Abstract
Contamination of freshwater ecosystems with nitrate is a growing global concern. Although nitrate pollution is recognized as a cause of aquatic eutrophication, few studies have examined the possible physiological impacts of nitrate exposure. In this study, we surveyed several reproductive variables of viviparous female Gambusia holbrooki (Poeciliidae) captured from eight springs in Florida. The eight springs represent a gradient of nitrate contamination (1–5 mg/L nitrate–nitrogen). We had two objectives in this study: to describe reproductive biology of female mosquitofish in the springs and to understand reproductive variation in the context of water quality, particularly the nitrate concentration. Our data show a significant negative association between nitrate and both dry weight of developing embryos and rate of reproductive activity among mature females. In addition, variation in Gambusia condition index and embryo number and dry weight was related to temperature variation, and hepatic weight was negatively related to dissolved oxygen concentration. Finally, we observed that many of the measured reproductive variables were interrelated and changeable, depending on gestational stage. Specifically, we provide evidence that maternal support of the embryo occurs at least during the first two thirds of gestation and that female fecundity is affected by an apparent tradeoff between embryo size and embryo number.
Keywords: endocrine disruption, fish, Gambusia, growth, larvae, matrotrophy, mosquitofish, nitrate, offspring, oxygen, reproduction, trade-off
Freshwater nitrate contamination is a growing international concern. Although the drinking water standard is 10 mg/L nitrate–nitrogen (NO3–N) in the United States and 11.3 mg/L NO3–N in Europe (European Council 1998; U.S. Environmental Protection Agency 1996), natural water bodies can exceed 100 mg/L nitrate [reviewed by Rouse et al. (1999)]. In Iowa a statewide well-water survey reported that 18% of rural drinking water wells were contaminated with nitrate concentrations that exceeded 10 mg/L NO3–N (Kross et al. 1993).
Nitrate usually enters surface and ground water in runoff from point and nonpoint sources, including fields, golf courses, private gardens, livestock feedlots, and sewage treatment facilities (Berndt et al. 1998; Katz et al. 1999). Under normal circumstances, aquatic nitrogen is naturally cycled by bacterial and plant communities. However, if these organisms are limited (e.g., low light, low phosphorus) and unable to remediate excess nitrate concentrations, nitrate can accumulate. Elevated aquatic nitrate potentially affects reproduction and survival of exposed animals by directly influencing their physiology [reviewed by Guillette and Edwards (2005)].
Aquatic animals are exposed to nitrate primarily through ingestion or epithelial absorption across gills or skin (Onken et al. 2003). In crabs, nitrate can cross the gills, sometimes against a concentration gradient, by substituting for chloride (Cl) in the chloride-bicarbonate exchange mechanism that normally regulates the osmotic and respiratory functions of the gill (Lee and Pritchard 1985; Onken et al. 2003). The ability of the gill epithelium of freshwater fish to accumulate Cl− suggests that nitrate can also accumulate, as shown in tiger prawns (Cheng and Chen 2002). Thus, as is the case with chloride, the circulating nitrate concentration can exceed that of the surrounding water.
Evidence suggests that sensitivity to nitrate is species-specific. Kincheloe et al. (1979) reported larval mortality of Chinook salmon, rainbow trout, and cutthroat trout at concentrations as low as 2.3–7.6 mg/L NO3–N. The 96-hr LC50 (median lethal concentration) for fathead minnow larvae is 1,341 mg/L NO3–N (Scott and Crunkilton 2000), and the lethal dose for adult and juvenile medaka is 100 mg/L NO3–N (Shimura et al. 2002).
A range of sublethal effects of nitrate has also been reported. For example, Greenlee et al. (2004) observed increased apoptosis and reduced cell number in cultured preimplantation mouse embryos exposed to 1 mg/L ammonium nitrate. In an accumulated nitrate test, in which nitrate built up over the course the experiment, Shimura et al. (2002) observed delayed hatching time and reduced fertilization and hatching rates of eggs produced by adult medaka exposed for 2 months to a maximum of 75 mg/L NO3–N. In that test, the offspring also exhibited reduced juvenile growth rates. At 50 mg/L NO3–N, Shimura et al. (2002) observed reduced spawning and fecundity (measured as egg number) among adult medaka exposed to nitrate as juveniles.
In mammals, nitrate can be converted by reversible reactions in vivo to nitrite and then nitric oxide (NO) (Kozlov et al. 1999; Lepore 2000; Panesar and Chan 2000; Samouilov et al. 1998; Weitzberg and Lundberg 1998). Several authors have suggested that nitrate influences vertebrate reproduction by affecting steroid hormone balance or NO regulation (DelPunta 1996; Panesar and Chan 2000; Vanvoorhis et al. 1994). For example, the mammalian ovarian cycle and ovulation are regulated, in part, by interactions among gonadotropins, progesterone, estradiol, and NO (Al-Hijji et al. 2001; Rupnow et al. 2001; Vanvoorhis et al. 1994; Yamagata et al. 2002). Essentially, NO appears to reduce steroid hormone synthesis by inhibiting several steroidogenic enzymes or other major factors in the steroidogenic pathway. These include steroidogenic acute regulatory protein (StAR), and the enzymes P450-sidechain cleavage (P450SCC), 3β-hydroxysteroid dehydrogenase (3βHSD), and aromatase (DelPunta et al. 1996; Panesar and Chan 2000; Stocco DM and Guillette LJ, unpublished data; Vanvoorhis et al. 1994; Weitzberg and Lundberg 1998; Yamagata et al. 2002).
Given the observed and hypothesized effects of nitrate on vertebrate reproduction and growth, we investigated the relationships between low concentrations of nitrate and several reproductive variables in wild female mosquitofish captured from eight Florida springs. The range of nitrate concentrations in the sampled springs (0.2–5.1 mg/L NO3–N) is representative of most Florida springs (Katz et al. 1999). We also considered the potential influence of four other environmental parameters: temperature, pH, conductivity, and dissolved oxygen. In addition to this primary objective, the second purpose of this study was to describe the reproductive biology of female Gambusia holbrooki from the sampled populations.
Methods
Field collections and water quality
Between 21 May and 7 June 2003, adult female G. holbrooki (eastern mosquitofish) were collected using 3-mm mesh dip nets or seines from eight Florida springs with varying degrees of nitrate contamination. The sampled springs are located along the Santa Fe and Suwannee Rivers in northcentral Florida. Fish were selected if they were mature. This was judged by size in the field and confirmed during necropsy based on presence of differentiated follicles. Mature fish from the sampled springs exhibited a standard length ≥ 2 cm.
As fish were captured, they were randomly parsed into one of two groups. Fish placed in the group for estradiol analysis (n = 13–17 per spring) were immediately chilled on ice. Fish used for necropsy (n = 30 per spring) were taken live to the laboratory, using aerated coolers filled with water taken from the capture site. Fish in the necropsy group were dissected within 1 day of capture to examine ovarian and hepatic weight, embryo number, and embryo dry and wet weight.
On the day of the collection, between 1200 and 1500 hr, water quality data were obtained at the location where fish were captured. Water temperature, pH, and conductivity were measured using a handheld Ultrameter (Model 6P; Myron L Company, Carlsbad, CA). Dissolved oxygen was measured using a YSI oxygen probe (Model 550A; YSI Life Sciences, Yellow Springs, OH). In addition, water samples were filtered through a 1-μm glass fiber filter (Millipore Cat. No. AP4004700), chilled on ice, and stored at −20°C until they were analyzed for nitrate using an auto-analyzer (Bran+Luebbe Technicon II with colorimeter; Bran+Luebbe, Buffalo Grove, IL). This method uses a copper–cadmium column to reduce nitrate to nitrite, which then reacts to form a colored solution that can be assayed colorimetrically. Therefore, nitrate concentrations are reported as parts per million (milligrams per liter) nitrogen in the form of nitrate and nitrite combined (NO3–N).
Unlike most surface water sites, spring water arises from ground water sources. Water quality and chemistry of spring water primarily reflect the composition of the underground aquifer rock with which it comes in contact during its time underground (residence time) (Scott et al. 2004). This fact suggests that water quality of spring water is more stable over time compared with that of other surface waters. Residence times range from several days to thousands of years, depending on the geology and flow rate of the spring [reviewed in Scott et al. (2004)]. Our study depends on water data taken only at the time of our fish collections; therefore, we cannot describe temporal variation in water quality. However, given the underground source of spring water, it is likely that our measured values are representative of spring conditions over the short term (weeks to months and possibly years) preceding our study. This statement is supported by other water data we collected during 2003 (unpublished data) and the emerging database on spring water quality initiated by Florida’s Suwannee River Water Management District (available online at http://www.srwmd.state.fl.us/water+data/surfacewater+quality/search+surfacewater+quality+data.asp?county_code=F001&Submit=GO).
Body size and dissections
Adult standard length (SL) was measured to the nearest 0.01 cm from the snout tip to the caudal peduncle using calipers. Fish were blotted dry and weighed with an electronic balance to the nearest milligram. Ovaries and livers were removed and weighed to the nearest 0.1 mg. Ovarian wet weight ranged from 1.6 to 874.2 mg, ovarian dry weight ranged from 0.3 to 200.3 mg, and hepatic weight ranged from 1.6 to 94.8 mg. Mature females were considered reproductive if their ovaries contained at least one vitellogenic (yellow rather than white) oocyte. To assess fecundity, we determined the developmental stage of the oocytes/embryos [based on Haynes (1995)], and counted embryos that were stage 3 or older (postfertilization). Counted embryos were dried in an oven for 24 hr at 40°C. In Gambusia, embryos develop within the ovary in synchronized waves and account for most of the ovarian weight. Therefore, mean embryo weight, both wet and dry, for each female was calculated by dividing the total wet and dry weight of a brood by the embryo number (Meffe and Snelson 1993). For stage 11 embryos (just before birth), wet weights are slightly exaggerated by the presence of yolked ovarian follicles under development as part of the subsequent brood.
Estradiol concentration
17β-Estradiol concentrations were measured on extracts of mosquitofish tissue using enzyme immunoassay (EIA) kits (Cat No. 582251) purchased from Cayman Chemical Company (Ann Arbor, MI) and validated in our lab for this purpose. All body tissue posterior to the gonad and anal fin was collected from each fish, and the fresh wet weight obtained after the caudal fin was removed. This tissue is primarily muscle and will be referred to as muscle for the remainder of the article. Tissue was stored at −80°C until it was thawed on ice, homogenized in 1 mL 65 mM borate buffer (pH 8.0), and extracted twice with 5 mL diethyl ether. For each extraction, the ether and homogenate were mixed for 2 min using a multitube vortex mixer. For the first extraction, tubes were allowed to settle for three minutes to separate phases. For the second extraction, phases were separated by centrifugation for 2 min. After phase separation, the aqueous portion was frozen in a methanol bath chilled to −25°C with dry ice. The lipophilic layers from both extractions were combined in a new tube, and the ether was evaporated under dry forced air. Dry extract was reconstituted in up to 4 mL EIA buffer and diluted as necessary (up to 1:100) so that samples would fall within the range of the standard curve. EIAs were run as recommended by Cayman with an 18-hr refrigerated incubation to increase sensitivity. Data were quantified against a standard curve that was linearized using a logit transformation of B/Bo (bound sample/maximum bound).
Statistics
At the beginning of our analysis, we intended to evaluate relationships between water quality factors, such as nitrate, and various measured reproductive variables. However, as we progressed through the analysis, it became clear that several response variables were interrelated and that these relationships needed to be described before we could examine the influence of water quality on reproduction.
Relationships among reproductive variables
To examine how different reproductive variables related to each other, we combined the study populations and constructed a correlation matrix based on data from individual fish. Estradiol concentrations were not included in the matrix because they were measured on a separate subset of fish (separate subsets were used to avoid altered sex steroid concentrations due to capture stress). To improve linearity, all data (except embryo stage) were log10 transformed. After the correlation analysis, co-linear pairwise combinations of reproductive variables were visualized using simple regression. Ovarian weight, embryo number, and embryo wet weight were strongly related to more than one other response variable. Therefore, for these variables, we used forward stepwise regression to rank the relative importance of each regressor.
Relationships among water quality parameters and reproductive variables
To determine which environmental parameters were important predictors of the measured reproductive variables, we used forward stepwise regression. Sampling order (expressed as days since first day of sampling) and the five water parameters (NO3–N, temperature, conductivity, dissolved oxygen, pH), expressed as a mean for each spring, were entered as independent variables. Their collective statistical influence was evaluated for each dependent variable, also expressed as a mean or adjusted mean. Adjusted means, based on a common regression slope, were calculated using ANCOVA following log-log transformation. Dependent variables included body size (SL, weight) and condition [expressed as mean (log10 weight) adjusted for (log10 SL)], estradiol concentration [log10 (E2 + 1)], embryo weight (wet and dry), number of nonreproductive, mature females captured (of 30 total from each spring), hepatic weight adjusted for body weight, and embryo number adjusted for standard length. Results from the correlation/simple regression analyses indicated that embryo number correlated positively with both standard length (r2 = 0.64, p < 0.0001) and maternal body weight (r2 = 0.74, p < 0.0001). However, compared with body weight, SL is a more appropriate covariate because it is independent of the response variable (embryo number).
When more than one independent variable entered into the stepwise model, we calculated partial correlation coefficients using a partial correlation matrix of the dependent and relevant independent variables.
Possible colinearities between pairs of independent variables were assessed using a correlation matrix. No significant colinearities were detected among water quality parameters (r2 < 0.41, p > 0.09 for all pairwise correlations). However, nitrate concentration and sampling date were (unintentionally) correlated (r2 = 0.5, p = 0.05); that is, the two high nitrate springs were sampled first.
At the conclusion of the stepwise analysis, we visualized the effects of single independent variables (water parameters) on individual response variables (averaged for each spring) using simple linear regression. We observed that temperature was an important predictor for several reproductive variables. However, for all these variables, particularly condition, the significant influence of temperature was driven by a lower temperature at Ruth Spring. The temperature of Ruth Spring was 0.9–1.8°C less than that of the other seven sites. Given that this difference is apparently small, we repeated the stepwise analysis after excluding temperature as an independent variable.
In addition to the above stepwise analysis, log10 (E2 + 1)-transformed estradiol concentrations were also compared among fish from the different springs using analysis of variance (ANOVA). Adjusted means were calculated (using a common regression slope for all sites) and compared using an analysis of covariance (ANCOVA) model in SPSS, version 12.0 (SPSS Inc., Chicago, IL). Homogeneity of slopes was confirmed for all tests. All other analyses were performed using Statview 5.0 (SAS Institute, Cary, NC), and results were considered significant at α = 0.05.
Outliers
During the analysis, we omitted three measured estradiol values (2.5%) that were more than three SD values from the mean for all fish in the study. One female from Ruth Spring was omitted because she exhibited unusually high fecundity compared with the mean for all females in the study (245 vs. an average of 27 embryos in ovario).
Results
Relationships among reproductive variables
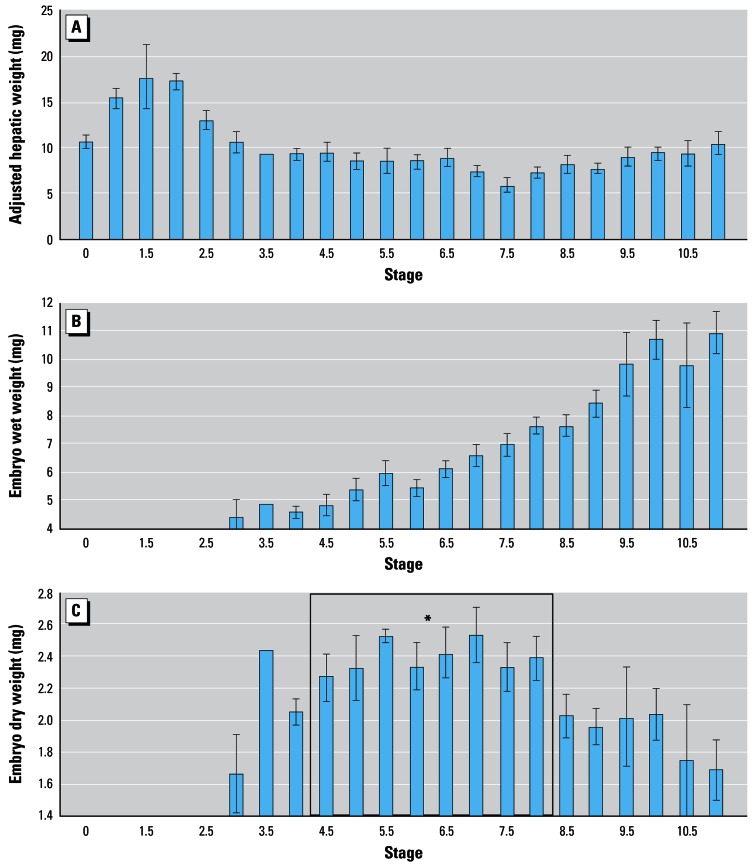
Standard length and female body mass (log10-log10 transformed) were highly correlated (r2 = 0.95) (Table 1). In addition, hepatic weight correlated positively with maternal body weight (r2 = 0.62) and embryo number correlated positively with SL (r2 = 0.64) (Tables 1 and 2). Adjusted hepatic weight was influenced by stage of embryonic development, being highest during the period of yolk deposition to the embryos (stages 0.5–2.5) and then dropping for the remainder of gestation (Figure 1A).
Table 1.
Linear relationships among response variables measured in adult female G. holbrooki collected from eight Florida springs.
| Response variable | Correlated with | r | r2 | p-value |
|---|---|---|---|---|
| Body weight | Standard length | 0.98 | 0.95 | < 0.0001 |
| Hepatic weight | Body weight | 0.79 | 0.62 | < 0.0001 |
| Embryo dry weighta | Embryo numbera | −0.32 | 0.10 | < 0.0001 |
Analyses involving embryo number or embryo weight include embryos at stage 3 or greater; staging based on Haynes (1995).
Table 2.
Results of forward stepwise regression analysis of response variables with more than one significant regressor. Data were measured in adult female G. holbrooki collected from eight Florida springs.
| Response variable | Step | r2 | p-value | Regressors | Partial r |
|---|---|---|---|---|---|
| Ovary weight | 1 | 0.86 | < 0.0001 | Body weight | 0.93 |
| 2 | 0.91 | < 0.0001 | Body weight | 0.92 | |
| Stage | 0.57 | ||||
| 3 | 0.97 | < 0.0001 | Body weight | 0.69 | |
| Stage | 0.82 | ||||
| Embryo number | 0.82 | ||||
| Embryo numbera | 1 | 0.64 | < 0.0001 | Standard length | 0.80 |
| 2 | 0.77 | < 0.0001 | Standard length | 0.86 | |
| Embryo dry weighta | −0.61 | ||||
| Embryo wet weighta, b | 1 | 0.66 | < 0.0001 | Stage | 0.81 |
Analyses involving embryo number or embryo weight include embryos at stage 3 or greater; staging based on Haynes (1995).
Embryo wet weight was positively correlated with both stage (shown) and maternal body weight (r2 = 0.21, p < 0.0001). However, when both are included in a stepwise regression model for embryo wet weight, only stage enters the model.
Figure 1.
(A) Mean maternal hepatic weight, adjusted for body weight; (B) embryo wet weight; and (C) embryo dry weight plotted by embryonic stage [stages based on Hayne (1995)]. Graphs represent data pooled from all eight springs. Data at stage 3.5 were limited to a single female. Embryo weights represent the sum of the embryo and yolk sac. We did not obtain oocyte/embryo weight data at stages younger than 3 because those oocytes are small, variable in size, and possibly unfertilized. Error bars indicate ± 1 SE.
*The collective mean dry weight of embryos between stages 4.5 and 8 (enclosed in box on graph) was significantly greater than the collective mean dry weights of embryos either younger or older (ANOVA: Scheffe’s post-hoc test, p < 0.05).
Ovarian weight and embryo number were also influenced by other life history variables (Table 2). Gambusia embryos develop inside the maternal ovary and, according to our data, consistently gain wet weight during the course of gestation (as stage increases) (Figure 1B). Embryo dry weight also increases at the beginning of gestation but stabilizes between stages 4.5 and 8, then decreases as offspring approach parturition (Figure 1C). There appears to be a tradeoff between embryo number and embryo dry weight (but not wet weight), such that a female may have many smaller embryos or fewer large ones (Tables 1 and 2).The outcome of this tradeoff is influenced by maternal body weight because larger females generally produce more offspring, and those offspring exhibit increased wet weights in a manner that may be stage-dependent (Table 2).
Water quality
Table 3 shows the collection sites and provides abiotic water data. Ranges across the eight springs for each water parameter were as follows: temperature: 21.4–23.2 °C; pH: 7.02–7.35; conductivity: 347–479 μS; dissolved oxygen: 0.39–5.22 mg/L; and NO3–N: 0.22–5.06 mg/L.
Table 3.
Florida collection sites for female G. holbrooki. Water parameter values (± 1 SE) were obtained at the time and location(s) of the fish collection.
| Spring and GPS location | Collection date (2003) | Temperature (°C) | pH | Conductivity (μS) | DO (mg/L) | NO3–N (mg/L) |
|---|---|---|---|---|---|---|
| Blue | ||||||
| N 29°49’49.2”; W 082°40’56.6” | 2 June | 23.2 | 7.27 | 346.5 | 5.22 | 1.51 |
| Fanning | ||||||
| N 29°35’15.0”; W 082°56’08.0” | 21 May | 22.60 ± 0.06 | 7.09 ± 0.01 | 470.9 ± 3.6 | 1.89 ± 0.20 | 4.03 ± 0.41 |
| Hart | ||||||
| N 29°40’30.4”; W 082°57’05.0” | 5 June | 22.35 ± 0.25 | 7.10 ± 0.01 | 402.1 ± 0.9 | 0.39 ± 0.07 | 0.81 ± 0.04 |
| Lily | ||||||
| N 29°49’48.6”; W 082°39’37.7” | 7 June | 22.3 | 7.19 | 425.1 | 0.84 | 0.32 |
| Manatee | ||||||
| N 29°29’20.6”; W 082°58’40.0” | 5 June | 22.85 ± 0.25 | 7.16 | 479.1 ± 0.6 | 1.94 ± 0.14 | 1.26 ± 0.16 |
| Peacock | ||||||
| N 30°07’18.0”; W 083°07’57.0” | 24 May | 22.50 ± 0.44 | 7.35 ± 0.07 | 362.2 ± 1.6 | 2.02 ± 0.27 | 1.69 ± 0.15 |
| Poe | ||||||
| N 29°49’33.0”; W 082°38’58.0” | 28 May | 22.40 ± 0.06 | 7.19 ± 0.01 | 415.1 ± 0.2 | 0.59 ± 0.28 | 0.22 ± 0.01 |
| Ruth | ||||||
| N 29°59’44.0”; W 082°58’38.0” | 24 May | 21.37 ± 0.50 | 7.02 ± 0.07 | 404.2 ± 4.3 | 1.17 ± 0.44 | 5.06 ± 0.61 |
Abbreviations: DO, dissolved oxygen; GPS, global positioning satellite.
Relationships between water quality and reproduction
Detailed results of the stepwise regression analyses are shown in Table 4. The direction of individual interactions is indicated by the partial correlation coefficients. With the five water parameters included, nitrate significantly predicted the number of non-reproductive females sampled from the springs (Figure 2). This correlation is confounded by sampling date as we inadvertently sampled the two high nitrate springs (Fanning and Ruth) first.
Table 4.
Relationships among water quality parameters and response variables measured in adult female G. holbrooki collected from eight Florida springs.
| Response variable | Step | r2 | p-value | Water parameter | Partial r |
|---|---|---|---|---|---|
| Standard length | 0 | ||||
| Body weight | 0 | ||||
| Condition | 1 | 0.56 | 0.03 | Temperature | −0.75 |
| Adjusted hepatic weight | 1 | 0.85 | 0.001 | Dissolved O2 | −0.92 |
| Adjusted embryo number | 1 | 0.76 | 0.005 | Temperature | −0.87 |
| Mean embryo dry weight | 1 | 0.68 | 0.012 | Temperature | 0.82 |
| 2 | 0.83 | 0.01 | Temperature | 0.78 | |
| NO3–N | −0.69 | ||||
| Mean embryo wet weight | 0 | ||||
| Estradiol | 0 | ||||
| Number of nonreproductive femalesa | 1 | 0.57 | 0.03 | NO3–N | 0.75 |
| With temperature removed from the analysisb | |||||
| Condition | 0 | ||||
| Adjusted hepatic weight | 1 | 0.85 | 0.001 | Dissolved O2 | −0.92 |
| Adjusted embryo number | 0 | ||||
| Mean embryo dry weight | 1 | 0.56 | 0.03 | NO3–N | −0.75 |
| Number of nonreproductive femalesa | 1 | 0.57 | 0.03 | NO3–N | 0.75 |
Number of nonreproductive females was also explained by sampling date, which correlated with nitrate concentration (see Table 3 for sampling dates).
With the exception of the robust relationship between temperature and embryo number, the significant influence of temperature is largely driven by a lower temperature at Ruth Spring, which is 0.9 to −1.8°C less than that at the other seven sites. Given that this is a seemingly small difference, we repeated the stepwise analysis after excluding temperature as an independent variable.
Figure 2.
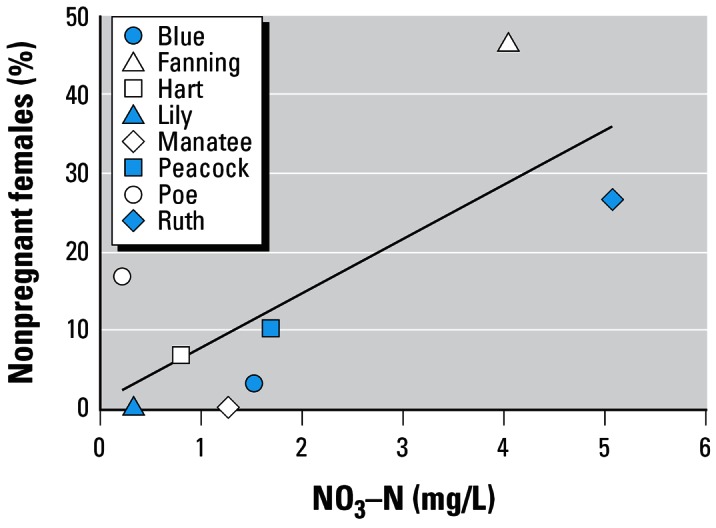
Percentage of nonreproductive, mature females sampled from Florida springs with varying nitrate concentrations. Fish were sampled during the reproductive season. Total samplings from each spring consisted of 30 mature females. r2 = 0.57, p = 0.03.
Temperature exhibited a negative relationship with condition and adjusted embryo number (Figure 3), and a positive relationship with embryo dry weight. Embryo dry weight was also related negatively to nitrate (Table 4). However, for these three variables, the influence of temperature appears to be driven by the Ruth Spring data point, which is cooler than the other sites by < 2°C. If Ruth Spring is excluded from the model, the negative relationship between temperature and adjusted embryo number becomes marginal (r2 = 0.53, p = 0.06), and the relationships between temperature and condition or embryo dry weight are lost (p > 0.3).
Figure 3.
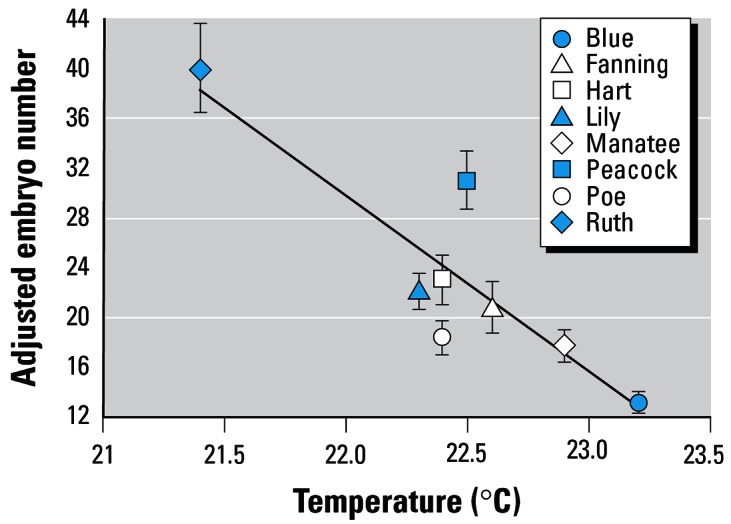
Mean embryo number, adjusted for maternal body weight for females captured in Florida springs with varying temperatures. Graph shows mean ± 1 SE. r2 = 0.76, p = 0.005.
With temperature excluded from the list of potential independent variables in the stepwise model, we found that nitrate still played a significant role in predicting embryo dry weight (negative relationship, Figure 4). We checked our data to be sure that this negative association between nitrate and embryo dry weight could not be explained by differences in stage among embryos from different springs (Figure 1C). However, this relationship is again driven by data from Ruth Spring. If Ruth Spring is removed from the analysis, the relationship between embryo dry weight and nitrate concentration becomes nonsignificant (p = 0.4).
Figure 4.
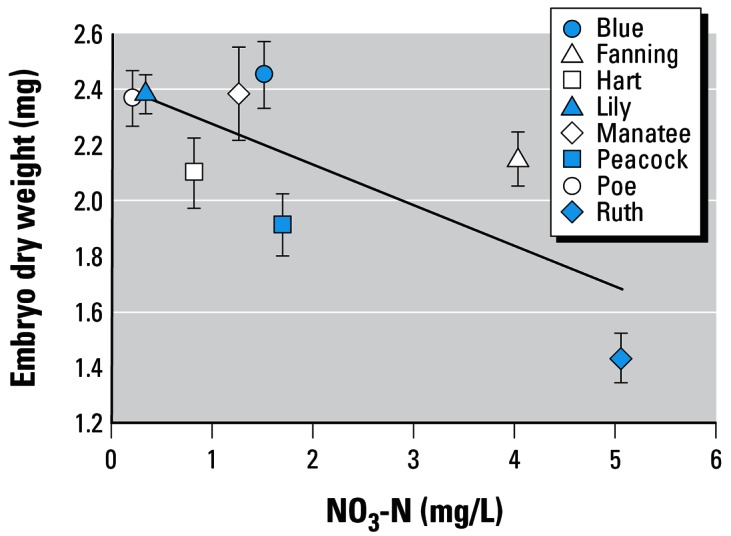
Embryo dry weight (mg) for embryos taken from females captured in Florida springs with varying concentrations of nitrate. Graph shows mean ± 1 SE. r2 = 0.56, p = 0.003.
Dissolved oxygen was the only variable to enter into the stepwise model for predicting adjusted hepatic weight (Figure 5). Again, this association between oxygen concentration and hepatic weight could not be explained by differences in developmental stage of the embryos (Figure 1A). Based on our data, embryo wet weight and maternal estradiol concentrations were not influenced by the water quality parameters we measured (Table 4, Figure 6). In addition, we did not observe significant differences in muscle estradiol concentrations among springs (p = 0.15).
Figure 5.
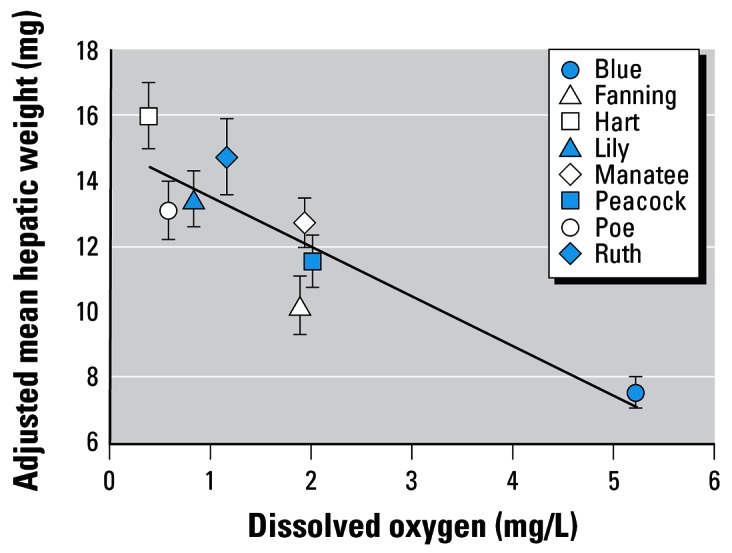
Mean hepatic weight, adjusted for body weight, for females captured in Florida springs with varying dissolved oxygen concentrations. Graph shows adjusted mean ± 1 SE. r2 = 0.85, p = 0.001.
Figure 6.

Muscle estradiol concentrations for females from each spring. Graph shows mean ± 1 SE. Means are not statistically different (ANOVA, p = 0.15). Numbers at base of data columns indicate sample size.
Discussion
At the outset of our study, we hypothesized that low concentrations of environmental nitrate would be related to changes in reproduction and growth of mosquitofish captured from Florida springs based on a hypotheses we had developed previously (Guillette and Edwards 2005). With all springs included, our data indicate a significant association between increasing nitrate and reduced embryo dry weight. We also observed a strong relationship between increased nitrate and reduced reproductive activity among mature females. In addition to these findings regarding nitrate, we observed that many of the measured reproductive variables were interrelated, as expected from previous studies of the reproductive biology of vertebrates. Finally, variation in Gambusia body size and embryo number and dry weight were related to temperature, and hepatic weight was related to dissolved oxygen concentration.
Relationship between nitrate and reduced embryo dry weight
We hypothesize that the observed negative relationship between nitrate and embryo dry weight is due to nitrate-induced alterations in endocrine function. It has been shown that nitrate can influence nitric oxide synthesis as well as cellular ion concentrations and enzyme actions. Our data did not pinpoint a mechanism of action for the observed change in embryo dry weight. One plausible mechanism could involve alterations in thyroid function. Although the relationship between nitrate and embryo dry weight was primarily driven by data obtained from Ruth Spring (which had the highest nitrate concentration of the springs tested), the observation is worthy of further consideration. Environmentally relevant concentrations of nitrate have been shown to reduce thyroid function, feeding behavior, and growth rate in a variety of vertebrates such as sharks, amphibians, and mammals (Allen et al. 1996; Crow et al. 1998; Jahreis et al. 1991; Schuytema and Nebeker 1999; Zaki et al. 2004; Zraly et al. 1997). Nitrate exposure has been associated with goiter and reductions in plasma thyroxine (T4), plasma triiodothyronine (T3), iodine availability, iodine uptake, hypothalamic concentrations of growth hormone releasing factor, and plasma concentrations of somatomedin-C and IGF1(insulin-like growth factor 1), which are part of the growth hormone axis (Crow et al. 1998; Jahreis et al. 1991; Kursa et al. 2000; Simon et al. 2000; Zraly et al. 1997). The importance of thyroid function during development and growth suggests that embryos, fetuses, and juveniles could be more susceptible than adults to the disruptive effects of nitrate exposure.
Relationship between nitrate and reduced reproductive activity
In addition to the observed relationship between low embryonic growth and nitrate, we noted that the number of reproductive females captured during sampling was negatively related to nitrate concentration. That is, as nitrate levels went up, fewer reproductive females (less than 54% in Fanning Spring) were captured relative to the total number of sexually mature females caught. This correlation is confounded by sampling date as we inadvertently sampled the two high nitrate springs (Fanning and Ruth) first. However, on the basis of other Gambusia life history studies, we reason that it is unlikely that differences in sampling date fully explain the observed variation in number of reproductively active females. First, Koya and Kamiya (2000) reported that vitellogenesis and pregnancy in Gambusia affinis require threshold temperatures of 14°C and 18°C, respectively, regardless of daylength. Data taken in February 2003 and again during collections in May 2003 indicate that water temperature in both springs exceeded 21°C in both February and May (Edwards TM, unpublished data; Suwannee River Water Management District online searchable database (http://www.srwmd.state.fl.us/water+data/surfacewater+quality/search+surfacewater+quality+data.asp?county_code=F001&Submit=GO). Second, data collected in 2001 and 2002 from G. holbrooki populations in lakes in central Florida predict that pregnancy rates in May should be ≥ 90% (Edwards 2005).
Because Gambusia incorporate yolk into oocytes before fertilization (Koya et al. 2000), our observation of reduced reproductive activity in association with increased nitrate exposure does not imply disrupted fertilization. Rather, it suggests that nitrate, or its metabolites (nitrite, nitric oxide) can influence some aspect of vitellogenesis or vitellogenin sequestering during oogenesis. Vitellogenesis occurs in the liver and is stimulated by estrogens (Tolar et al. 2001). If estrogens are decreased by nitrate or by its metabolites (as hypothesized in our introduction), then vitellogenesis could be similarly decreased. Yamagata et al. (2002) demonstrated that in vitro steroidogenesis by rat granulosa cells could be decreased by exposure to a nitric oxide donor. We did not find a relationship between estradiol concentration in the body tissue and nitrate concentrations in the springs. Nor did we observe a relationship between estradiol and frequency of reproductive females. However, it is possible that nitrate may alter the action of estradiol in the liver. Alternatively, the reduced frequency of reproductively active females could be due to a delayed onset of seasonal reproductive activity among some females in the population. Both hypotheses require further testing.
Interrelated reproductive variables—implications for the gonadosomatic index
Our data show that the relationship between ovarian weight and body mass, traditionally expressed as the gonadosomatic index (GSI), is complicated by gestational wet weight gain and also embryo number, which in turn is influenced by maternal body mass and embryo dry weight (Table 2). Since females in any given population are not synchronized with regard to gestational stage, it could be misleading to compare populations using GSI as a singular measure of reproductive health or fecundity (as is a common practice in the piscine literature) without knowing the gestational stage or degree of tradeoff between embryo size and number. We discourage the use of GSI for this purpose in future mosquitofish studies.
Relationship between hepatic weight and dissolved oxygen
Hypoxia in the springs was related to increased adjusted hepatic weight and explained 84% of the variance in this variable. In cultured mammalian hepatocytes and whole animals, hypoxia and related acidosis stimulate sodium accumulation in liver cells, which causes cell swelling. If this swelling is not excessive, the cell membrane will remain intact and the cell will avoid necrosis (Carini et al. 1999). In addition, necrosis is also avoided if hepatocytes are preconditioned by early but intermittent exposure to hypoxia (Carini et al. 2001), as may be the case for wild Gambusia. We informally screened hepatic histology for several male fish captured with the females in our study and did not observe necrotic cells in fish from low or high oxygen sites.
Matrotrophy
We have noted in the literature that Gambusia are classified as lecithotrophs (Constantz 1989). However, our data suggest that Gambusia are matrotrophic, at least during the first two thirds of development (through stage 8), when both embryo dry weight and wet weight are stable or rising (Figures 1B, C). Meffe and Snelson (1993) observed a similar increase in Gambusia embryo dry weight during gestation. In our study, hepatic weight (adjusted for body weight) was highest during the period of yolk deposition to the embryos (stages 0.5–2.5) (Figure 1A) and then dropped for the remainder of gestation. This suggests that vitellogenesis is greatest at the beginning of gestation (Koya et al. 2000). Although vitellogenesis apparently drops by stage 3, embryos gain or maintain dry weight through to stage 8. Between stages 8 and 11, the yolk sac diminishes rapidly and some dry weight is lost (Figure 1C). On the basis of these observations, we suggest that Gambusia exhibit some direct, matrotrophic support of embryo growth during the first two thirds of development and rely on egg yolk reserves for the completion of gestation. This observation of matrotrophy in Gambusia is supported by other recent evidence of maternal nutrient transfer (Marsh-Matthews et al. 2001). The gain in wet weight before birth (a 4-fold increase on average) could be adaptive in that larger larvae often exhibit better survivorship (Hare and Cowen 1997).
Conclusion
Our data suggest that growth and reproductive parameters in Gambusia are highly interrelated and subject to influence from a variety of environmental factors, including nitrate, temperature, and dissolved oxygen. In particular, nitrate exposure is related to reduced dry weight of developing Gambusia embryos during gestation and reduced rate of reproductive activity among mature females. These findings, along with those of other studies cited here, suggest that nitrate could act as an endocrine disruptor as previously suggested (Guillette and Edwards 2005), thereby affecting signaling patterns associated with the thyroid, liver, and gonad. The mechanisms associated with these alterations require extensive study because nitrate contamination of aquatic ecosystems is a major global concern.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
We are grateful for the superb help of R. Emrich and J.M. Thro. We also thank S. Keller, L. Chapman, B. Moore, and the many undergraduates in our laboratory.
Funding was provided by the Florida Department of Environmental Protection (agreement no. S0028).
The views expressed herein are those of the authors and do not necessarily reflect the views of the Florida Department of Environmental Protection.
References
- Al-Hijji J, Larsson I, Batra S. Effect of ovarian steroids on nitric oxide synthase in the rat uterus, cervix, and vagina. Life Sci. 2001;69:1133–1142. doi: 10.1016/s0024-3205(01)01204-8. [DOI] [PubMed] [Google Scholar]
- Allen AL, Townsend HGG, Doige CE, Fretz PB. A case-control study of the congenital hypothyroidism and dysmaturity syndrome of foals. Can Vet J Rev Vet Can. 1996;37:349. [PMC free article] [PubMed] [Google Scholar]
- Berndt MP, Hatzell HH, Crandall CA, Turtora M, Pittman JR, Oaksford ET. 1998. Water Quality in the Georgia-Florida Coastal Pain. Tallahassee, FL:U.S. Geological Survey.
- Carini R, Autelli R, Bellomo G, Albano E. Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp Cell Res. 1999;248:280–293. doi: 10.1006/excr.1999.4408. [DOI] [PubMed] [Google Scholar]
- Carini R, De Cesaris MG, Splendore R, Albano E. Stimulation of p38 map kinase reduces acidosis and Na+ overload in preconditioned hepatocytes. FEBS Lett. 2001;491:180–183. doi: 10.1016/s0014-5793(01)02189-5. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Chen JC. Accumulations of nitrite and nitrate in the tissues of Penaeus monodon exposed to a combined environment of elevated nitrite and nitrate. Arch Environ Contam Toxicol. 2002;43:64–74. doi: 10.1007/s00244-001-0056-8. [DOI] [PubMed] [Google Scholar]
- Constantz GD. 1989. Reproductive biology of poeciliid fish. In: Ecology and Evolution of Livebearing Fish (Poeciliidae), (Meffe GK, Snelson FF, eds). Englewood Cliffs, NJ:Prentice Hall, 33–50.
- Crow GL, Atkinson MJ, Ron B, Atkinson S, Skillman ADK, Wong GTF. Relationship of water chemistry to serum thyroid hormones in captive sharks with goiters. Aquat Geochem. 1998;4:469–480. [Google Scholar]
- DelPunta K, Charreau EH, Pignataro OP. Nitric oxide inhibits Leydig cell steroidogenesis. Endocrinology. 1996;137:5337–5343. doi: 10.1210/endo.137.12.8940355. [DOI] [PubMed] [Google Scholar]
- Edwards TM. 2005. Environmental Influences on Mosquitofish Reproduction [PhD Dissertation]. Gainesville, FL:University of Florida.
- European Council. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off J Eur Commun. 1998;L330:32–54. [Google Scholar]
- Greenlee AR, Ellis TM, Berg RL. Low-dose agrochemicals and lawn-care pesticides induce developmental toxicity in murine pre-implantation embryos. Environ Health Perspect. 2004;112:703–709. doi: 10.1289/ehp.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ, Edwards TM. Is nitrate an ecologically relevant endocrine disruptor in vertebrates? Integr Comp Biol. 2005;45:19–27. doi: 10.1093/icb/45.1.19. [DOI] [PubMed] [Google Scholar]
- Hare JA, Cowen RK. Size, growth, development, and survival of the planktonic larvae of Pomatomus saltatrix (Pisces: Pomatomidae) Ecology. 1997;78:2415–2431. [Google Scholar]
- Haynes JL. Standardized classification of poeciliid development for life-history studies. Copeia. 1995;1:147–154. [Google Scholar]
- Jahreis G, Hesse V, Rohde W, Prange H, Zwacka G. Nitrate-induced hypothyroidism is associated with a reduced concentration of growth hormone releasing factor in hypothalamic tissue of rats. Exp Clin Endocrinol. 1991;97:109–112. doi: 10.1055/s-0029-1211049. [DOI] [PubMed] [Google Scholar]
- Katz BG, Hornsby D, Bohlke JF, Mokray MF. 1999. Sources and Chronology of Nitrate Contamination in Spring Waters, Suwannee River Basin, Florida. Water-Resources Investigations Report No. 99–4252. Tallahassee,FL:U.S. Geological Survey.
- Kincheloe JW, Wedemeyer GA, Koch DL. Tolerance of developing salmonid eggs and fry to nitrate exposure. Bull Environ Contam Toxicol. 1979;23:574–578. doi: 10.1007/BF01770006. [DOI] [PubMed] [Google Scholar]
- Koya Y, Inoue M, Naruse T, Sawaguchi S. Dynamics of oocyte and embryonic development during ovarian cycle of the viviparous mosquitofish (Gambusia affinis) Fish Sci. 2000;66:63–70. [Google Scholar]
- Koya Y, Kamiya E. Environmental regulation of annual reproductive cycle in the mosquitofish, Gambusia affinis. J Exp Zool. 2000;286:204–211. doi: 10.1002/(sici)1097-010x(20000201)286:2<204::aid-jez12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- Kross BC, Hallberg GR, Bruner DR, Cherryholmes K, Johnson JK. The nitrate contamination of private well water in Iowa. Am J Public Health. 1993;83:270–272. doi: 10.2105/ajph.83.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursa J, Travnicek J, Rambeck WA, Kroupova V, Vitovec J. Goiterogenic effects of extracted rapeseed meal and nitrates in sheep and their progeny. Vet Med. 2000;45:129–140. [Google Scholar]
- Lee SH, Pritchard JB. Bicarbonate-chloride exchange in gill plasma membranes of blue crab. Am J Physiol. 1985;249:R544–R550. doi: 10.1152/ajpregu.1985.249.5.R544. [DOI] [PubMed] [Google Scholar]
- Lepore DA. Nitric oxide synthase-independent generation of nitric oxide in muscle ischemia-reperfusion injury. Nitric Oxide. 2000;4:541–545. doi: 10.1006/niox.2000.0308. [DOI] [PubMed] [Google Scholar]
- Marsh-Matthews E, Skierkowski P, DeMarais A. Direct evidence for mother-to-embryo transfer of nutrients in the live-bearing fish Gambusia geiseri. Copeia. 2001;1:1–6. [Google Scholar]
- Meffe GK, Snelson FF. Lipid dynamics during reproduction in 2 livebearing fish, Gambusia holbrooki and Poecilia latipinna. Can J Fish Aquat Sci. 1993;50:2185–2191. [Google Scholar]
- Onken H, Tresguerres M, Luquet CM. Active NaCl absorption across posterior gills of hyperosmoregulating Chasmagnathus granulatus. J Exp Biol. 2003;206:1017–1023. doi: 10.1242/jeb.00227. [DOI] [PubMed] [Google Scholar]
- Panesar NS, Chan KW. Decreased steroid hormone synthesis from inorganic nitrite and nitrate: studies in vitro and in vivo. Toxicol Appl Pharmacol. 2000;169:222–230. doi: 10.1006/taap.2000.9079. [DOI] [PubMed] [Google Scholar]
- Rouse JD, Bishop CA, Struger J. Nitrogen pollution: an assessment of its threat to amphibian survival. Environ Health Perspect. 1999;107:799–803. doi: 10.1289/ehp.99107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circul Physiol. 2001;280:H1699–H1705. doi: 10.1152/ajpheart.2001.280.4.H1699. [DOI] [PubMed] [Google Scholar]
- Samouilov A, Kuppusamy P, Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch Biochem Biophys. 1998;357:1–7. doi: 10.1006/abbi.1998.0785. [DOI] [PubMed] [Google Scholar]
- Schuytema GS, Nebeker AV. Comparative toxicity of ammonium and nitrate compounds to Pacific tree frog and African clawed frog tadpoles. Environ Toxicol Chem. 1999;18:2251–2257. doi: 10.1002/etc.5620181019. [DOI] [PubMed] [Google Scholar]
- Scott G, Crunkilton RL. Acute and chronic toxicity of nitrate to fathead minnows (Pimephales promelas), Ceriodaphnia dubia, and Daphnia magna. Environ Toxicol Chem. 2000;19:2918–2922. [Google Scholar]
- Scott TM, Means GH, Meegan RP, Means RC, Upchurch SB, Copeland RE, et al. Springs of Florida. Florida Geol Surv. 2004;66:15–24. [Google Scholar]
- Shimura R, Ijiri K, Mizuno R, Nagaoka S. Aquatic animal research in space station and its issues—focus on support technology on nitrate toxicity. Adv Space Res. 2002;30:803–808. doi: 10.1016/s0273-1177(02)00399-x. [DOI] [PubMed] [Google Scholar]
- Simon C, Bostedt H, Adams W. Juvenile goiter in a herd of goats in northwest Germany. Schweiz Arch Tierheilkd. 2000;142:339–347. [PubMed] [Google Scholar]
- Tolar JF, Mehollin AR, Watson RD, Angus RA. Mosquitofish (Gambusia affinis) vitellogenin: identification, purification, and immunoassay. Comp Biochem Physiol C Pharmacol Toxicol Pharmacol. 2001;128:237–245. doi: 10.1016/s1532-0456(00)00194-0. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. 1996. Drinking Water Regulations and Health Advisories. Washington, DC:U.S. Environmental Protection Agency
- Vanvoorhis BJ, Dunn MS, Snyder GD, Weiner CP. Nitricoxide—an autocrine regulator of human granulosaluteal cell steroidogenesis. Endocrinology. 1994;135:1799–1806. doi: 10.1210/endo.135.5.7525252. [DOI] [PubMed] [Google Scholar]
- Weitzberg E, Lundberg JON. Non-enzymatic nitric oxide production in humans. Nitric Oxide. 1998;2:1–7. doi: 10.1006/niox.1997.0162. [DOI] [PubMed] [Google Scholar]
- Yamagata Y, Nakamura Y, Sugino N, Harada A, Takayama H, Kashida S, et al. Alterations in nitrate/nitrite and nitric oxide synthase in preovulatory follicles in gonadotropin-primed immature rat. Endocr J. 2002;49:219–226. doi: 10.1507/endocrj.49.219. [DOI] [PubMed] [Google Scholar]
- Zaki A, Chaoui AA, Talibi A, Derouiche AF, Aboussaouira T, Zarrouck K, et al. Impact of nitrate intake in drinking water on the thyroid gland activity in male rat. Toxicol Lett. 2004;147:27–33. doi: 10.1016/j.toxlet.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Zraly Z, Bendova J, Svecova D, Faldikova L, Veznik Z, Zajicova A. Effects of oral intake of nitrates on reproductive functions of bulls. Vet Med-Czech. 1997;42:345–354. [PubMed] [Google Scholar]