter gene constructs (Orlando et al. 2004; Parks et al. 2001). The feedlot operator indicated that trenbolone acetate was used in the production facility, so our second objective was to evaluate the samples with instrumental analyses to determine the possible presence and concentrations of 17α- and 17β-trenbolone in the site samples.
Methods and Materials
Study site
The beef cattle feeding operation was located in southwest central Ohio. It was constructed in the mid-1960s and consists of eight cattle buildings on about 96 ha. The cattle barns have a compacted clay floor, which is cleaned periodically and lined with fresh hardwood chips to absorb urine and provide comfort to the animals as insulation from the bare ground. Manure and urine fall on the bedding material. The waste is handled as a solid material with typical moisture content of 40–50%. Shallow drains from the buildings collect groundwater, including wastes that seep from the chips. As of early 2002, the feeding operation had a capacity to hold and feed 9,800 head of cattle. Revalor S implants (Hoechst-Roussel Agri-Vet Co., Sommerville, NJ), which contain both trenbolone acetate and 17β-estradiol, are used at this facility.
Three locations on a river adjacent to the feedlot were chosen for sample collection: a) 572 m upstream from all drainage from the facility, b) a discharge drain that collects run-off from the two sets of buildings, and c) 381 m downstream from the discharge drain. Samples were collected in amber 1- or 4-L (methanol rinsed) glass containers, placed on ice, and shipped overnight from the U.S. Environmental Protection Agency (EPA) laboratory in Cincinnati, Ohio, to U.S. EPA laboratories in Research Triangle Park, North Carolina, and Duluth, Minnesota. Upon arrival, the samples were stored at 4°C in the dark until analyzed. Samples were collected on nine different occasions and were designated by sample identifier and date as follows: A-February 2002, B-March 2002, C-June 2002, D-July 2002, E-September 2002, F-October 2002, G-March 2003, H-April 2003, and I-June 2003.
CV-1 assay
Androgenic activity in water samples was determined as described by Parks et al. (2001). Briefly, CV-1 cells (monkey kidney cell line; American Type Culture Collection no. CCL-70) were transiently cotransfected with human androgen receptor vector [pCMVhAR; Wong et al. (1995)] and MMTV (mouse mammary tumor virus)-luciferase reporter using Fugene reagent (Boehringer, Mannheim, Germany). Twenty-four hours after transfection, the original medium (Dulbecco’s modified Eagle’s medium; Gibco Invitrogen Corp., Carlsbad, CA, USA) with 5% dextran charcoal-stripped serum (Hyclone, Logan, UT, USA) was removed from the cell culture and replaced with medium prepared from water samples that had been passed through a 0.2-μm nylon filter. After 24 hr, cells were harvested, and relative light intensity was determined using a luminometer. Relative luciferase light data were analyzed and expressed as fold response relative to media. Hence, a fold induction of one is a negative response, equivalent to the media-only control. Each group of CV-1 assays of site samples was conducted simultaneously with a dihydrotestosterone (DHT) positive control (27 ng/L) and an untreated media negative control.
The CV-1 assay was also performed on two separate occasions with varying concentrations of both 17α- and 17β-trenbolone to estimate the detection limit of the assay for each compound. These studies were conducted using solutions of 17α- and 17β-trenbolone prepared in water under the conditions described above. In these studies, CV-1 cells were incubated with 17α-trenbolone (at 0, 2.7, 13.6, 18.9, 27, and 54 ng/L) or with 17β-trenbolone (at 0, 0.27, 5.4, 13.6, 27, and 40 ng/L), again with the positive DHT control. The chemicals for the CV-1 assays were obtained from Hayashi Pure Chemical Industries [Osaka, Japan; 17α-trenbolone (80657-17-6), 99.9% purity] and Sigma Chemical Co. [St. Louis, MO, USA; 17β-trenbolone (10161-33-8), 98% purity].
Because of logistical constraints, many of the CV-1 assays with the site samples were performed only once. However, with four samples (samples A, B, F, and G), the CV-1 assays were performed on three different occasions, together with a set of co-treatments with the androgen receptor antagonist 2-hydroxyflutamide (292 μg/L). Data from the A, B, F, and G sampling periods were used for statistical analyses of the data. In these analyses, CV-1 luciferase activity data were log10 transformed, and analyzed using PROC GLM available in SAS, version 8.2 (SAS Institute, Cary, NC, USA). Overall statistical significance was determined using the F-value from analyis of variance, followed by a least squares means analysis, which compares the means of individual groups with one another by t-tests. Differences between treatments were considered significant at p ≤ 0.05.
Instrumental analyses
Water samples for the instrumental analyses were processed by filtration (1-μm glass fiber filter) followed by C18 solid-phase extraction (SPE) concentration. A Bakerbond 6-mL HC C18 SPE column (JT Baker, Philipsburg, NJ, USA) was prepared by washing with 20 mL of acetonitrile and then conditioned with 20 mL of methanol followed by 20 mL of deionized water. One liter of filtered site water was pumped onto the conditioned SPE column at a rate of about 5 mL/min. The SPE column was then rinsed with 20 mL of 50% methanol–water (discarded) and allowed to vacuum dry for 2 min. The SPE column was eluted with two aliquots of 2 mL of methanol. The two aliquots were combined and, at this point, used for chemical analyses.
Trenbolone (both 17α and 17β) was measured in concentrated site samples by HPLC with fluorescence detection and, in a subset of samples, by gas chromatography–mass spectrometry (GC-MS). The HPLC determinations of trenbolone were made by injecting 40-μL aliquots onto a Nucleosil 100-Å, 5-μm C18 AB (Macherey-Nagel Inc., Easton, PA, USA) column (4.6 mm × 250 mm) on an Agilent 1100 HPLC (Agilent Technologies, Wilmington, DE, USA) at a flow rate of 0.9 mL/min. The solvent gradient started at 35% methanol–water and changed linearly over 3 min to 65% methanol–water, followed by isocratic elution with 93% methanol–water. Fluorescence detection was used with excitation and emission wavelengths at 359 and 458 nm, respectively. External standard quantitations were made. Detection limits were usually about 4 ng/L in the water samples. Standards used for the HPLC and GC–MS analyses were obtained from Aventis Pharma S.A. (Antony, France; 17α-trenbolone, 99.6% purity) and Sigma (17β-trenbolone).
Trenbolone measurements were made using GC/MS by injecting a 10-μl Aliquot of the SPE extract onto a 30 m × 0.25 mm Delta-3 GC column (Machery-Nagel, Oensingen, Switzerland) with a 0.25-μm film using an Apex large-volume injector (Apex, Philadelphia, PA, USA). The injector was equipped with a 2-mm quartz liner and a plain glass insert and was heated ballistically from 80°C to 250°C in 50 sec. The column oven was programmed from 80°C (3-min hold) to 250 °C at 15°C/min, followed by 250°C to 325°C at 5 °C/min, then held at 325°C for 5 min. A mass spectrometer (Agilent Technologies) was used in the single ion monitoring (SIM) mode at mass 270 and in the full scan mode for structural confirmation with an external standard method of quantitation. Detection limits for the GC-MS analyses were rather variable, ranging from slightly < 10 to about 100 ng/L.
Results
Androgenic activity of 17α- and 17β-trenbolone in the CV-1 cell line is shown in Figure 1A,B. In the case of 17α-trenbolone, androgenic activity was increased to about 3-fold over media at test concentrations of 12–30 ng/L, but the concentration–response relationship for the assay was comparatively flat above that, increasing to about 4-fold over media above 50 ng/L (Figure 1A). Androgenic activity in the cell line was increased by 5- to 8-fold over media by 17β-trenbolone concentrations of 6–40 ng/L (Figure 1B). The concentration activity curve of 17β-trenbolone in the CV-1 cells had an initial rise followed by a plateau at about 8-fold above media at a test concentration of 27 ng/L (Figure 1B). Statistical significance (above the media control) was achieved with ≥ 5.4 ng/L of 17β-trenbolone and ≥ 13.6 ng/L of 17α-trenbolone.
Figure 1.
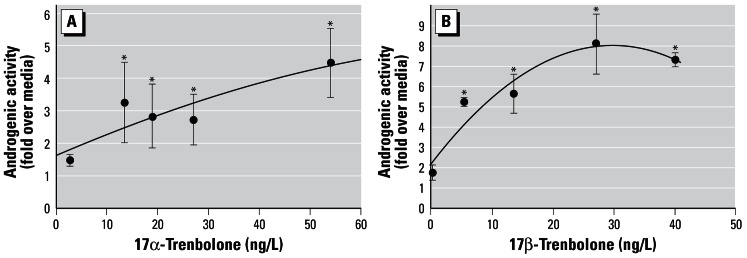
Androgenic activity in CV-1 cells of (A) 17α-trenbolone, and (B) 17β-trenbolone. Variability bars (± SE) reflect duplicate determinations, expressed as fold over media. The trend lines on A and B are a polynomial regression of the second order.
*Samples that have statistically significant androgenic activity compared with the media control.
When we assessed the samples, we found the positive control DHT (27 ng/L) consistently produced a significant induction of androgenic activity in the CV-1 assay, with a mean value (± SE, n = 9) over the course of the 16-month study of 16.2 ± 1.1-fold over media (Figure 2). Figure 2 also presents the effects of the environmental samples on the CV-1 cells. For four of the nine sampling periods (E, F, H, I), no clear difference was observed among the upstream, downstream, or discharge samples. For the other sampling periods (A, B, D, G), the discharge sample had elevated androgenic activity in the CV-1 cells, while neither the upstream nor the downstream samples (A, B, G) displayed consistently elevated androgenic activity (Figure 2). Based on statistical analysis of the four sampling periods for which CV-1 assays were conducted on multiple occasions (A, B, F, G), androgenic activity from the discharge samples but not from the upstream and downstream sites was significantly elevated. Co-treatments of the CV-1 cells with 2-hydroxyflutamide on these dates blocked increases in luciferase activity caused by either DHT or discharge samples from the feedlot, indicating that both responses were mediated via the androgen receptor (data not shown).
Figure 2.
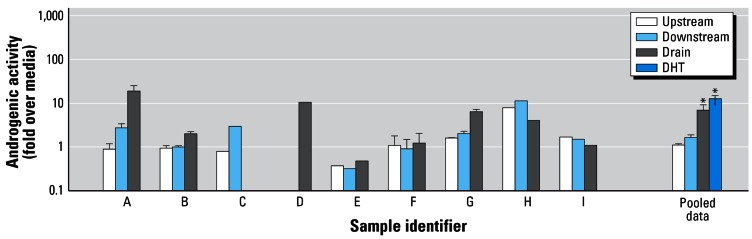
Androgenic activity in CV-1 cells of upstream, downstream, and discharge samples expressed relative to media. Only a discharge sample was collected on D, and only upstream and downstream samples were collected on C. Pooled data are from samples A, B, F, and G, for which the CV-1 assays were conducted in triplicate. Variability bars for these samples reflect the SE of individual replicate analyses, while for the pooled sample set, the bars indicate variability of all data for samples A, B, F, and G. The mean (± SE) DHT positive controls from all assays are also shown with pooled data.
*Samples that have statistically significant androgenic activity compared with the media control.
Concentrations of chemicals in the samples with elution times (and fluorescence properties) similar to those of 17α- and 17β-trenbolone are indicated in Figure 3A,B. As was true for the androgenic activity, in most instances when the two analytes were detected by HPLC, they were in the discharge rather than in the upstream or downstream samples. The 17α-trenbolone was detected more frequently and at higher concentrations than the 17β-trenbolone, which is consistent with a greater excretion of the 17α- versus 17β-isomer by cattle treated with trenbolone acetate (Schiffer et al. 2001). Overall, 17α-trenbolone was detected in six of nine samples from the discharge with concentrations ranging from < 10 to about 120 ng/L. Two of the nine discharge samples had detectable 17β-trenbolone at concentrations of about 10 and 20 ng/L. There were small amounts of 17α-trenbolone and/or 17β-trenbolone detected in some of the upstream (A, B, E, F) and in some of the downstream samples (A, E, F), but these concentrations were predominantly much lower than those found in the discharge samples.
Figure 3.
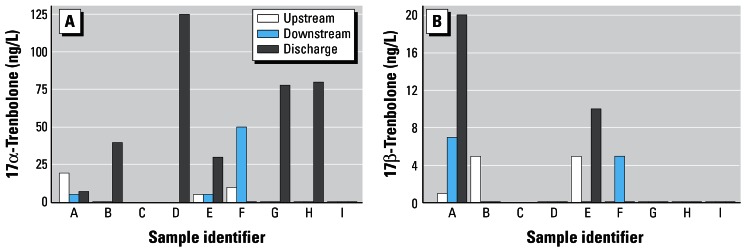
Concentrations (nanograms per liter) of 17α-trenbolone (A), and 17β-trenbolone (B) in upstream, downstream, and discharge samples as determined by HPLC.
Figure 4A–C depict confirmatory GC-MS data for the two trenbolone acetate metabolites in water collected from the feedlot discharge. The top panel of the figure shows a GC-MS scan for the chemical standards, the middle panel depicts GC-MS data for 17α-and 17β-trenbolone spiked into water from the discharge (at 50 ng/L) of sample E, and the bottom panel shows data for the two metabolites detected in the discharge of sample E. Quantitation of 17α- and 17β-trenbolone via GC-MS indicated concentrations of 8 and 12 ng/L, respectively, in the sample. HPLC analysis of this same sample produced respective concentrations of 30 and 10 ng/L. Neither 17α- nor 17β-trenbolone was detected in samples analyzed by GC-MS from other dates (sample ID:detection limit; F: 10 ng/L, G: 30 ng/L, H: 25 ng/L, and I: 40 ng/L).
Figure 4.
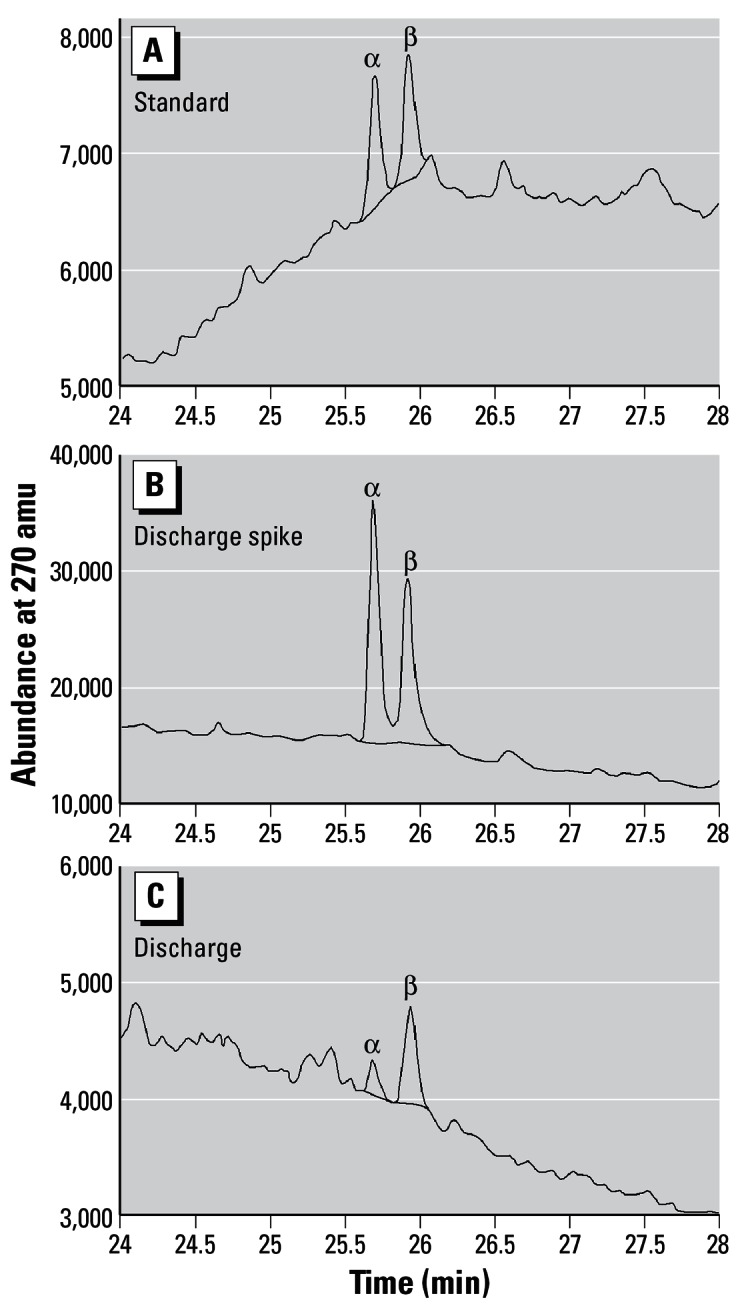
GC-MS trace of 17α- and 17β-trenbolone standards (A), 17α- and 17β-trenbolone in fortified discharge sample E (B), and discharge sample E (C).
Discussion
Schiffer et al. (2001) reported that half-lives for degradation of 17α- and 17β-trenbolone in liquid cattle manure were on the order of 260 days. Hence, it is not unreasonable to expect that the two trenbolone acetate metabolites would be detectable in runoff or discharges from a feedlot that uses the material as a growth promoter. However, this finding is, to our knowledge, the best- documented example of the occurrence of trenbolone in surface waters of the United States. Both trenbolone metabolites bind with high affinity to androgen receptors in mammals and fish (Wilson et al. 2002, 2004), and they are potent androgens in vivo; for example, they cause masculinization and decreases in fecundity in fish at nanograms per liter concentrations comparable to those detected in the discharge sample from this study (Ankley et al. 2003; Jensen K, unpublished data).
Assays of the water with the CV-1 cell line indicated an elevated level of androgenic activity in several samples collected over time from the discharge. The fact that hydoxyflutamide blocked the increase in gene expression in the CV-1 cells demonstrates that this induction was androgen-receptor mediated. Orlando et al. (2004) also reported that water from a pond adjacent to a beef feedlot located in Nebraska stimulated androgenic activity in the cell line, but they were unable to identify chemicals responsible for the activity. It would be tempting to speculate that 17α- and/or 17β-trenbolone were responsible for the androgenic activity observed in the present study; however, the data collected in our experiments do not permit this type of extrapolation. First, because of variations in efficiency of transfection of the receptor/reporter gene construct from experiment to experiment, even with the DHT positive control, the CV-1 assay is probably best considered a semiquantitative measure of androgenic activity. Second, given the detection limit for 17β-trenbolone (5 ng/L) in the CV-1 assay, it is possible that a positive androgenic response caused by 17β-trenbolone may not be consistently verifiable even by HPLC. Finally, in the present study, while performance of the HPLC method remained reasonably constant over time, the varying GC-MS detection limits (i.e., 10–100 ng/L) did not allow a sufficient number of confirmatory analyses to validate the HPLC data from a quantitative perspective. Further work at this site and/or at other feedlots is needed to ascertain the identity of chemicals (including natural steroids, such as testosterone, that may be excreted by the animals) that may contribute to androgenic activity in water associated with the sites. A number of variables, including sex, age, and reproductive status of the livestock, can affect androgenic activity in animal wastes (Lorenzen et al. 2004). A biologically based fractionation approach, based on a system such as the CV-1 cells, would be a logical method to employ for further studies (Durhan et al. 2002; Hewitt et al. 2003; Jenkins et al. 2001). Given the seeming variability we observed in the nature of samples from the site, this type of work might be most productively conducted at a location(s) where inputs/discharges can be better controlled (or documented).
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
We thank D. Hammermeister and I. Knoebl for manuscript review.
This work was funded wholly by the U.S. EPA, and is approved for publication. The contents do not necessarily reflect the views of the agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, et al. Effects of the androgenic growth promoter 17β-trenbolone on fecundity and reproductive endocrinology of the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2003;22:1350–1360. [PubMed] [Google Scholar]
- Bauer ERS, Daxenberger A, Petri T, Sauerwein H, Meyer HHD. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin, and bovine gestagen receptor. Acta Pathol Microbiol Immunol Scand. 2000;108:838–846. doi: 10.1111/j.1600-0463.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Durhan EJ, Lambright C, Wilson V, Butterworth BC, Kuehl DW, Orlando EF, et al. Evaluation of androstenedione as an androgenic component of river water downstream of a pulp and paper mill effluent. Environ Toxicol Chem. 2002;21:1973–1976. [PubMed] [Google Scholar]
- Hewitt LM, Pryce AC, Parrott JL, Marlatt V, Wood C, Oakes K, et al. Accumulation of ligands for aryl hydrocarbon and sex steroid receptors in fish exposed to treated effluent from a bleached sulfite/groundwood pulp and paper mill. Environ Toxicol Chem. 2003;22:2890–2897. doi: 10.1897/02-180. [DOI] [PubMed] [Google Scholar]
- Jenkins R, Angus RA, McNatt H, Howell WM, Kemppainen JA, Kirk M, et al. Identification of androstenedione in a river containing paper mill effluent. Environ Toxicol Chem. 2001;20:1325–1331. doi: 10.1897/1551-5028(2001)020<1325:ioaiar>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lorenzen A, Hendel JG, Conn KL, Bittman S, Kwabiah AB, Lazarovitz G, et al. Survey of hormone activities in municipal biosolids and animal manures. Environ Toxicol. 2004;19:216–225. doi: 10.1002/tox.20014. [DOI] [PubMed] [Google Scholar]
- Meyer HHD. Biochemistry and physiology of anabolic hormones used for improvement of meat production. APMIS. 2001;109:1–8. doi: 10.1111/j.1600-0463.2001.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Neumann F. 1976. Pharmacological and endocrinological studies on anabolic agents. In: Anabolic Agents in Animal Production (Lu FC, Rendal J, eds). Stuttgart:Verlag Georg Thieme, 253–264. [PubMed]
- Orlando EF, Kolok AS, Binzcik GA, Gates JL, Horton MK, Lambright CS, et al. Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ Health Perspect. 2004;112:353–358. doi: 10.1289/ehp.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottier J, Cousty C, Heitzmann RJ, Reynolds IP. Differences in the biotransformation of a 17α-hydroxylated steroid, trenbolone acetate, in rat and cow. Xenobiotica. 1981;11:489–500. doi: 10.3109/00498258109045859. [DOI] [PubMed] [Google Scholar]
- Parks LG, Lambright CS, Orlando EF, Guillette LJ, Ankley GT, Gray LE. Masculinization of female mosquitofish in kraft mill effluent contaminated Fenholloway River water is associated with androgen receptor agonist activity. Toxicol Sci. 2001;62:257–267. doi: 10.1093/toxsci/62.2.257. [DOI] [PubMed] [Google Scholar]
- Raloff J. Hormones: Here’s the beef. Sci News. 2002;161:10–12. [Google Scholar]
- Roche JF, Quirke JF. 1986. The effects of steroid hormones and xenobiotics on growth of farm animals. In: Control and Manipulation of Animal Growth (Buttery PJ, Haynes NB, Lindsay DB, eds). London:Butterworths, 39–51.
- Schiffer B, Daxenberger A, Meyer K, Meyer HHD. The fate of trenbolone acetate and melengestrol acetate after application as growth promoters in cattle: environmental studies. Environ Health Perspect. 2001;109:1145–1151. doi: 10.1289/ehp.011091145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VS, Cardon M, Thornton J, Korte JJ, Ankley GT, Welch J, et al. Cloning and in vitro expression and characterization of the androgen receptor and isolation of estrogen receptor alpha from the fathead minnow (Pimephales promelas) Environ Sci Technol. 2004;38(23):6314–6321. doi: 10.1021/es049771j. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright CS, Ostby J, Gray LE. In vitro and in vivo effects of 17 β-trenbolone: a feedlot effluent contaminant. Toxicol Sci. 2002;70:202–211. doi: 10.1093/toxsci/70.2.202. [DOI] [PubMed] [Google Scholar]
- Wong C, Kelce WR, Sar M, Wilson EM. Androgen receptor antagonist versus agonist activities of the fungicide vinvin-clozolin relative to hydroxyflutamide. J Biol Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]


