Abstract
It had been observed that many male Sitka black-tailed deer (Odocoileus hemionus sitkensis) on Kodiak Island, Alaska, had abnormal antlers, were cryptorchid, and presented no evidence of hypospadias. We sought to better understand the problem and investigated 171 male deer for phenotypic aberrations and 12 for detailed testicular histopathology. For the low-lying Aliulik Peninsula (AP), 61 of 94 deer were bilateral cryptorchids (BCOs); 70% of these had abnormal antlers. Elsewhere on the Kodiak Archipelago, only 5 of 65 deer were BCOs. All 11 abdominal testes examined had no spermatogenesis but contained abnormalities including carcinoma in situ–like cells, possible precursors of seminoma; Sertoli cell, Leydig cell, and stromal cell tumors; carcinoma and adenoma of rete testis; and microlithiasis or calcifications. Cysts also were evident within the excurrent ducts. Two of 10 scrotal testes contained similar abnormalities, although spermatogenesis was ongoing. We cannot rule out that these abnormalities are linked sequelae of a mutation(s) in a founder animal, followed by transmission over many years and causing high prevalence only on the AP. However, based on lesions observed, we hypothesize that it is more likely that this testis–antler dysgenesis resulted from continuing exposure of pregnant females to an estrogenic environmental agent(s), thereby transforming testicular cells, affecting development of primordial antler pedicles, and blocking transabdominal descent of fetal testes. A browse (e.g., kelp) favored by deer in this locale might carry the putative estrogenic agent(s).
Keywords: antler dysgenesis, CIS, cryptorchidism, Leydig cell tumor, microlithiasis, rete carcinoma, seminoma, Sertoli cell tumor
Sitka black-tailed deer (SBTD; Odocoileus hemionus sitkensis) were introduced into the Kodiak Archipelago before 1935 [Supplemental Material Figure 1 and “Background” (http://www.ehponline.org/members/2005/8052/supplemental.pdf)]. There were occasional reports of cryptorchidism in SBTD on Kodiak Island, often with abnormal antlers (i.e., sharp tips, atypical/bizarre shape, retention of velvet) or an atypical (enlarged, convex) bony antler pedicle after antlers were shed. Apparently, incidence of such abnormal deer on the Aliulik and Hepburn Peninsulas increased after 1990 (Van Daele and McDonald 2001). Without scrutiny of the problem, wildlife personnel attributed the cryptorchidism to inbreeding (Lewis 1991).
Figure 1.
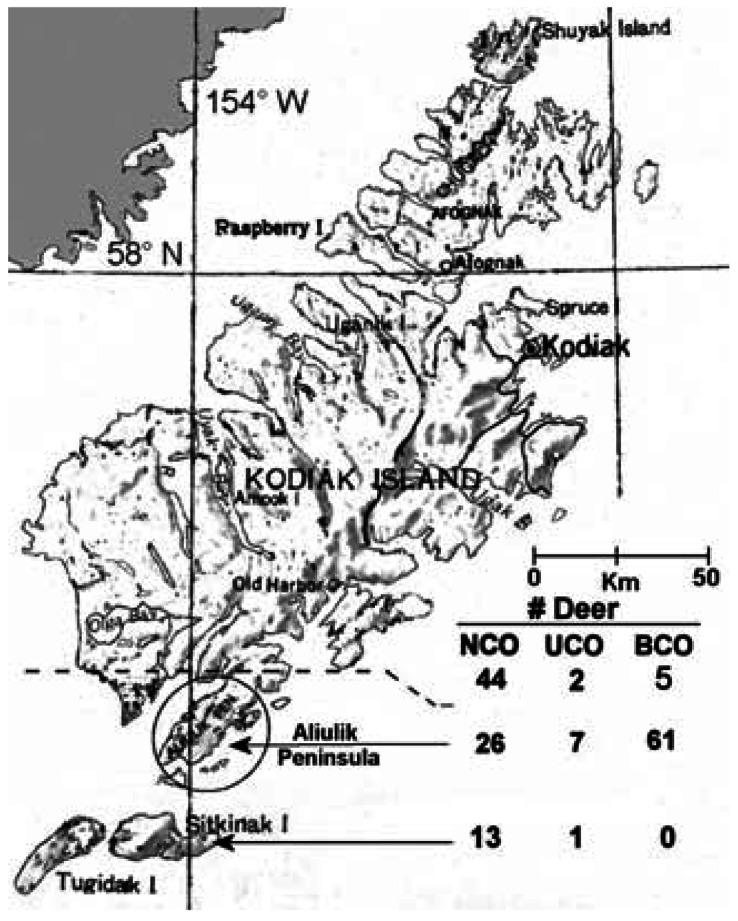
Kodiak Archipelago, showing the AP of Kodiak Island and numbers of NCO, UCO, and BCO SBTD shot at sites above the dashed line, on the AP and on Sitkinak Island. Note predominance of BCOs on the AP.
Elsewhere, deer with abnormal antlers and atrophic scrotal testes have been reported (Chapman et al. 1984; Tiller et al. 1997). Occasionally, males of other deer species lacking scrotal testes have been reported (Hofmann 1968; Leader-Williams 1979; Marburger et al. 1967), but they had normal antlers. Hoy et al. (2002) found a high proportion of males with malpositioned testes among road-killed deer in an area of Montana, but they apparently had normal antlers. Reported abnormalities are different from those reported herein for SBTD. Cryptorchidism also occurs in domesticated animals or humans, with prevalence ranging from 3 to 20% (Klonisch et al. 2004; Nielen et al. 2001).
A lay publication (Jacobson 2003) and earlier reports (Bubenik et al. 2001; Bubenik and Jacobson, 2002) of unusually high incidence of cryptorchidism in SBTD led to the current study. To determine if there really is a problem of cryptorchid SBTD with abnormal antlers in the Kodiak Archipelago, we gathered additional data and compiled and scrutinized all data. We were intrigued concerning the possible cause(s). Because we suspected a problem during fetal development and because analyses of maternal blood serum or fat for an array of potential endocrine disruptors was expensive, we focused on collection of properly fixed testicular tissue and critical evaluation thereof (Higuchi et al. 2003; Veeramachaneni 2000; Veeramachaneni et al. 1986, 2001). We anticipated that these evaluations would: a) determine if there were many unusual deer on a portion of Kodiak Island; b) point to the cause of the unexplained phenomenon (genetic basis vs chemical agent); and c) provide a database, critical for any future study.
Materials and Methods
Population sampling
This study involved observations on 171 male SBTD legally hunted in mid-October through December during 1999–2004. Hunting occurred in the vicinity of 36 general sites [Supplemental Material Figure 2 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)], 8 of which were on the Aliulik Peninsula (AP) of Kodiak Island and 28 elsewhere on Kodiak Island or nearby islands of the Kodiak Archipelago. Hunters decided what deer to shoot, and we must assume that they represented all in that geographic area. SBTD in this study include those in earlier reports (Bubenik et al. 2001; Bubenik and Jacobson 2002), but we did not rely on histological observations in the latter report.
Figure 2.
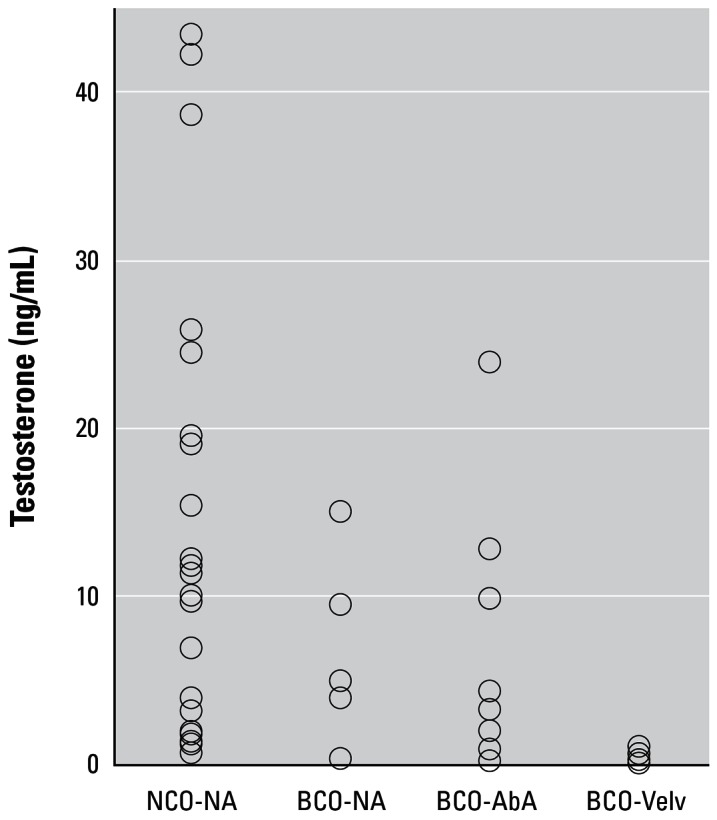
Concentrations of testosterone (ng/mL) in serum prepared from blood collected shortly after death from NCO and BCO deer. Abbreviations: BCO-NA, BCO deer with normal antlers; BCO-AbA, BCO deer with abnormal antlers without velvet; and BCO-Velv, BCO deer whose antlers retained velvet. Data grouped as NCO-NA, NCO deer with normal antlers. Geometric means were 3.81, 1.73, 2.03, and 0.22 ng/mL, respectively.
Tissue sampling, processing, and evaluation
For 2003 and 2004 our goal was to obtain blood serum and tissue from at least six cryptorchid and six noncryptorchid (NCO) males annually. Soon after a male was shot, an ID number was assigned, normalcy of penis and scrotum was recorded, and blood was taken and allowed to clot. Serum was separated and stored at –20°C.
Testes and epididymides were photographed alongside a ruler. Slices of testes at two loci were fixed in Bouin’s fluid or 4% glutaraldehyde. A cross-section through the cauda epididymidis was fixed in Bouin’s fluid. Tissues were fixed in the field, usually < 3 hr after death. Tissues were processed for light and transmission electron microscopy and evaluated based on criteria described by Markwald (1968) and Veeramachaneni et al. (1986). Briefly, 50- to 100-tubule cross-sections per testis were classified as one of eight grades denoting progressive loss of germ cells and degenerative changes in seminiferous epithelium, and the relative degree of germinal epithelial loss (DGEL) for each section of testis was calculated [Supplemental Material (http://www.ehponline.org/members/2005/8052/supplemental.pdf)].
Detailed evaluations of morphology of the four cell types (germ cells, Sertoli cells, Leydig cells, and stromal cells) within each testis were performed. Emphasis was placed on normalcy of differentiating germ cells; presence of atypical germ cells resembling carcinoma in situ (CIS; also called intratubular germ cell neoplasia) (Skakkebaek 1972) or proliferating as a tumor; abnormal/neoplastic supporting or Sertoli cells; and abnormal/neoplastic Leydig cells and stromal cells (Mostafi 1977). Thin sections prepared from selected blocks, thought to represent an area of special interest, were examined using transmission electron microscopy.
Quantification of serum testosterone
We measured concentration of testosterone using a validated radioimmunoassay (Berndtson et al. 1974) in all available serum samples (n = 86) of male SBTD shot during 2000–2004. Sensitivity of the assay was 0.024 ng testosterone/mL serum. Based on duplicate aliquots of each sample, intraassay variation was 4.4%. The data summary also included values (n = 19) from Bubenik et al. (2001) for males shot in 1999.
Statistics
We summarized descriptive data within and across sites for males with both, one, or no testes within the scrotum and for normalcy of antlers. Statistics were not applied to these descriptive data or to semiquantitative classifications of testicular histology and DGEL. Values for estimated age for males shot at sites on or not on the AP were compared using a t-test. Testosterone concentrations were subjected to a log10 transformation, and geometric means are presented. Differences were compared with a t-test or analysis of variance or, alternatively, a Mann-Whitney or Kruskal-Wallis test. Differences between certain ratios [unilateral cryptorchid (UCO) + bilateral cryptorchid (BCO) vs. NCO deer] were substantial, but the nonsystematic harvest of SBTD made statistical testing illogical.
Results
Biometric data
Estimated age was available for 112 of the 171 SBTD and averaged 5.3 years (range 1.5–12.5 years). On average, deer shot on the AP were older than those shot elsewhere (5.8 vs 4.6 years; p < 0.01; n = 64, 48).
Numbers of NCO, UCO, and BCO deer at each site [Figure 1; details in Supplemental Material Table 1 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)] revealed that cryptorchid deer represented 72% of the population on AP but only 12% averaged across all other sites. Across all sites the ratio of UCO to BCO was 1:6.6. Occasional cryptorchid deer were far distant from the AP and likely were present at low incidence throughout the archipelago. Prevalence of cryptorchidism on the AP might have increased since 1999. Numbers of NCO, UCO, and BCO were respectively 9, 3, and 10 in 1999 compared with 10, 2, and 25 in 2003–2004. Respective ratios of NCO:UCO + BCO deer were 1:1.4 versus 1:2.7.
Table 1.
Histological characteristics of testes of NCO and cryptorchid SBTD.
| Testisa
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade (%)
|
||||||||||||
| Male | Age (years) | Antlers | Site | Side | Location | 0 | 1–3 | 4 | 6 | 7 | DGEL | Special observations |
| 03-01 | 7.5 | N | 7 | L | S | 27 | 64 | 10 | 0 | 0 | 36 | Multinucleate germ cells; focal foamy LC hyperplasia |
| R | S | 60 | 40 | 0 | 0 | 0 | 14 | Occasional multinucleate germ cell; foamy eosinophilic LC | ||||
| 03-10 | 8.5 | N | 7 | L | S | 64 | 26 | 10 | 0 | 0 | 18 | Two clusters of CIS-like cells; areas with spermatids sharing acrosome |
| R | S | 65 | 23 | 12 | 0 | 0 | 20 | Divergent quality in two loci; areas with spermatids sharing acrosome; microlithiasis | ||||
| 03-12 | 6.5 | N | 7 | L | S | 25 | 20 | 55 | 0 | 0 | 68 | Occasional spermatids sharing acrosome |
| R | S | 12 | 18 | 70 | 0 | 0 | 80 | Many SCO tubules; CIS cells | ||||
| 03-51 | 2.5 | N | 19 | L | S | 36 | 60 | 4 | 0 | 0 | 28 | No overt lesion |
| R | S | 46 | 26 | 25 | 3 | 0 | 37 | Several SCO tubules | ||||
| 03-02 | 4.5 | N | 7 | L | A | Hunter did not find testis | ||||||
| R | S | 74 | 24 | 2 | 0 | 0 | 11 | Two CIS-like cells | ||||
| 03-09 | 4.0 | N | 7 | L | S | 67 | 31 | 2 | 0 | 0 | 12 | Normal except for few SCO tubules |
| R | A | 0 | 0 | 25 | 25 | 50 | 100 | Fibrosis with microlithiasis; SCO tubules replaced by stromal cells | ||||
| 03-03 | 8.5 | VA | 8 | L | A | 0 | 0 | 100 | 0 | 0 | 100 | LC hyperplasia and tumor; ST replaced by microlithiasis and solid cords; concretions in IT |
| R | A | 0 | Hunter did not find testis | |||||||||
| 03-06 | 9.0 | VA | 8 | L | A | 0 | 0 | 100 | 0 | 0 | 100 | Stromal tumor; SC tumor nodule; rete testis adenoma, cysts and microlithiasis |
| R | A | 0 | 0 | 100 | 0 | 0 | 100 | Regressed LC clusters; rete testis hypertrophic | ||||
| 03-07 | 1.5 | N | 7 | L | A | 0 | 0 | 100 | 0 | 0 | 100 | One CIS-like cell; occasional foamy LC; microlithiasis |
| R | A | 0 | 0 | 100 | 0 | 0 | 100 | Conspicuous lipid globules in some LC | ||||
| 03-11 | 5.5 | VA | 7 | L | A | 0 | 0 | 50 | 50 | 0 | 100 | Nodular LC tumors; SC tumors; extensive microlithiasis; rete testis adenoma and carcinoma |
| R | A | 0 | 0 | 50 | 50 | 0 | 100 | Nodular LC tumors; extensive microlithiasis | ||||
| 03-13 | 8.5 | VA | 7 | L | A | 0 | 0 | 50 | 50 | 0 | 100 | CIS-like cells; beginning seminoma; nodular LC tumors around SCO tubules |
| R | A | 0 | 0 | 50 | 50 | 0 | 100 | CIS-like cells; rete testis hypertrophy and extensive microlithiasis | ||||
| 03-14 | 3.5 | N | 7 | L | A | 0 | 0 | 50 | 50 | 0 | 100 | CIS-like cells; extensive microlithiasis |
| R | A | 0 | 0 | 50 | 50 | 0 | 100 | Clusters of CIS-like cells; seminoma | ||||
Abbreviations: A, abdominal testis; DGEL, degree of germinal epithelial loss; IT, interstitial tissue; L, left; LC, Leydig cells; N, normal antlers; R, right; S, scrotal testis; SC, Sertoli cell; SCO, Sertoli cell only; ST, seminiferous tubule; VA, velvet-covered antlers.
Grade 5 tubules were not found except for a 1% incidence in deer 03-01.
Two of 4 abdominal testes in UCO deer and 40 of 58 in BCO deer were recovered between the kidney and inner inguinal ring [Supplemental Material Figure 3 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)]. The other 20 abdominal testes were not found or sought by the hunter. In no case was a testis found within the inguinal canal. BCO deer lacked a scrotum.
Figure 3.
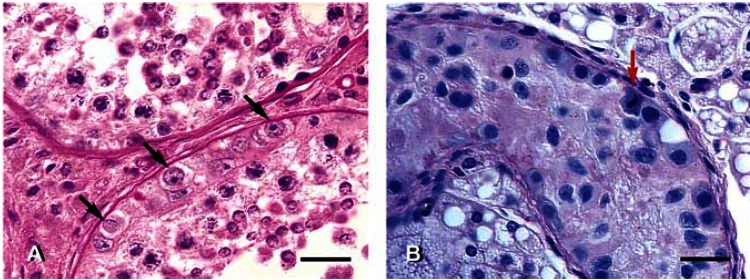
Germ cell atypia. (A) Atypical germ cells (arrows), characterized by abnormal nuclei, pale cytoplasm, and perinuclear cytoplasmic inclusions, in the testis of NCO deer 03-10. These abnormal germ cells are CIS-like cells, also called intratubular germ cell neoplasia. Note the location of these cells in the basal compartment of a seminiferous tubule (where premeiotic germ cells are normally located). Primary spermatocytes and spherical spermatids are seen adluminally. PAS and hematoxylin staining. (B) Atypical germ cells in a seminiferous tubule lacking any normal germ cell. Arrow designates a mitotic figure. Note foamy, hypertrophic Leydig cells. Differential interference contrast microscopy. Scale bars = 25 μm.
For 2003–2004, we estimated testis size from photographs that included a scale. Maximum diameter and length averaged 31 and 47 mm for scrotal testes (n = 37) and 13 and 21 mm for abdominal testes (n = 20). By applying the formula for a prolate spheroid to dimensions of each testis (Amann 1990) and assuming a density of 1.038 g/cc, we estimated that the average scrotal testis weighed 24 g, or 12 times the average of 2 g for abdominal testes. There was no obvious difference in estimated weight of scrotal testes for deer sampled on the AP versus elsewhere.
Observations on antlers were summarized for 125 SBTD. Antlers were normal [Supplemental Material Figure 4 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)] for 60 of 61 NCO deer and all 8 UCO deer; antlers on 1 NCO deer had sharp polished tips. However, only 17 of 56 BCO deer, including 15 on the AP, had normal antlers. Among abnormal antler sets from BCO deer [Supplemental Material Figure 4 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)], 15 had sharp polished tips, 4 had an atypical/bizarre shape, 16 had an atypical/bizarre shape and also remained in velvet, and 4 remained in velvet. Hence, 62% of these BCO males had a shape defect, 36% a velvet defect, and 29% both a shape and velvet defect.
Figure 4.
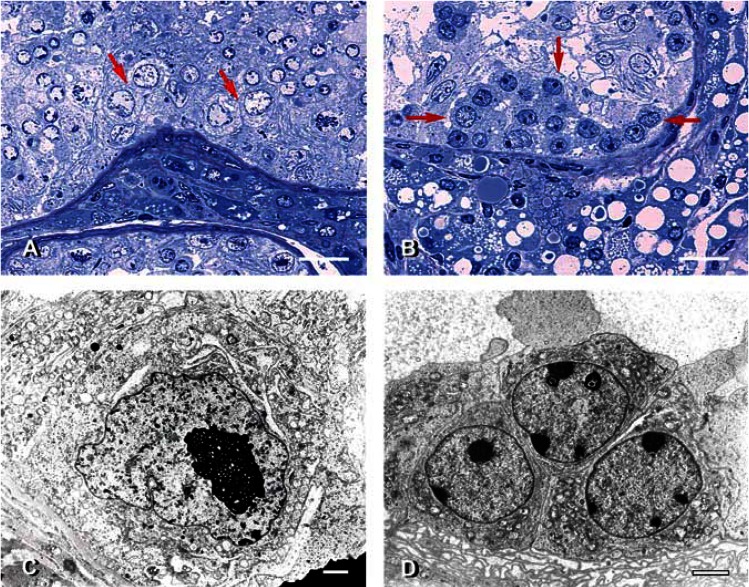
Testicular CIS. (A) CIS-like cells (arrows) in the basal aspect of a seminiferous tubule from the testis of NCO deer 03-12. Compare the size and abnormal contours of the nuclei in these cells with those of spermatogonia in the lower tubule. Methylene blue and Safranin-O staining. (B) An intratubular tumorous nodule (designated by arrows) containing CIS-like cells in BCO deer 03-14. Note the foaminess and hypertrophy of Leydig cells and compare with those in A. Methylene blue and Safranin-O staining. (C) Transmission electron micrograph of an atypical germ cell from the testis of BCO deer 03-7. Note abnormal nuclear contours, chromatin clumps, and altered nucleolonema in large nucleolus, swollen mitochondria, and unusual membranous profiles. (D) Transmission electron micrograph of a cluster of CIS-like cells from the testis of BCO deer 03-6. Note meandering, marginated nucleoli typical of seminomatous lesions and swollen mitochondria in each of these cells. Scale bars: A and B = 25 μm; C and D = 2 μm.
On average (geometric means), serum concentrations of testosterone were similar for NCO and BCO deer whose antlers had shed velvet (3.8 vs 1.9 ng/mL; n = 34, 27; p = 0.09). Values for 7 UCO deer averaged 1.7 ng/mL. All but 1 NCO deer had normal antlers. Regarding BCOs (Figure 2), testosterone concentrations for deer whose antlers were covered with velvet were lower than those for deer whose antlers were abnormal without velvet or were normal. Geometric means were 0.2, 2.0, or 1.7 ng/mL; the latter two values were not significantly different.
Testicular histopathology
Testicular tissue from 12 deer hunted in 2003 was examined (4 NCO, 2 UCO, 6 BCO). Normal progression of spermatogenesis [Supplemental Material Figure 5A (http://www.ehponline.org/members/2005/8052/supplemental.pdf)] was evident in scrotal testes of all NCO and UCO deer. However, degenerative changes in the seminiferous epithelium ranging from focal vacuolation (because of desquamation of immature germ cells) to complete loss of germ cells leaving only Sertoli cells [Supplemental Material Figure 5B (http://www.ehponline.org/members/2005/8052/supplemental.pdf)] also were observed. The DGEL in scrotal testes of the 4 NCO and 2 UCO deer ranged from 11 to 80 (Table 1); the higher the value, the more the degeneration. A defect that occurs during spermiogenesis, sharing of a single acrosome between two or more spermatids, was encountered occasionally in 2 of the 4 NCO deer.
Figure 5.
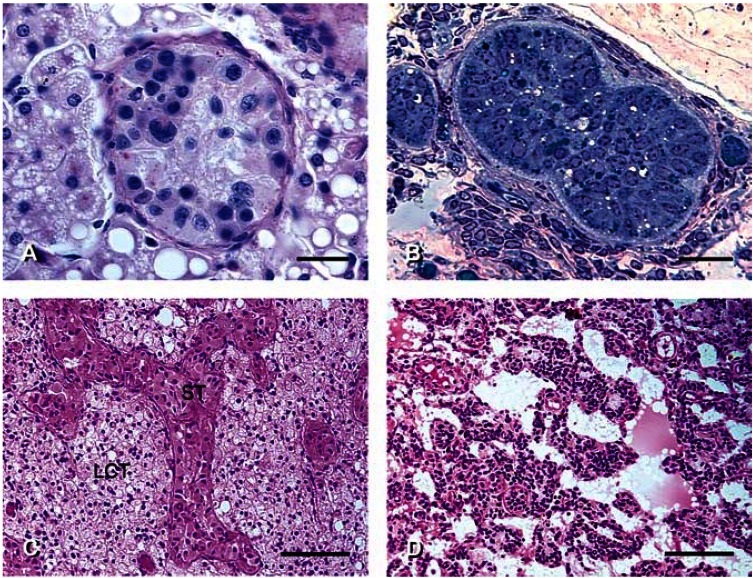
Testicular tumors. (A) Intratubular seminoma-like cells in BCO deer 03-14. Note considerable variation in size and shape of nuclei; such tumors have been designated as anaplastic. PAS and hematoxylin staining; differential interference contrast microscopy. (B) Intratubular, solid Sertoli cell tumor in the testis of BCO deer 03-6. Note regressed Leydig cells in the interstitium and compare with foamy, hypertrophic Leydig cells in association with the seminoma in A. Methylene blue and Safranin-O staining. (C) Leydig cell tumor (LCT) causing distortion of seminiferous tubules (ST) containing only Sertoli cells in the testis of BCO deer 03-03. (D) Stromal cell tumor in the testis of BCO deer 03-6. H&E staining. Scale bars: A and B = 25 μm; C and D = 100 μm.
In undescended testes, there was no spermatogenesis [as expected, because of higher abdominal temperature; Supplemental Material Figure 6 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)]. The DGEL in all abdominal testes was 100 (Table 1). Atypical germ cells characterized by abnormal nuclei and periodic acid–Schiff-positive (PAS-positive) cytoplasmic inclusions (glycogen) were found in two scrotal testes (Figure 3A) and four abdominal testes (Figure 3B ). They were found individually or in small clusters (Figure 4A, B). At the electron microscopic level, they had ultrastructural features typical of CIS cells, namely, irregular nuclear contours, chromatin clumps, meandering nucleolonema, marginated nucleoli, swollen mitochondria, and unusual membranous profiles (Figure 4C, D). CIS cells are known to be a precursor of seminoma in humans, but as the fate of these atypical germ cells in SBTD is not known, hereafter they are termed CIS-like cells. Intratubular seminoma-like lesions were found in two abdominal testes (Figure 5A).
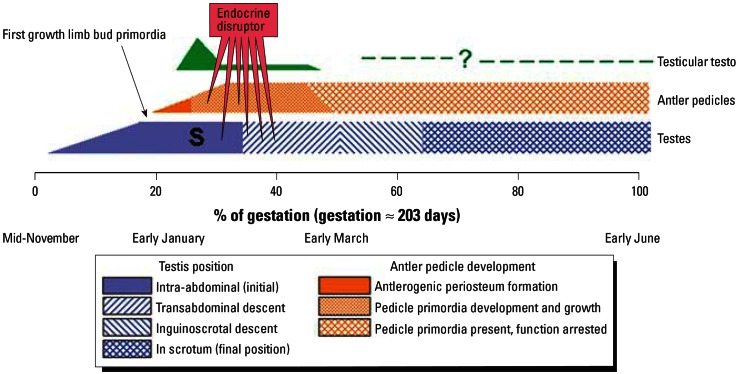
Figure 6.
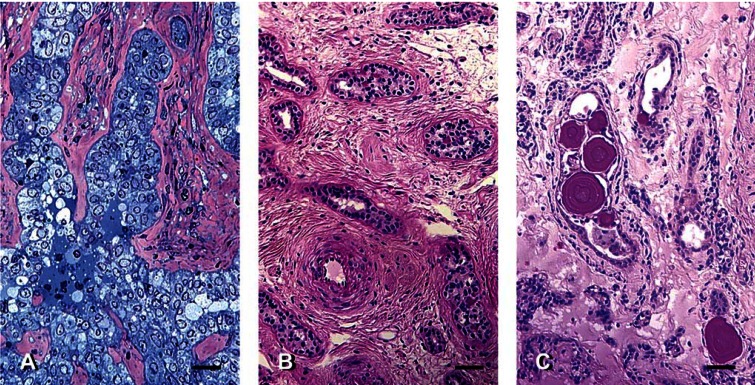
Neoplastic lesions and concretions in the rete testis. (A) Carcinoma, with proliferation of dysplastic epithelial cells (normally cuboidal) of the rete testis invading the stromal elements, in the testis of BCO deer 03-11. Methylene blue and Safranin-O staining. (B) Adenoma of rete tubules in the testis of BCO deer 03-6. Hypertrophic glandular epithelial cells with PAS-positive secretions are evident. PAS and hematoxylin staining. (C) Microlithiasis within rete testis tubules in the testis of BCO deer 03-13. PAS and hematoxylin staining. Scale bars: A = 25 μm; B and C = 50 μm.
Hyperplastic Sertoli cells, often arranged in rosettes and sometimes with neoplastic changes, were found in several abdominal testes [Supplemental Material Figure 6 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)]. A proteinaceous material, sometimes including mineralized concretions, was found in association with these hyperplastic cells. Similar proteinaceous material also was found in a scrotal testis in the lumina of seminiferous tubules predominantly containing Sertoli cells [Supplemental Material Figure 5B (http://www.ehponline.org/members/2005/8052/supplemental.pdf)]. Solid, intratubular Sertoli cell tumors (Figure 5B) were found in two abdominal testes.
Focal Leydig cell hyperplasia and hypertrophy were common findings. Large, foamy Leydig cells containing fat globules were found in several scrotal as well as abdominal testes [Table 1; Figures 4B, 5A; Supplemental Material Figure 6D (http://www.ehponline.org/members/2005/8052/supplemental.pdf)]. This phenomenon indicates impaired steroidogenesis resulting in underutilization and consequent accumulation of lipids. Nodular Leydig cell tumors were found in four abdominal testes; Figure 5C shows a diffuse tumor distorting the seminiferous tubules. A stromal cell tumor with intermittent serosanguinous areas was found in an abdominal testis (Figure 5D).
Lesions characterized by hyperplasia, hypertrophy, and neoplasia were found in the excurrent ducts. Carcinoma of the rete testis (Figure 6A), a rarely occurring lesion, was found in an abdominal testis. Adenoma of the rete testis (Figure 6B) was found in the same and another abdominal testis. Rete tubules containing concretions (Figure 6C) and cysts filled with proteinaceous material [Supplemental Material Figure 7A (http://www.ehponline.org/members/2005/8052/supplemental.pdf)] were occasionally found. Focal areas of adenomatous principal cells with PAS-positive secretions were found in the caput epididymidis of an NCO deer, and cysts filled with proteinaceous material [Supplemental Material Figure 7B (http://www.ehponline.org/members/2005/8052/supplemental.pdf)] were found in the caput epididymidis of a BCO deer.
Figure 7.
Timing during ontogeny of the testes, antler pedicles, and limbs in SBTD and postulated actions of an endocrine disruptor to transform testicular cells, alter the antler pedicle primordia, and disrupt transabdominal descent of the testes. Testicular testo: testosterone concentration in total testicular tissue. At “S,” the future scrotum is visible externally as scrotal swellings. Timing of development in SBTD based on data for mule deer (Hudson and Browman 1959) and red deer (Li and Suttie 2001; Lincoln 1973) scaled to gestation length; duration of gestation assumed as 203 days in SBTD and mule deer and 233 days in red deer. In model animals, transabdominal descent of the testes is blocked by estrogens, whereas passage through the inguinal canal is blocked by antiandrogens (Klonisch et al. 2004). Insulin-like peptide-3 (Insl3) is involved in transabdominal testicular descent; hence, altered expression of Insl3 and or a Hoxa gene could cause cryptorchidism. Products of the fibroblast growth factor gene family (i.e., FGFs) promote early outgrowth of limb buds, with axis patterning controlled by the sonic hedgehog (Shh) gene. FGF genes as well as Shh have been detected in the growth region of regenerating antlers of red deer postnatally (Li and Suttie 2001). The primary agent causing abnormalities such as those seen in SBTD, if caused by an endocrine disruptor, might be a phytoestrogen, estrogenic mycotoxin, alkyl phenol, organochlorine, or polychlorinated biphenyl (see text). An antiandrogen might be a co-agent.
Discussion
The weight of evidence [Table 1, Figures 1–6; Supplemental Material Table 1, Figures 1–7 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)] is that many male SBTD have multiple yet similar abnormalities and that the incidence of collective occurrence is not trivial. Hence, we concluded that something was affecting SBTD on the AP.
Dilemma and hypothesis
How do antler malformation, cryptorchidism, induction of abnormalities in all four primordial testicular cell types, and lack of hypospadias tie together? Leydig cells, stromal elements, and supporting cells (primordia of Sertoli cells) differentiate around primordial germ cells early in gonadogenesis; testosterone secretion follows. Bubenik (1990a) recognized that the antler pedicle primordia, obligatory for postnatal antler development, underwent differential growth early in gestation of male red deer (Cervus elaphus) coincident with initial production of testosterone. Testosterone was maximal at 24–29% of gestational length (233 days in red deer) and undetectable (< 0.1 ng/g testis) after 45% of gestation (Lincoln 1973). The scrotal swelling was evident by 26% of gestation. The importance of Lincoln’s 1973 observation that early development of primordial antler pedicles was initiated at approximately 26% of gestational length, shortly before the testes initiated their transabdominal descent (at ~ 34% of gestational length), apparently escaped earlier workers studying abnormal deer. Primordial antler pedicles are conspicuous in mule deer (Odocoileus hemionus hemionus) by 36% of gestation (Hudson and Browman 1959). Li and Suttie (2001) confirmed timing of the initial appearance of antler pedicle primordia in red deer.
Accepting this ontogenic scenario, the underlying cause of abnormalities in many SBTD could be a mutation(s) in an originator female/male, directly or indirectly causing all defects, with the mutation(s) transmitted to progeny over many generations (i.e., genetic). Alternatively, the cause could be continuing exposure of female deer and their developing fetus(es) to an environmental agent that affected the fetus(es). Regardless, female SBTD also might be affected phenotypically.
In our data, the abnormalities were found almost exclusively on the AP (68 of 76 BCO or UCO males) (Figure 1). However, occasional SBTD lacking obvious scrotal testes, some with abnormal antlers, have been shot in lowlands near the western end of Olga Bay (northwest of AP; Figure 1) or nearby Hepburn Peninsula in the mid-1990s and also on Shuyak Island (northernmost island in Figure 1) (Van Daele and McDonald 2001).
UCO or BCO males found elsewhere might have been descendants of the same originator animal or exposed to the same agent(s) at sites distant from the AP (e.g., low coastal area with similar vegetation, wash-up of oceanborne plants or matter, or contaminated fog). Alternatively, and regardless of cause, they or their dam might have migrated from the AP to the site where they were shot. Importantly, 66 of 76 cryptorchid males were BCOs and therefore could not sire offspring.
For reasons presented below, we do not favor transmission of a mutation(s) causing a) cryptorchidism plus abnormal antlers and induced abnormalities in all four cell types in the testis; or b) cryptorchidism, with abdominal location of testes (not the mutation per se) indirectly causing deformations of antlers and transformation of testicular cells. Rather, we hypothesize that male SBTD on the Kodiak Archipelago with cryptorchidism, antler malformation, and alteration of testicular cells were impacted by an endocrine disruptor while developing as a fetus during the first 40% of gestation. Arguably, the same agent(s) prevented descent of one or both testes into the potential scrotum and, in 70% of BCO males, altered the primordial antler pedicle to affect shape and/or retention of velvet. Alternatively, retention of velvet on 36% of BCO but on no NCO males might be an indirect consequence of changes induced in fetal Leydig cells (hampering proliferation and/or postnatal function), thereby resulting in insufficient testosterone postnatally to promote shedding of velvet (Bubenik 1990a). This latter scenario could not explain altered antler shape. Concentration and/or timing of an agent(s) impinging on the early fetus could result in variable expression of multiple defects.
Deer on AP are unique
Cryptorchid deer represented 72% of the population shot on AP, with 9 times more BCO than UCO males. This is an unusually high prevalence of cryptorchidism and predominance of BCO over UCO (McEntee 1990). Further, 62% of BCO deer for which data were available had a shape defect of the antlers, 36% a velvet defect, and 29% both a shape and velvet defect. This combination of cryptorchidism and antler malformation is different from earlier reports of deer with abnormal antlers or cryptorchidism. Most reports of abnormal antlers (Carrasco et al. 1997; DeMartini and Connolly 1975; Robinette and Jones 1959; Taylor et al. 1964; Tiller et al. 1997) were for animals with atrophy of scrotal testes. Further, available evidence suggests that cryptorchidism in deer typically is not accompanied by abnormal antlers [Supplemental Material (http://www.ehponline.org/members/2005/8052/supplemental.pdf)].
Testicular histopathology
The totality of lesions found in testes from both NCO and BCO or UCO deer on the AP is very unusual and is inconsistent with appearance of testicular tissue in naturally occurring cryptorchids of other species. A variety of antiandrogenic and estrogenic chemicals can induce cryptorchidism (Gray et al. 2001; Toppari et al. 1996), but the nature of lesions in the undescended testes depends on the agent. Typically, estrogenic chemicals induce mitogenic effects causing proliferative lesions, whereas antiandrogenic chemicals cause regressive changes.
For example, in rabbits, normal descent of testes into the scrotum can be blocked a) by in utero exposure to the antiandrogen flutamide, estrogenic octylphenol, or estradiol per se; b) by active immunization against gonadotropin-releasing hormone (ultimately reducing testosterone production); or c) surgically (Veeramachaneni et al. 2001). The spectrum of lesions in the undescended testis was different depending on the etiological factor. CIS-like, atypical germ cells were found in the undescended testes of octylphenol- or estradiol-exposed animals but not in those of remaining groups. Similarly, in utero exposure to dibutylphthalate (Higuchi et al. 2003) resulted in CIS-like cells in the undescended testes, as did perinatal exposure to the insecticide p,p′-DDT [1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane] (some estrogenic action, predominantly antiandrogenic; Veeramachaneni 2000) or its metabolite p,p′-DDE [1,1-dichloro-2,2-bis(p-chlorophenyl) ethylene] (Veeramachaneni 2006). Thus, abnormal development of germ cells in males can result from direct in utero exposure to chemical pollutants during critical periods of gonadal differentiation. However, neonatal surgery preventing transabdominal descent of the testes, keeping them within the abdominal cavity thereafter, did not induce atypical cells within any of the four specialized cell types that form a testis (Veeramachaneni et al. 2001). Although a variety of genetic mutations or chemical agents can block normal testicular descent, not all cause germ cell transformation leading to CIS-like cells and development of tumors.
Testicular dysgenesis, an increasingly common developmental disorder, is an important cause of reproductive failure in men (Skakkebaek et al. 2001) and has been associated with a variety of environmental factors. Dysgenetic gonads are predisposed to the development of tumors or tumorlike proliferations of both germ cells and sex cord/stromal elements (Mostafi 1977) similar to those found in SBTD.
Exposure of pregnant mice to diethylstilbestrol resulted in adenocarcinoma of the rete testis of male offspring (Newbold et al. 1985) and interstitial cell tumors (Newbold et al. 1987) but not germ cell tumors. These proliferative lesions and tumors in the rete testis occur transgenerationally without further exposure of descendents (Newbold et al. 2000). Furthermore, microlithiasis in seminiferous tubules and adenomatous lesions in rete tubules similar to lesions found in these SBTD were found in South African eland (Tragelaphus oryx) that had high body burdens of estrogenic nonylphenol (Bornman et al. 2004). The occurrence of extremely rare rete carcinoma or stromal cell tumors in 3 of 6 BCO SBTD, together with other testicular tumors, suggests exposures to estrogenic chemicals. Considering that increased susceptibility for diethylstilbestrol-induced lesions in the rete testis is transmitted transgenerationally (Newbold et al. 2000), could one or more pregnant SBTD have been exposed to an estrogenic agent causing similar transmission of a propensity for proliferative lesions and tumors to successive generations, perhaps eventually concentrated by inbreeding?
Antlerogenesis
The discussion of antlerogenesis presented in Supplemental Material (http://www.ehponline.org/members/2005/8052/supplemental.pdf) is the basis for our interpretation of the combination of testicular and antler dysgenesis. Descriptions and illustrations of sequelae of experimental hormonal manipulations (Bubenik 1990b; Goss 1990; Jaczewski 1990; Kolle et al. 1993) are not similar to atypical/bizarre-shaped antlers commonly found in SBTD on the AP. In deer, blockage of testosterone secretion by administration of cyproterone acetate can prolong growth and delay mineralization (Bubenik et al. 1975) and in some males result in antlers with sharp tips (Bubenik GA, personal communication). However, it is not evident that a simple deficiency in secretion of testosterone, independent of any alteration of androgen receptors or other changes in the periosteum, caused elongated sharp antler tips in these SBTD. Serum from 7 of 12 SBTD with sharp antler tips had ≥ 1.5 ng/mL testosterone, 5 had ≥ 3.3 ng/mL, and all 12 averaged 1.2 ng/mL. Based on published reports (Bubenik 1990a; McMillin et al. 1974; Suttie et al. 1984), apparently approximately 1.5 ng/mL testosterone is sufficient for hardening of antler bone. Accidental or experimental injury to an antler bud in otherwise normal males is followed by exuberant growth of the antlers, thereby resulting in structures (Figure 119 in Goss 1983) similar to many found in SBTD [Supplemental Material Figure 4 (http://www.ehponline.org/members/2005/8052/supplemental.pdf); Figures 4 and 5 in Bubenik et al. (2001)]. However, it is illogical that most BCO and no NCO males would have received injuries of a type causing bizarre antlers, as BCO deer are unlikely to be aggressive if they have low testosterone.
Consideration of a genetic cause of cryptorchidism
If there were one originator animal produced by a spontaneous mutation(s) of one or more genes, could the mutation be rapidly transmitted among deer on the AP, concomitant with sufficient inbreeding, to produce 72% cryptorchidism? Historically, there was disagreement whether cryptorchidism in a population was transmitted as a single or multiple dominant or recessive gene(s). However, a multigenetic recessive cause now is accepted (Nielen et al. 2001). For example, Rothschild et al. (1988) found that their pig data were consistent with recessive genes at two or more loci [Supplemental Material (http://www.ehponline.org/members/2005/8052/supplemental.pdf)].
Information on genes involved in testicular descent and limb-bud formation is evolving, especially for mice and humans (Klonisch et al. 2004). For humans, more than 20 different causes of cryptorchidism in humans have been traced to one or more specific loci of seven different chromosomes. In brief, a mutation in the Insl3, Great, or Hoxa-10/Hoxa-11 genes might block transabdominal testicular descent, and products of the Insl3, Great, Hoxa, or Hoxd gene families might be important for normal formation of the primordial antler pedicle, as with limb buds in mice. Desrt probably could not be the culprit, as it affects the inguinoscrotal phase of testicular descent rather than transabdominal descent. Dhh apparently has effects different from those we observed, although it is involved in differentiation of Leydig cells. Insl3 is produced in fetal Leydig cells at the time of transabdominal testicular descent. Male Insl3−/− mice are cryptorchid because of failure of the gubernaculum to develop. However, adult Insl3−/− male mice have normal concentrations of serum testosterone and apparently normal osteogenesis of the penial bone and limbs (Nef and Parada 1999). Importantly, testes in Insl3−/− males have normal spermatogenesis in most tubules if surgically placed into the scrotum postnatally (Zimmermann et al. 1999). Hence, although mutation of the Insl3 gene could block transabdominal descent of testes, it is unlikely that such a mutation could: a) induce CIS-like cells (transformed germ cells) and/or abnormal Leydig (mesenchymal) and Sertoli (epithelial) cells; and b) cause abnormal antler primordia. However, as discussed in “Testicular histopathology,” transgenerational transmission of a propensity for CIS-like cells has not been demonstrated, although in mice a propensity for tumors of the rete testis can be inherited. In the case of SBTD, such a change would have to be derived from a gene mutation(s) responsible for cryptorchidism.
In humans and mice (Ferlin et al. 2003; Gorlov et al. 2002), Insl3 peptide binds to a receptor transcribed by the Great gene. Gubernacular tissue contains an extraordinarily high concentration of this receptor. Hence, a mutation of either Insl3 or Great genes could cause cryptorchidism. However, among 82 cryptorchid men (43% BCOs), only 8.5% had a mutation or deletion of the Insl3 or Great genes (Ferlin et al. 2003).
Although populations of deer tend to retain heterozygosity (Chesser and Smith 1987), the genetics of reproductive development or cryptorchidism in deer has not been studied. However, in a study based on 747 males born in 457 litters of boxer dogs, Nielen et al. (2001) found an 11% prevalence of cryptorchidism. When they assumed transmission of cryptorchidism via a polygenic recessive model, estimated heritability was 0.23. In families of related dogs, prevalence ranged from 0 to 20%. Penetrance was estimated at 5–8% in heterozygotic or noncarrier males but approximately 23% in homozygous recessive males [Supplemental Material (http://www.ehponline.org/members/2005/8052/supplemental.pdf)]. If there were a hypothetical SBTD that underwent a spontaneous mutation of one specific gene (e.g., Insl3 or Hoxa), such an originator might have produced carrier female and male descendants in numbers sufficient to produce the current prevalence of cryptorchidism over 15–30 years but could that cause all the abnormalities?
The apparent incidence of cryptorchidism on the AP doubled within 5 years (1.4:1 in 1999 vs. 2.7:1 in 2003–2004). If concentration of a historic mutation via inbreeding was the cause of the problem of cryptorchidism on the AP, this would require virtually no movement of breeding animals onto or away from the affected region. Otherwise, the disparity in prevalence of cryptorchidism on the AP versus elsewhere in the archipelago (72% vs. 12%, considering all data) would not exist. In fact, however, SBTD migrated to populate the archipelago, including the AP. Furthermore, analyses of microsatellite DNA at 12 loci for 76 SBTD on the AP, northern Kodiak Island, or adjacent Uganik Island revealed that deer in all three regions represented a single random-breeding population (Amann RP, Paetkau D, unpublished data). Together, these observations make a genetic cause of the cryptorchid problem on the AP unlikely.
Cryptorchidism as a cause of testicular neoplasia or antler deformation
It is generally accepted that cryptorchid testes are more prone to develop neoplasia, although such defects also occur in NCO males (McEntee 1990; Mostafi 1977). However, this does not establish cryptorchidism per se as the factor causing CIS-like cells or tumors and exclude an agent independently causing both cryptorchidism and testicular dysgenesis. The distinction is important. Indeed, emerging knowledge of gene defects in males (Klonisch et al. 2004) suggests a common cause for cryptorchidism and sensitization to a tumor-inducing agent.
It is generally considered that retention of velvet on deer antlers after the normal time of shedding is a direct result of insufficient testosterone in blood (Bubenik 1990a). Abdominal testes of other species apparently have reduced capability to secrete testosterone, although some reports are contradictory (Nef and Parada 1999; Setchell 1978). As described in “Antlerogenesis,” approximately 1.5 ng/mL testosterone is the threshold needed for final hardening of antlers and shedding velvet. Accepting the limitation of single blood samples for deer in our study, 18 of 45 (40%) BCOs had ≥ 1.5 ng/mL serum, as did 24 of 34 (71%) NCO deer (Figure 2). The 10 NCO deer with < 1.5 ng/mL testosterone did not have retained velvet, although each BCO deer with retained velvet had ≤ 1 ng/mL. Serum from BCO deer bearing normal antlers or abnormal antlers without velvet averaged (geometric mean) 3.8 and 1.9 ng/mL testosterone (Figure 2). The contribution, if any, from Leydig cell tumors (Table 1) to blood testosterone in BCO or NCO deer is unknown.
Considering all SBTD whose serum testosterone was measured, 28 of 45 BCOs (62%) had polished antlers when shot. This suggests that relatively low production of testosterone by a BCO deer is not inconsistent with shedding of velvet. Indeed, scrutiny of available data for NCO red deer (Suttie et al. 1984) or Odocoileus sp. (McMillin et al. 1974; West and Nordan 1976) led to the conclusion that the testosterone stimulation associated with hardening and then shedding of velvet represents an increase from typical values of < 0.3 ng/mL to > 2 ng/mL (as high as10 ng/mL; Suttie et al. 1984). However, the critical question is not what concentration of testosterone is there but rather what is the minimal concentration of testosterone required to drive the response; it is difficult to determine the value of “enough” [see Figure 2 in Amann and Hammerstedt (1993)]. Demonstration of cause and effect, with respect to association of cryptorchidism and diminished testosterone production or diminished testosterone production and retention of velvet, is not provided by retention of velvet by 38% of BCO deer on the AP.
Consideration of an environmental agent
For reasons given above, we cannot rationalize a spontaneous mutation(s) in one originator animal, followed by increased prevalence due to inbreeding, as the cause of cryptorchidism plus transformed testicular cells and abnormally shaped antlers. If a hypothetical genetic mutation was the cause of all defects, with only 27% NCO males on the AP in 2003–2004, most males and females must carry the altered gene(s). Therefore, in a few years, deer should be absent from the Peninsula because virtually all males will be BCOs. Time will tell. The argument that local extinction would be precluded by inward movement from distant areas of deer lacking the hypothetical mutation argues equally well against the notion of an inbreeding problem. Emigration or transplantation of only a few animals lacking a genetic defect can rapidly eliminate a problem (Keller and Waller 2002).
Alternatively, it could be hypothesized that concurrent expression of two or three independent causes (gene mutations) was the cause of all defects. Of 94 deer shot on the AP, 61 were BCOs, of which 43 had abnormal antlers (70%), and 6 of 6 BCOs studied had detected testicular tumors and/or CIS-like cells (100%). Laws of chance make independent causes very unlikely.
By elimination, but especially because of what is known about endocrine disruptors and ontogeny of the reproductive system and antlers, one must consider that a common causative agent(s) acted on male fetuses to cause all three defects. This hypothesis is illustrated in Figure 7 and is supported by observations for other species. Although we hypothesize that the endocrine disruptor(s) causes damage during a window narrower than 25–40% of gestation, we suspect that it is present in the female deer throughout much of gestation (i.e., winter), if not year-round. Alternatively, the observed dysgenesis might result from an epigenetic effect transmitted over generations (Anway et al. 2005).
Numerous studies have reported sequelae of in utero exposure of male fetuses to a multiplicity of agents including estrogenic and antiandrogenic chemicals (Anway et al. 2005; Colborn et al. 1993; Gray et al. 2001; Higuchi et al. 2003; McMahon et al. 1995; Nef et al. 2000; Newbold et al. 1985, 1987, 2000; Safe et al. 2001; Toppari et al. 1996; Veeramachaneni 2000; Wilson et al. 2004). Because hypospadias, a long-known symptom of decreased action of androgens during the development of reproductive system, was not encountered in SBTD, we speculate that the putative endocrine disruptor is estrogenic rather than antiandrogenic or with some other action. Supporting this hypothesis is the fact that lesions observed in the testes and excurrent ducts of affected SBTD are proliferative and dysplastic (typical of estrogenic stimulation) and not regressive (typical of antiandrogens).
Certain estrogenic molecules can down-regulate the Insl3 gene and disrupt normal testicular descent (Emmen et al. 2000; Nef et al. 2000), so testes remain abdominal [Supplemental Material Figure 3 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)] and also cause dysplastic and/or neoplastic lesions in the male reproductive tract similar to those seen in SBTD [Figures 3–6; Supplemental Figures 6, 7 (http://www.ehponline.org/members/2005/8052/supplemental.pdf)]. Additionally, products of the Insl3 gene could be among factors affecting formation of the primordial antler pedicle (as for limb buds; Klonisch et al. 2004).
We suspect that the postulated estrogenic endocrine disruptor molecule affecting SBTD on the AP is associated with contaminated fog (Chernyak et al. 1996), washed-up ocean matter deposited on deer browse, or ocean plants (e.g., kelp) deposited on shore. See Supplemental Material (http://www.ehponline.org/members/2005/8052/supplemental.pdf) for description of topography of AP. Kelp includes molecules binding to estradiol receptors in vitro (Skibola et al. 2005), but the nature of the molecule(s) or its potency in kelp was not determined. It is unlikely that ingested tundra plants or brush per se contain the estrogenic molecule because leaves and twigs of similar plants [described by Smith (1979)] and lichens are eaten by SBTD elsewhere without obvious deleterious affects. However, coastal grass (including rye) could include estrogenic molecules or host fungi with estrogenic activity (e.g., zeralenone-like). Speculation on identity of a possible molecule would be meaningless.
Assuming the endocrine disruptor hypothesis is correct, study of altered gene expression in affected postpubertal male SBTD might be rewarding. Ultimately, epigenetic mechanisms should be studied in fetuses early in pregnancy (15–45% of gestation) or in young fawns and their dams.
Conclusions and Inferences
A majority of SBTD on the AP manifest bilateral cryptorchidism, abnormalities in all four primordial testicular cell types, and antler malformation. Most SBTD elsewhere in the Kodiak Archipelago are unaffected.
Microsatellite DNA analyses showed that affected AP deer and SBTD in distant locales in the Kodiak Archipelago apparently all are one population. Hence, localized expression of a genetic mutation(s) as the cause of abnormal SBTD on the AP is unlikely.
The AP differs from other areas of the Kodiak Archipelago in low topography, localized ocean currents favoring deposition of ocean plants or materials on beaches, and extensive ingestion by SBTD of copious kelp. Kelp, coastal grass, or fog might provide estrogenic molecules.
We hypothesize that testis–antler dysgenesis results from exposure of pregnant females to an estrogenic environmental agent(s) acting epigenetically to transform testicular cells and also to alter the primordial antler pedicles and affect expression of the Insl3 or other genes to block transabdominal descent of fetal testes in most animals. Only the latter is evident at birth.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
Supplemental Material is available online (http://www.ehponline.org/members/2005/8052/supplemental.pdf).
We thank J. Palmer, C. Moeller, G. Sammonds, and J. Yamane for technical assistance.
References
- Amann RP. 1990. Management of bulls to maximize sperm output. In: Proceedings of the Thirteenth Technical Conference, 20–21 April 1990, Milwaukee, WI. Columbia, MO: National Association of Animal Breeders, 84–91.
- Amann RP, Hammerstedt RH. In vitro evaluation of sperm quality: an opinion. J Androl. 1993;14:397–406. [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndtson WE, Pickett BW, Nett TM. Reproductive physiology of the stallion. IV. Seasonal changes in the testosterone concentration of peripheral plasma. J Reprod Fertil. 1974;39:115–118. doi: 10.1530/jrf.0.0390115. [DOI] [PubMed] [Google Scholar]
- Bornman MS, Barnhoorn IEJ, Dreyer L, Veeramachaneni DNR, De Jager C. 2004. Testicular degeneration coincident with fat residues of nonylphenol in the common eland (Tragelaphus oryx): a possible link to endocrine disruption [Abstract]? In: Proceedings of the CREDO Cluster Workshop on Ecological Relevance of Chemically-Induced Endocrine Disruption in Wildlife, 5–7 July 2004, Exeter, UK. Exeter, UK:University of Exeter, 59.
- Bubenik GA. 1990a. Neuroendocrine regulation of the antler cycle. In: Horns, Pronghorns, and Antlers (Bubenik GA, Bubenik AB, eds). New York:Springer-Verlag, 265–297.
- Bubenik GA. 1990b. The role of the nervous system in the growth of antlers. In: Horns, Pronghorns, and Antlers (Bubenik GA, Bubenik AB, eds). New York:Springer-Verlag, 339–358.
- Bubenik GA, Bubenik AB, Brown GM, Wilson DA. The role of sex hormones in the growth of antler bone tissue. J Exp Zool. 1975;194:349–358. doi: 10.1002/jez.1401940202. [DOI] [PubMed] [Google Scholar]
- Bubenik GA, Jacobson JP. Testicular histology of cryptorchid black-tailed deer (Odocoileus hemionus sitkensis) of Kodiak Island, Alaska. Z Jagdwiss. 2002;48:234–243. [Google Scholar]
- Bubenik GA, Jacobson JP, Schams KD, Barto? L. Cryptorchidism, hypogonadism and antler malformation in black-tailed deer (Odocoileus hemionus sitkensis) of Kodiak Island. Z Jagdwiss. 2001;47:241–252. [Google Scholar]
- Carrasco L, Fierro Y, Sánchez-Castillejo JM, Hervás J, Perez J, Gómez-Villamandos JC. Abnormal antler growth associated with testicular hypogonadism in red deer. J Wildlife Dis. 1997;33:670–672. doi: 10.7589/0090-3558-33.3.670. [DOI] [PubMed] [Google Scholar]
- Chapman DI, Chapman NG, Horwood MT, Masters EH. Observations on hypogonadism in a perruque Sitka deer (Cervus nippon) J Mammal. 1984;49:579–584. [Google Scholar]
- Chernyak SM, Rice CP, McConnell LL. Evidence of currently-used pesticides in air, ice, fog, sea water and surface microlayer in the Baring and Chukchi seas. Mar Pollut Bull. 1996;32:410–419. [Google Scholar]
- Chesser RK, Smith MH. 1987. Relationship of genetic variation to growth and reproduction in the white-tailed deer. In: Biology and Management of Cervidae (Weimmer CM, ed). Washington DC:Smithsonian Institute Press, 168–177.
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini JC, Connolly GE. Testicular atrophy in Columbian black-tailed deer in California. J Wildl Dis. 1975;11:101–106. doi: 10.7589/0090-3558-11.1.101. [DOI] [PubMed] [Google Scholar]
- Emmen JMA, McLuskey A, Adham I, Engel W, Verhoef-Post M, Themmen AP, et al. Involvement of insulin-like factor 3 (Insl3) in diethylstilboestrol-induced cryptorchidism. Endocrinology. 2000;141:846–849. doi: 10.1210/endo.141.2.7379. [DOI] [PubMed] [Google Scholar]
- Ferlin A, Simonato M, Bartoloni L, Rizzo G, Bettella A, Dottorini T, et al. The INSL3-LGR8/GREAT ligand-receptor pair in human cryptorchidism. J Clin Endocrinol Metab. 2003;88:4273–4279. doi: 10.1210/jc.2003-030359. [DOI] [PubMed] [Google Scholar]
- Gorlov IP, Makat A, Bogatcheva NV, Jones E, Lamb DJ, Truong A, et al. Mutations of the GREAT gene cause cryptorchidism. Hum Mol Genet. 2002;11:2309–2318. doi: 10.1093/hmg/11.19.2309. [DOI] [PubMed] [Google Scholar]
- Goss RJ. 1983. Deer Antlers. New York:Academic Press.
- Goss RJ. 1990. Of antlers and embryos. In: Horns, Pronghorns, and Antlers (Bubenik GA, Bubenik AB, eds). New York:Springer-Verlag, 298–312.
- Gray LE, Jr, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update. 2001;7:248–264. doi: 10.1093/humupd/7.3.248. [DOI] [PubMed] [Google Scholar]
- Higuchi TT, Palmer JS, Gray LE, Jr, Veeramachaneni DNR. Effects of dibutyl phthalate in male rabbits following in utero, adolescent, or postpubertal exposure. Toxicol Sci. 2003;72:301–313. doi: 10.1093/toxsci/kfg036. [DOI] [PubMed] [Google Scholar]
- Hofmann W. Ein Beitrag zum abdominalen Kryptorchismus beim Rehbock [in German] Z Zagdwiss. 1968;14:28–30. [Google Scholar]
- Hoy JA, Hoy R, Seba D, Kerstetter TH. Genital abnormalities in white-tailed deer (Odocoileus virginianus) in west-central Montana: pesticide exposure as a possible cause. J Environ Biol. 2002;23:189–197. [PubMed] [Google Scholar]
- Hudson P, Browman LG. Embryonic and fetal development of the mule deer. J Wildl Manage. 1959;23:295–304. [Google Scholar]
- Jacobson JP. 2003. Sterile bucks concern Kodiak Island hunters. Alaska, 69 August:57.
- Jaczewski Z. 1990. Experimental induction of antler growth. In: Horns, Pronghorns, and Antlers (Bubenik GA, Bubenik AB, eds). New York:Springer-Verlag, 371–395.
- Keller LF, Waller DM. Inbreeding effects in wild populations. Trends Ecol Evol. 2002;17:230–241. [Google Scholar]
- Klonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Dev Biol. 2004;270:1–18. doi: 10.1016/j.ydbio.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Kolle R, Kierdorf U, Fischer K. Effects of an antiandrogen treatment on morphological characteristics and physiological functions of male fallow deer (Dama dama L) J Exp Zool. 1993;267:288–298. doi: 10.1002/jez.1402670307. [DOI] [PubMed] [Google Scholar]
- Leader-Williams N. Abnormal testes in reindeer, Rangifer tarandus. J Reprod Fertil. 1979;57:127–130. doi: 10.1530/jrf.0.0570127. [DOI] [PubMed] [Google Scholar]
- Lewis JP. 1991. Assessment of the Exxon Valdez Oil Spill on the Sitka Black-Tailed Deer in Prince William Sound and the Kodiak Archipelago: Terrestrial Study Number 1. Anchorage, AK:Alaska Department of Fish and Game.
- Li C, Suttie JM. Deer antlerogenic periosteum: a piece of postnatally retained embryonic tissue? Anat Embryol. 2001;204:375–388. doi: 10.1007/s004290100204. [DOI] [PubMed] [Google Scholar]
- Lincoln GA. Appearance of antler pedicles in early foetal life in red deer. J Embryol Exp Morph. 1973;29:431–437. [PubMed] [Google Scholar]
- Marburger RG, Robinson RM, Thomas JW. Genital hypoplasia of white-tailed deer. J Mammal. 1967;48:674–676. [PubMed] [Google Scholar]
- Markwald RR. 1968. Histologic and Histochemical Study of Testicular Periodicity in Mule Deer (Odocoileus hemionus hemionus) [MS Thesis]. Fort Collins, CO:Colorado State University.
- McEntee K. 1990. Reproductive Pathology of Domestic Mammals. New York:Academic Press.
- McMahon DR, Kramer AS, Husmann DA. Antiandrogen induced cryptorchidism in the pig is associated with failed gubernacular regression and epididymal malformations. J Urol. 1995;154:553–557. doi: 10.1097/00005392-199508000-00068. [DOI] [PubMed] [Google Scholar]
- McMillin JM, Seal US, Keenlyne KD, Erickson AW, Jones JE. Annual testosterone rhythm in the adult white-tailed deer (Odocoileus virginianus borealis) Endocrinology. 1974;94:1034–1040. doi: 10.1210/endo-94-4-1034. [DOI] [PubMed] [Google Scholar]
- Mostafi FK. 1977. Histological Typing of Testis Tumours. Geneva:World Health Organization.
- Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nat Genet. 1999;22:295–299. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- Nef S, Shipman T, Parada LF. A molecular basis for estrogen-induced cryptorchidism. Dev Biol. 2000;224:354–361. doi: 10.1006/dbio.2000.9785. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Bullock BC, McLachlan JA. Lesions of the rete testis in mice exposed prenatally to diethylstilbestrol. Cancer Res. 1985;45:5145–5150. [PubMed] [Google Scholar]
- Newbold RR, Bullock BC, McLachlan JA. Testicular tumors in mice exposed in utero to diethylstilbestrol. J Urol. 1987;138:1446–1450. doi: 10.1016/s0022-5347(17)43672-x. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 2000;21:1355–1363. [PubMed] [Google Scholar]
- Nielen AL, Janss LL, Knol BW. Heritability estimates for diseases, coat color, body weight, and height in a birth cohort of Boxers. Am J Vet Res. 2001;62:1198–1206. doi: 10.2460/ajvr.2001.62.1198. [DOI] [PubMed] [Google Scholar]
- Robinette WL, Jones DA. Antler anomalies of mule deer. J Mammal. 1959;40:96–108. [Google Scholar]
- Rothschild MF, Christian LL, Blanchard W. Evidence for multigene control of cryptorchidism in swine. J Hered. 1988;79:313–314. [PubMed] [Google Scholar]
- Safe SH, Pallaroni L, Yoon K, Gaido K, Ross S, Saville B, et al. Toxicology of environmental estrogens. Reprod Fertil Dev. 2001;23:307–315. doi: 10.1071/rd00108. [DOI] [PubMed] [Google Scholar]
- Setchell BT. 1978. The Mammalian Testis. Ithaca, NY:Cornell University Press.
- Skakkebaek NE. Possible carcinoma in situ of the testis. Lancet. 1972;2:516–517. doi: 10.1016/s0140-6736(72)91909-5. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod. 2001;16:972–978. doi: 10.1093/humrep/16.5.972. [DOI] [PubMed] [Google Scholar]
- Skibola CF, Curry JD, VandeVoort C, Conley A, Smith MT. Brown kelp modulates endocrine hormones in female Sprague-Dawley rats and in human granulosa cells. J Nutr. 2005;135:296–300. doi: 10.1093/jn/135.2.296. [DOI] [PubMed] [Google Scholar]
- Smith RB. 1979. History and current status of Sitka black-tailed deer in the Kodiak Archipelago. In: Sitka Black-Tailed Deer, Proceedings of a Conference (Wallmo OC, Schoen SW, eds), 22–24 February 1979, Juneau, AK. USDA Series No R10-48. Juneau, AK:Alaska Department of Fish and Game, 184–195.
- Suttie JM, Lincoln GA, Kay RNB. Endocrine control of antler growth in red deer stags. J Reprod Fertil. 1984;71:7–15. doi: 10.1530/jrf.0.0710007. [DOI] [PubMed] [Google Scholar]
- Taylor DO, Thomas JW, Marburger RG. Abnormal antler growth associated with hypogonadism in white-tailed deer in Texas. Am J Vet Res. 1964;25:179–184. [PubMed] [Google Scholar]
- Tiller BL, Dagle G, Cadwell LL. Testicular atrophy in a mule deer population. J Wildl Dis. 1997;33:420–429. doi: 10.7589/0090-3558-33.3.420. [DOI] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104(suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Daele LJ, McDonald MG. 2001. Kodiak and adjacent islands. In: Deer (Hicks MV, ed). Survey Management Report for July 1998 to June 2000. Juneau, AK:Alaska Department of Fish and Game, 93–108.
- Veeramachaneni DNR. Deteriorating trends in male reproduction: idiopathic or environmental? Anim Reprod Sci. 2000;60–61:121–130. doi: 10.1016/s0378-4320(00)00113-5. [DOI] [PubMed] [Google Scholar]
- Veeramachaneni DNR. Germ cell atypia in undescended testes hinges on the aetiology of cryptorchidism but not the abdominal location per se. Int J Androl. 2006;29:235–240. doi: 10.1111/j.1365-2605.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- Veeramachaneni DNR, Ott RS, Heath EH, McEntee K, Bolt DJ, Hixon JE. Pathophysiology of small testes in beef bulls: relationship between scrotal circumference, histopathologic features of testes, and epididymides, seminal characteristics, and endocrine profiles. Am J Vet Res. 1986;47:1988–1999. [PubMed] [Google Scholar]
- Veeramachaneni DNR, Palmer JS, Tessari JD, Awoniyi CA. Cellular manifestations of undescended testes depend upon the etiology of cryptorchidism but not the abdominal location per se [Abstract] J Androl. 2001;22(suppl):168. [Google Scholar]
- West NO, Nordan HC. Hormonal regulation of reproduction and antler cycle in the male Columbian black-tailed deer (Odocoileus hemionus columbianus). Part I. Seasonal changes in the histology of the reproductive organs, serum testosterone, sperm production, and the antler cycle. Can J Zool. 1976;54:1617–1636. doi: 10.1139/z76-189. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Furr J, Ostby J, Wood C, Held G, et al. Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicol Lett. 2004;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JMA, Brinkmann AO, Nayernia K, Holsetin AF, et al. Targeted disruption of the insl3 gene causes bilateral cryptorchidism. Mol Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]