Abstract
High concentrations of vitellogenin (VTG; egg yolk protein) have previously been found in male flounder (Platichthys flesus) from several UK estuaries; these levels have been ascribed to the presence of estrogenic endocrine-disrupting compounds (EDCs). Gonadal abnormalities, including intersex, have also been recorded in these estuaries. However, there is no firm evidence to date that these two findings are causally linked or that the presence of estrogenic EDCs has any adverse population effects. In the present study, we examined the relationship between concentrations of VTG and sex steroids (11-oxotestosterone in males and 17β-estradiol in females) in specimens of flounder captured from the estuary of the River Mersey. We first questioned whether the high concentrations of VTG in male and immature female flounder were indeed caused by a direct effect of exogenous EDCs and not indirectly via the endogenous secretion of 17β-estradiol. The data favored the direct involvement of estrogenic EDCs. We then questioned whether the presence of estrogenic EDCs not only stimulated inappropriate VTG synthesis but whether it might also have had a negative effect on endogenous steroid secretion. It should be noted that the predicted consequences of a drop in steroid secretion include smaller gonads, smaller oocytes, fewer numbers of sperm, and depressed spawning behavior. This question was more difficult to answer because of the strong effect of the seasonal reproductive cycle and stage of maturation on steroid concentrations. However, matched by month of capture and stage of maturation, both 17β-estradiol in females and 11-keto-testosterone in males were in most cases significantly lower in those years when VTG concentrations were higher.
Keywords: 17β-estradiol, endocrine disruption, estrogens, flounder, sex steroids, vitellogenin
Surveys of vitellogenin (VTG; egg yolk) induction in male flounder (Platichthys flesus) in the United Kingdom (Allen et al. 1999a, 1999b; Kirby et al. 2004a; Kleinkauf et al. 2004c; Lye et al. 1997, 1998; Matthiessen et al. 2002) and Dutch (Vethaak et al. 2002) estuaries; in male flounder (Pleuronectes yokohamae) in Tokyo Bay, Japan (Hashimoto et al. 2000); and in male common goby (Acanthogobius flavimanus) and grey mullet (Mugil cephalus) in coastal areas of Japan (Hara et al. 2001; Ohkubo et al. 2003) have shown that these species have been and still are being exposed to estrogenic endocrine-disrupting compounds (EDCs). In the United Kingdom, France, and Japan as well, a small proportion of male flatfish (Allen et al. 1999b; Hashimoto et al. 2000; Kirby et al. 2004a; Minier et al. 2000) and shad (Konosirus punctatus) (Cho et al. 2003) have been shown to exhibit the intersex condition (presence of ovotestis). Over 7 years of survey in the United Kingdom, there have been some estuaries where male flounder have shown little or no sign of the presence of estrogenic EDCs and others where, in certain years, concentrations of VTG have approached and even exceeded those found in fully reproductively mature females. In the estuaries of the rivers Tyne and Mersey, there is also good evidence of a statistically significant decrease in the presence of estrogenic EDCs over the 7-year sampling period (Kirby et al. 2004a; Kleinkauf et al. 2004c), a finding in line with improvements in water quality that occurred during that period. Male flounder with elevated VTG concentrations in their plasma have also been caught in coastal (as opposed to estuarine) waters (Allen et al. 1999b). However, all evidence suggests these fish have migrated from contaminated estuaries rather than receiving their exposure in the open sea. The half-life of VTG in plasma of male flounder is 14 days (Allen et al. 1999b; George et al. 2004).
In 2002 it was stated by Matthiessen et al. (2002), in relation to the evidence for estrogenic EDCs and ovotestis induction in flounder in some UK estuaries [obtained during the Endocrine Disruption in the Marine Environment (EDMAR) program], that “although reproductive success may be impaired in some cases, implications for fish populations are still unclear.” This statement is still true today. Marine fish are not easy to study in the field. The closest we can get to answering the question about population effects in flounder is to examine whether overtly important reproductive parameters, such as fecundity and fertility, are related to the presence of EDCs in estuaries. Few such studies have been carried out in the rivers Tyne, Dee, and Mersey in the United Kingdom (Gill et al. 2002; Kleinkauf et al. 2004a, 2004b; Lye et al. 1997, 1998; Simpson et al. 2000). They all show that flounder from contaminated estuaries (i.e., where fish have consistently high VTG concentrations) also exhibit more signs of disrupted gonadal development (listed in “Discussion”) than flounder from uncontaminated estuaries.
The only aspect of gonadal development examined in any detail as part of the EDMAR program was the presence or absence of intersex. In an effort to obtain further evidence for possible adverse effects of estrogenic EDCs in fish collected during the course of EDMAR, we decided to investigate sex steroid concentrations in plasma. In another species, the white sucker (Catostomus commersoni) living in polluted waters, the circulating concentrations of sex steroids are significantly lower than circulating concentrations in the same fish living in unpolluted waters (Munkittrick et al. 1991). These sex steroid concentrations also appear to be related to increased age, maturation, reduced secondary sexual characteristics, and reduced gonadal development (all important “population effects”). Several other researchers have examined sex steroid concentrations specifically in relation to EDCs (Ankley et al. 1998; Kime 1995; McMaster et al. 2001). These studies support a decrease in sex steroid concentrations in response to the presence of EDCs, although none of the studies indicate whether this decrease is a result of EDCs acting directly on the activity of steroidogenic enzymes, indirectly through effects on feedback regulation within the hypothalamo–pituitary–gonadal axis, or by affecting the catabolism and excretion of the organism. Whatever the mechanism, sex steroid concentrations do appear to be sensitive to pollutants (Folmar et al. 1996; Jobling et al. 1996; Kime 1995; Munkittrick et al. 1998), especially in fish exposed to sewage treatment works or pulp mill effluents.
Most of the publications cited above highlight a major problem in interpreting sex steroid concentrations; that is, that they are highly dependent on the reproductive state of the fish. Thus, if fish from the same site are to be compared in different years, sampling has to be conducted at exactly the same point of their reproductive cycle and the fish have to be of a matching reproductive status. Because of this constraint, we decided in the present study to examine sex steroid concentrations in flounder caught only in the Mersey estuary, where seven sampling trips (six of which could be paired for month of sampling) were made between 1997 and 2003. Another major advantage of using this estuary as a case study was that the amount of apparent estrogenic contamination was different from year to year (Kirby et al. 2004a). Without such differences, it would have been difficult to test any association between sex steroid concentrations and VTG concentrations in a field situation. The steroids we have chosen to measure are 17β-estradiol (E2, the main estrogen of female fish) and 11-oxotestosterone [also known as 11-ketotestosterone (11-KT), the major androgen of male fish]. In addition to asking the question as to whether high estrogenic EDC levels mean low steroid concentrations, we also ask the question as to whether the estrogenic EDCs induce VTG directly or via stimulation of endogenous E2 secretion.
Materials and Methods
The site of capture of flounder in the Mersey estuary (and in nearby Liverpool Bay), the method of capture, and the method of sample collection are described in previous articles (Allen et al. 1999a, 1999b; Kirby et al. 2004a, 2004b). Data on VTG concentrations used in the present study were taken from these previous studies. However, the analysis of data by stage of maturation in the present article is new. The histological sections of gonads were also prepared during the course of the previous studies (Allen et al. 1999a). We re-examined these sections for stage of maturity. This determination was based upon the presence of the most advanced stage of gamete development that could be seen in histological sections of the gonads described below.
Ovaries: F1, primary oocytes only; F2, cortical alveoli-stage oocytes as well as primary oocytes; F3a, a mixture of cortical alveoli-stage and secondary (i.e., vitellogenic) oocytes; F3b, predominantly secondary oocytes.
Testes: M1, spermatogonia only; M2a, secondary spermatogonia; M2b, primary spermatocytes through to spermatids; M3a, some spermatozoa in addition to spermatocytes and spermatids; M3a, spermatozoa only.
To increase the numbers of fish in each grouping (for statistical purposes), we combined the subcategories (i.e., a and b) for the analyses of VTG, E2, and 11-KT concentrations.
For steroid assay, plasma aliquots (100 μL) were shaken vigorously with 50 μL distilled water and 4 mL diethyl ether for 2 min. The aqueous layer was allowed to settle, then frozen in liquid nitrogen. The solvent phase was poured into a glass tube (12 mm × 75 mm), then evaporated in a water bath at 45ºC. The residue was redissolved in 400 μL radioimmunoassay (RIA) buffer and measured as described previously for E2 and 11-KT (Scott et al. 1980, 1984). Two 100-μL aliquots were used for assay of E2, and an additional two 50-μL aliquots were used for assay of 11-KT. A plasma sample “spiked” with 2 ng/mL E2 was included in every E2 assay. The coefficient of interassay variation was < 10%. Sixty plasma samples from flounder collected in 1997 were also assayed twice (and by two different people) 4 years apart. The correlation coefficient between values generated by the two assays was 0.93, and the slope of the relationship was 1.03.
All data are expressed as means ± SE. Data were statistically analyzed by analysis of variance followed by comparison of means using Duncan’s multiple range test at a significance level of 0.05. Logarithmic transformation was performed when necessary to ensure homogeneity of variance.
Results
The numbers of fish caught at each maturation stage and at each sampling time in the Mersey estuary between 1996 and 2003 are shown in Table 1. For the purpose of analyzing steroid and VTG concentrations, we divided fish of both sexes into three groups that roughly equated to “immature,” “on the borderline between immaturity and maturity,” and “mature.” This last category encompassed the whole period of time from the first appearance of secondary oocytes and secondary spermatocytes up until the fish were ready to spawn. The histological and sex steroid data indicate that the secondary maturation phase in flounder lasts from September until March.
Table 1.
Numbers of flounder at each stage of maturation caught in the Mersey estuary at each sampling time.
| Year/month | F1 | F2 | F3a | F3b | M1 | M2a | M2b | M3a | M3b | Intersex |
|---|---|---|---|---|---|---|---|---|---|---|
| 1996 December | 2 | 1 | 9 | 24 | 4 | – | – | 13 | 8 | – |
| 1997 September | 41 | 19 | 22 | – | 56 | – | 4 | – | – | 5 |
| 1999 September | 10 | – | – | – | 1 | – | – | – | – | – |
| 2000 November | 13 | 1 | 5 | 4 | 8 | 5 | 4 | 1 | – | – |
| 2001 February | 13 | 1 | – | 6 | 5 | – | 1 | 3 | 4 | 3 |
| 2001 September | 18 | 3 | 3 | – | 14 | 2 | – | – | – | – |
| 2002 December | 4 | – | – | 21 | 1 | – | – | – | 25 | – |
| 2003 February | 6 | – | 1 | 24 | 3 | – | – | – | 8 | 1 |
| 1997 Marcha | – | – | – | – | 8 | – | – | – | 16 | – |
–, no fish.
Caught in Liverpool Bay.
Mean concentrations of E2, 11-KT, and VTG in males and females, categorized by sampling date and stage of maturation, are summarized in Tables 2 and 3. When only one or two fish were at a particular stage of maturation, the data were not included in the tables. Data from intersex fish (n = 9) were also not included. The data in Tables 2 and 3 were used to explore the relationship between sex, stage of maturation, and time of year versus VTG and sex steroid concentrations in flounder.
Table 2.
Mean ± SE concentrations of E2 (ng/mL), 11-KT (ng/mL), and VTG (μg/mL) in male flounder from the Mersey estuary by year, month, and stage of maturity.
| Year/month | Stage | n | E2 | 11-KT | VTG |
|---|---|---|---|---|---|
| Mersey estuary | |||||
| 1997 September | M1 | 56 | 0.09 ± 0.01a | 0.89 ± 0.11a | 780 ± 339a |
| M3 | 4 | 0.08 ± 0.01a | 0.57 ± 0.05a | 1307 ± 614a | |
| 2001 September | M1 | 14 | 0.07 ± 0.01 | 0.79 ± 0.2 | 0.32 ± 0.3 |
| 2000 November | M1 | 8 | 0.12 ± 0.01a | 0.37 ± 0.05a | 192 ± 76a |
| M3 | 9 | 0.20 ± 0.03b | 2.65 ± 0.45b | 527 ± 380a | |
| 1996 December | M1 | 4 | 0.13 ± 0.03a | 0.18 ± 0.07a | 10,847 ± 6,533a |
| M3 | 19 | 0.22 ± 0.02a | 3.29 ± 0.49b | 20,262 ± 9,133a | |
| 2002 December | M3 | 25 | 0.16 ± 0.01 | 7.48 ± 1.48 | 1107 ± 603 |
| 2001 February | M1 | 5 | 0.09 ± 0.01a | 0.54 ± 0.4a | 2,842 ± 2373a |
| M3 | 7 | 0.10 ± 0.01a | 15.2 ± 11.7b | 601 ± 421a | |
| 2003 February | M1 | 3 | 0.22 ± 0.01a | 0.61 ± 0.11a | 283 ± 259a |
| M3 | 8 | 0.22 ± 0.01a | 19.1 ± 5.6b | 47 ± 46a | |
| Liverpool Bay | |||||
| 1997 March | M3 | 16 | 0.09 ± 0.01 | 52.9 ± 12.5 | 780 ± 750 |
Values with the same letters within year/month are not significantly different from each other.
Table 3.
Mean ± SE concentrations (ng/mL) of E2, 11-KT, and VTG in female flounder from the Mersey estuary by year, month, and stage of maturity.
| Year/month | Stage | n | E2 | 11-KT | VTG |
|---|---|---|---|---|---|
| Mersey estuary | |||||
| 1997 September | F1 | 41 | 0.15 ± 0.02a | 0.24 ± 0.02a | 1,080 ± 342a |
| F2 | 19 | 0.32 ± 0.04b | 0.33 ± 0.12a | 900 ± 400a | |
| F3 | 22 | 0.34 ± 0.05b | 0.24 ± 0.03a | 5,797 ± 1,009b | |
| 1999 September | F1 | 10 | 0.14 ± 0.02 | 0.35 ± 0.04 | 292 ± 196 |
| 2001 September | F1 | 18 | 0.21 ± 0.06a | < 0.5* | 29 ± 16a |
| F2 | 3 | 0.87 ± 0.15b | < 0.5 | 8,096 ± 2,159b | |
| F3 | 3 | 1.41 ± 0.28b | < 0.5 | 4,443 ± 1,199b | |
| 2000 November | F1 | 13 | 0.19 ± 0.02a | 0.27 ± 0.03a | 312 ± 202a |
| F3 | 8 | 1.2 ± 0.23b | 0.37 ± 0.01a | 4,795 ± 629b | |
| 1996 December | F3 | 33 | 2.79 ± 0.4 | 0.17 ± 0.03 | 18,319 ± 3,139 |
| 2002 December | F1 | 4 | 0.18 ± 0.02a | < 0.5 | 32 ± 31a |
| F3 | 21 | 5.64 ± 1.2b | < 0.5 | 6,186 ± 2,161b | |
| 2001 February | F1 | 13 | 0.15 ± 0.01a | 0.53 ± 0.05a | 1,208 ± 822a |
| F3 | 6 | 5.92 ± 0.83b | 0.71 ± 0.09a | 2,831 ± 1,719b | |
| 2003 February | F1 | 6 | 0.22 ± 0.02a | 0.26 ± 0.06a | 581 ± 323a |
| F3 | 25 | 19.9 ± 4.5b | 1.02 ± 0.10b | 7,239 ± 921b | |
| Liverpool Bay | |||||
| 1997 March | F3 | 8 | 22.3 ± 6.6 | 0.59 ± 0.1 | 8,081 ± 1287 |
Values with the same letters within year/month are not significantly different from each other.
A high detection limit of 0.5 ng/mL was set in some of the 11-KT assays; all values less than this are shown.
Mean E2 concentrations in males were low (between 0.08 and 0.22 ng/mL, Table 2) and displayed no obvious relationship to the time of year or stage of sexual maturity. Most important, there was no evidence that high VTG concentrations were caused by high E2 concentrations; in fact, there was no relationship (r2 = 0.03; n = 158) between E2 and VTG concentrations in males (Figure 1). We found no obvious trend in VTG concentrations of either mature or immature males throughout the year (Figure 2) and no statistical difference between fish at different stages of maturation (Table 2). In contrast, mean 11-KT concentrations in males showed a regular month-on-month increase between September and March (Figure 3) and were also different between mature and immature fish (see statistics in Table 2).
Figure 1.

Lack of correlation between VTG concentrations and E2 concentrations in individual male flounder captured in the Mersey estuary between 1996 and 2003.
Figure 2.
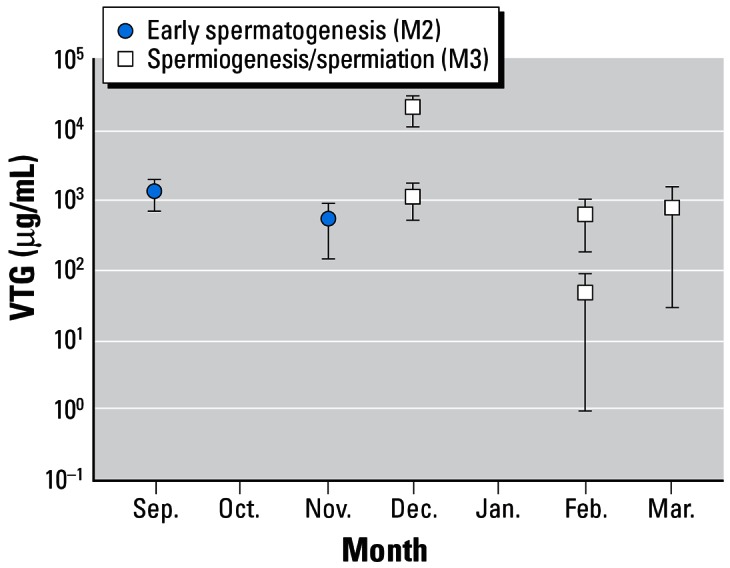
Mean concentrations (± SE) of VTG in male flounder by month of capture in the Mersey estuary.
Figure 3.
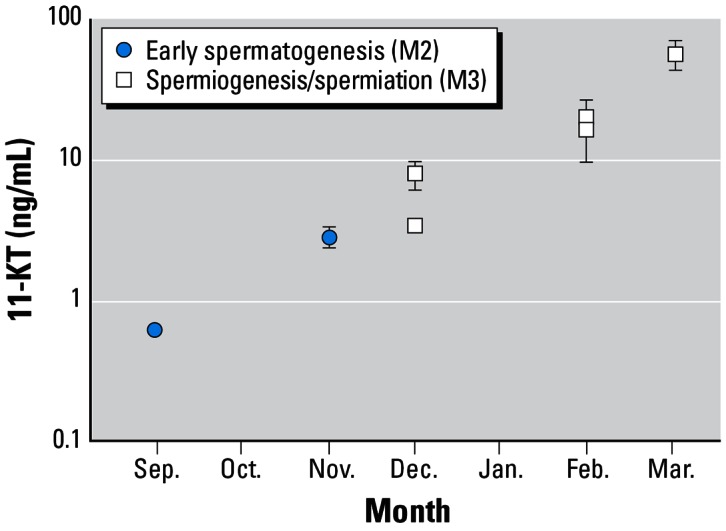
Mean concentrations (± SE) of 11-KT in male flounder by month of capture in the Mersey estuary.
Mean E2 concentrations in females were strongly influenced by stage of maturation (Table 3). E2 concentrations in immature females were never higher than those concentrations in either immature or mature males. In mature females, however, concomitant with the beginning of secondary oocyte development in September, E2 concentrations started to rise and reached a peak of 20 ng/mL at spawning time in March (Figure 4). As with males, we saw no obvious trend or pattern in VTG concentrations between September and March (Figure 5). Values fluctuated between 2,800 and 18,000 μg/mL. These concentrations were, nevertheless, in all cases higher than those found in immature females sampled at the same time (Table 3). Also, VTG concentrations in immature females were never at any sampling time significantly different from VTG concentrations in mature or immature males (analysis not shown).
Figure 4.

Mean (± SE) concentrations of E2 in female flounder by month of capture in the Mersey estuary.
Figure 5.

Mean concentrations of VTG (± SE) in female Mersey estuary flounder by month of capture.
Mature males caught in December 1996 had significantly lower mean 11-KT concentrations than mature males caught in December 2002 (Table 4). However, there was no significant difference between mean 11-KT concentrations in males caught in February 2001 and those caught in February 2003 (Table 4). Mature females caught in December 1996 had significantly lower E2 concentrations than mature females caught in December 2002 (Table 4). We also observed a significant difference between E2 concentrations in mature females caught in February 2001 and those caught in February 2003 (Table 4). In all cases, the lower E2 concentrations were found in the years when VTG concentrations in males were higher, which suggests that the presence of EDCs is associated with lower endogenous steroid synthesis.
Table 4.
Matching mean ± SE (n) concentrations of VTG in male, E2 in mature female (F3), and 11-KT in mature male (M3) Mersey estuary flounder by month and year.
| Year/month | VTG in all males (μg/mL) | E2 in mature females (ng/mL) | 11-KT in mature males (ng/mL) |
|---|---|---|---|
| 1997 September | 794 ± 318a (6) | 0.34 ± 0.05a (22) | — |
| 2001 September | 0.3 ± 0.3b (14) | 1.41 ± 0.28b (3) | — |
| 1996 December | 18,625 ± 7,614a (23) | 2.79 ± 0.40a (33) | 3.29 ± 0.49a (19) |
| 2002 December | 1,107 ± 603b (25) | 5.64 ± 1.20b (21) | 7.48 ± 1.48b (25) |
| 2001 February | 1,463 ± 940a (12) | 5.92 ± 0.83a (6) | 15.2 ± 11.7a (7) |
| 2003 February | 103 ± 53b (11) | 19.9 ± 4.5b (25) | 19.1 ± 5.6a (8) |
—, absence of M3 males in September. Values with the same letters within month are not significantly different from each other.
Discussion
We firmly concluded from this study that the VTG found in the plasma of male flounder in the Mersey estuary is not induced by endogenous E2. Although this conclusion seems naïve, substantial concentrations of E2 (> 1 ng/mL) have been found in blood plasma of reproductively mature males of a closely-related species, the North Sea plaice (Pleuronectes platessa) (Scott et al. 2000; Wingfield and Grimm 1977). The concentrations of E2 in male flounder in the present study, however, were < 0.22 ng/mL, never higher than those found in immature females, and thus were unlikely to be a trigger for VTG synthesis. Exogenous compound(s), acting directly on the estrogen receptor, are the more likely cause.
The number of fish caught at each maturity stage, plus steroid concentration data, in each month clearly indicates that the maturity cycle in both males and females begins about September and culminates in spawning (in the open sea) in March and April. The same conclusion can be reached by examining VTG concentrations (Kleinkauf et al. 2004c) and gonadosomatic indices (Kleinkauf et al. 2004a) of females caught in both the river Mersey and the river Dee estuaries between 1998 and 2000. Unlike in those studies, VTG concentrations in mature females in the present study showed no relationship to month of capture. The reason for this finding is probably that our samples have been collected over a period of 7 years, during which the level of estrogenic contamination has varied dramatically from a high in 1996 to a relative low in 2003 (Kirby et al. 2004a). This high variability in contamination has probably obscured the cycle. A previous observation that estrogen-induced VTG concentrations in male flounder also have a seasonal cycle (Kirby et al. 2004a; Kleinkauf et al. 2004c) was also obscured by the high year-to-year variations in estrogenic EDCs in the Mersey.
If, as seems likely, elevated VTG concentrations are mainly caused by estrogenic EDCs, then one might hypothesize that these same EDCs have a negative feedback effect on the secretion of gonadotropin from the pituitary and, hence, on 11-KT secretion by the testis in the males. The high dependence of 11-KT concentrations on stage of maturation and month of capture meant that, despite the large overall number of observations, it was only possible to examine this hypothesis via two pairs of data with the same month of capture and with a sufficient number of mature males for statistical analysis (Table 4). In December 1996, when VTG concentrations were high, 11-KT concentrations were significantly lower than in December 2002, when VTG concentrations were 20 times lower. In February 2001 and 2003 though, when VTG concentrations were much closer together (although still significantly different), 11-KT concentrations were not significantly different from each other. In females in September, December, and February, mean E2 concentrations were significantly lower in the years when mean VTG concentrations were higher.
Although supported by statistical analysis, the finding that mean steroid concentrations are in most cases lower in the years when the effects of exogenous estrogens are higher must be interpreted with caution. At the moment, it is an association only and needs to be tested in a laboratory experiment. There is no evidence yet of cause and effect. There are many other factors that could be the cause of 2- to 3-fold differences in steroid concentrations between years. These possibilities include water temperature (Onuma et al. 2003), diet (Cerda et al. 1995, 1997), and time of day. The sampling in the present study covered 7 years; therefore, another possible reason is that the differences were caused by deterioration of steroids during long-term storage. However, as indicated in “Materials and Methods,” there was no evidence of any deterioration of immunoreactivity of E2 in plasma samples that were stored for 4 years.
Bearing in mind that in no case is there necessarily a causal link, high plasma concentration of VTG in flounder have so far been associated with higher incidences of testicular malformation (Gill et al. 2002; Lye et al. 1997, 1998), higher amounts of oocyte malformation (Lye et al. 1998), more sperm abnormalities (Gill et al. 2002), more pathological lesions in the liver and kidney (Simpson et al. 2000), higher sperm motility (Kleinkauf et al. 2004b), lower gonadosomatic index (Kleinkauf et al. 2004a), and lower sex steroid concentrations (this study). However, there has been no clear link with degree of intersex (Allen et al. 1999a; Kirby et al. 2004a; Simpson et al. 2000), estrogen receptor concentrations in the liver (Kleinkauf et al. 2004b), hepatocyte proliferation markers (Kleinkauf et al. 2004b), ethoxyresorufin O-deethylase induction (Kirby et al. 2004b), or even with VTG mRNA (Craft et al. 2004; George et al. 2004). We stress the lack of a causal link because there are many other xenobiotics in estuaries (not just estrogens) that work through different mechanisms. These other compounds could cause the observed changes (without themselves affecting VTG concentrations).
An important practical outcome of the present study was that VTG concentrations in immature females (i.e., those shown by histological examination to possess only primary oocytes) were not statistically different from those found in males. This finding confirms the results of an earlier study (Kleinkauf et al. 2004c). The importance of this finding is that, until now, estuary surveys (Kirby et al. 2004a) have largely ignored data on VTG concentrations in females because of the uncertainty about the possible role of endogenous E2. However, our data suggest that, provided females can be proved immature by histological examination, their VTG concentrations can be pooled with those of males to increase the overall number of observations at each site.
Finally, an important lesson we have learned from the present study is that future field surveys should, if possible, be rigidly planned to catch flounder at the same places and at the same times each year. Only by ensuring such consistency is there any point in measuring sex steroid concentrations in flounder. We have also already concluded that only with rigid scheduling of field surveys is it possible to obtain clear-cut data on long-term trends in estrogenic EDC contamination in estuaries (Kirby et al. 2004a).
Conclusions
Few studies have been conducted on the population consequences of estrogenic EDCs on marine species. In the present article, we show that high VTG concentrations in male and female flounder caught in the Mersey estuary are not caused by endogenous E2. Rather, evidence indicates that elevated VTG concentrations are associated with a reduction in the synthesis of E2 by females and of 11-KT by males. Several mechanisms are possible, but the most likely is that the exogenous estrogenic EDCs not only enhance the production of VTG by the liver but also lower sex steroid production by negative feedback on the hypothalamic–pituitary axis. Work on other species suggests that reductions of sex steroid levels may have an adverse effect on fecundity and fertility. Any such causal link in flounder remains to be established, however.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
This study relied heavily on the samples collected during the course of the Endocrine Disruption in the Marine Environment (EDMAR) program. Many people, too numerous to list, from CEFAS and other organizations contributed to the collection and processing of the fish plasma and gonad samples. We thank them all.
We gratefully acknowledge the financial support of the Department for Environment, Food and Rural Affairs (DEFRA), the Scotland and Northern Ireland Forum for Environmental Research (SNIF-FER), and the Environment Agency (EA). We also thank P. Matthiessen for his direction of the EDMAR program.
References
- Allen Y, Matthiessen P, Scott AP, Haworth S, Feist S, Thain JE. The extent of oestrogenic contamination in the UK estuarine and marine environments—further surveys of flounder. Sci Tot Environ. 1999a;233:5–20. doi: 10.1016/s0048-9697(99)00175-8. [DOI] [PubMed] [Google Scholar]
- Allen Y, Scott AP, Matthiessen P, Haworth S, Thain JE, Feist S. Survey of estrogenic activity in United Kingdom estuarine and coastal waters and its effects on gonadal development of the flounder Platichthys flesus. Environ Toxicol Chem. 1999b;18:1791–1800. [Google Scholar]
- Ankley G, Mihaich E, Stahl R, Tillitt D, Colborn T, McMaster S, et al. Overview of a workshop on screening methods for detecting potential (anti-)estrogenic/androgenic chemicals in wildlife. Environ Toxicol Chem. 1998;17:68–87. [Google Scholar]
- Cerda J, Zanuy S, Carrillo M. Evidence for dietary effects on plasma levels of sexual steroids during spermatogenesis in the sea bass. Aquacult Int. 1997;5:473–477. [Google Scholar]
- Cerda J, Zanuy S, Carrillo M, Ramos J, Serrano R. Short-and long-term dietary effects on female sea bass (Dicentrarchus labrax): seasonal changes in plasma profiles of lipids and sex steroids in relation to reproduction. Comp Biochem Physiol C Pharmacol Toxicol Endocrino. 1995;111:83–91. [Google Scholar]
- Cho S-M, Kurihara R, Strussmann CA, Uozumi M, Yamakawa H, Yamasaki T, et al. Histological abnormalities in the gonads of konoshiro gizzard shad (Konosirus punctatus) from coastal areas of Japan. Environ Sci. 2003;10(1):25–36. [Google Scholar]
- Craft JA, Brown M, Dempsey K, Francey J, Kirby M, Scott AP, et al. Kinetics of vitellogenin protein and mRNA induction and depuration in fish following laboratory and environmental exposure to oestrogens. Mar Environ Res. 2004;58:419–423. doi: 10.1016/j.marenvres.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Folmar LC, Denslow ND, Rao V, Chow M, Crain DA, Enblom J, et al. Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environ Health Perspect. 1996;104:1096–1101. doi: 10.1289/ehp.961041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Gubbins M, MacIntosh A, Reynolds W, Sabine V, Scott A, et al. A comparison of pollutant biomarker responses with transcriptional responses in European flounders (Platichthys flesus) subjected to estuarine pollution. Mar Environ Res. 2004;58:571–575. doi: 10.1016/j.marenvres.2004.03.047. [DOI] [PubMed] [Google Scholar]
- Gill ME, Spiropoulus J, Moss C. Testicular structure and sperm production in flounders from a polluted estuary: a preliminary study. J Exp Mar Biol Ecol. 2002;281:41–51. [Google Scholar]
- Hara A, Matsubara H, Soyano K 2001. Endocrine and sexual disruption in wild grey mullet. In: UK-Japan Research Cooperation on Endocrine Disrupting Chemicals (Japan OECC, eds). Tokyo:Ministry of the Environment of Japan, 42–46.
- Hashimoto S, Bessho H, Hara A, Nakamura M, Iguchi T, Fujita K. Elevated serum vitellogenin levels and gonadal abnormalities in wild male flounder (Pleuronectes yokohamae) from Tokyo Bay, Japan. Mar Environ Res. 2000;49:37–53. doi: 10.1016/s0141-1136(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Jobling S, Sheahan DA, Osborne JA, Matthiessen P, Sumpter JP. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ Toxicol Chem. 1996;15:194–202. [Google Scholar]
- Kime DE. The effects of pollution on reproduction in fish. Rev Fish Biol Fisher. 1995;5:52–96. [Google Scholar]
- Kirby MF, Allen YT, Dyer RA, Feist SW, Katsiadaki I, Matthiessen P, et al. Surveys of plasma vitellogenin and intersex in male flounder (Platichthys flesus) as measures of endocrine disruption by estrogenic contamination in United Kingdom estuaries: temporal trends, 1996 to 2001. Environ Toxicol Chem. 2004a;23:748–758. doi: 10.1897/03-166. [DOI] [PubMed] [Google Scholar]
- Kirby MF, Neall P, Bateman TA, Thain JE. Hepatic ethoxyresorufin O-deethylase (EROD) activity in flounder (Platichthys flesus) from contaminant-impacted estuaries of the United Kingdom: continued monitoring 1999–2001. Mar Pollut Bull. 2004b;49:71–78. doi: 10.1016/j.marpolbul.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kleinkauf A, Connor L, Swarbreck D, Levene C, Walker P, Johnson PJ, et al. General condition biomarkers in relation to contaminant burden in European flounder (Platichthys flesus) Ecotox Environ Safe. 2004a;58:335–355. doi: 10.1016/j.ecoenv.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Kleinkauf A, Macfarlane C, Yeates S, Simpson MG, Leah RT. A biomarker approach to endocrine disruption in flounder-estrogen receptors, hepatocyte proliferation, and sperm motility. Ecotox Environ Safe. 2004b;58:324–334. doi: 10.1016/j.ecoenv.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Kleinkauf A, Scott AP, Stewart C, Simpson MG, Leah RT. Abnormally elevated VTG concentrations in flounder (Platichthys flesus) from the Mersey Estuary (UK)—a continuing problem. Ecotox Environ Safe. 2004c;58:356–364. doi: 10.1016/j.ecoenv.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Lye CM, Frid CLJ, Gill ME. Seasonal reproductive health of flounder Platicthys flesus exposed to sewage effluent. Mar Ecol Prog Ser. 1998;170:249–260. [Google Scholar]
- Lye CM, Frid CLJ, Gill ME, McCormick D. Abnormalities in the reproductive health of flounder Platichthys flesus exposed to effluent from a sewage treatment works. Mar Pollut Bull. 1997;34:34–41. [Google Scholar]
- Matthiessen P, Allen YT, Bamber S, Craft J, Hurst MR, Hutchinson TH, et al. The impact of oestrogenic and androgenic contamination on marine organisms in the United Kingdom—summary of the EDMAR programme. Mar Environ Res. 2002;54:645–649. doi: 10.1016/s0141-1136(02)00135-6. [DOI] [PubMed] [Google Scholar]
- McMaster ME, Jardine JJ, Ankley GT, Benson WH, Greeley MS, Jr, Gross TS, et al. An interlaboratory study on the use of steroid hormones in examining endocrine disruption. Environ Toxicol Chem. 2001;20:2081–2087. doi: 10.1897/1551-5028(2001)020<2081:aisotu>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Minier C, Levy F, Rabel D, Bocquene G, Godefroy D, Burgeot T, et al. Flounder health status in the Seine Bay. A multibiomarker study. Mar Environ Res. 2000;50:373–377. doi: 10.1016/s0141-1136(00)00059-3. [DOI] [PubMed] [Google Scholar]
- Munkittrick KR, McMaster ME, McCarthy LH, Servos MR, Van Der Kraak GJ. An overview of recent studies on the potential of pulp mill effluents to later reproductive parameters in fish. J Toxicol Environ Health B Crit Rev. 1998;1:347–371. doi: 10.1080/10937409809524558. [DOI] [PubMed] [Google Scholar]
- Munkittrick KR, Portt CB, Van der Kraak GJ, Smith IR, Rokosh DA. Impact of bleach kraft mill effluent on population characteristics, liver MFO activity, and serum steroid levels of a Lake Superior white sucker (Catostomus commersoni) population. Can J Fish Aquat Sci. 1991;48:1371–1380. [Google Scholar]
- Ohkubo N, Mochida K, Adachi S, Hara A, Hotta K, Nakamura Y, et al. Estrogenic activity in coastal areas around Japan evaluated by measuring male serum vitellogenins in Japanese common goby Acanthogobius flavimanus. Fish Sci. 2003;69:1135–1145. [Google Scholar]
- Onuma T, Higashi Y, Ando H, Ban M, Ueda H, Urano A. Year-to-year differences in plasma levels of steroid hormones in pre-spawning chum salmon. Gen Comp Endocrinol. 2003;133:199–215. doi: 10.1016/s0016-6480(03)00171-0. [DOI] [PubMed] [Google Scholar]
- Scott AP, Bye VJ, Baynes SM, Springate JRC. Seasonal variations in plasma concentrations of 11-ketotestos-terone and testosterone in male rainbow trout (Salmo gairdneri Richardson) J Fish Biol. 1980;17:495–505. [Google Scholar]
- Scott AP, Mackenzie DN, Stacey NE. Endocrine changes during natural spawning of the white sucker, Catostomus commersoni. II. Steroid hormones. Gen Comp Endocrinol. 1984;56:349–359. doi: 10.1016/0016-6480(84)90077-7. [DOI] [PubMed] [Google Scholar]
- Scott AP, Stewart C, Allen Y, Matthiessen P. 2000. 17β-Oestradiol in male flatfish. In: Proceedings of the Sixth International Symposium on Reproductive Physiology of Fish, 4–9 July 1999, Bergen, Norway (Norberg B, Kjesbu OS, Taranger GL, Andersson E, and Stefansson SO, eds). Bergen Norway:John Grieg As, 382.
- Simpson MG, Parry M, Kleinkauf A, Swarbreck D, Walker P, Leah RT. Pathology of the liver, kidney and gonad of flounder (Platicthys flesus) from a UK estuary impacted by endocrine disrupting chemicals. Mar Environ Res. 2000;50:283–287. doi: 10.1016/s0141-1136(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Vethaak AD, Lahr J, Kuiper RV, Grinwis GCM, Rankouhi TR, Giesy JP, et al. Estrogenic effects in fish in The Netherlands: some preliminary results. Toxicol. 2002;181–182:147–150. doi: 10.1016/s0300-483x(02)00271-8. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Grimm AS. Seasonal changes in plasma cortisol, testosterone and oestradiol-17β in the plaice, Pleuronectes platessa L. Gen Comp Endocrinol. 1977;31:1–11. doi: 10.1016/0016-6480(77)90184-8. [DOI] [PubMed] [Google Scholar]


