Abstract
The human cytomegalovirus (HCMV) glycoprotein US2 specifically binds to major histocompatibility complex (MHC) class I heavy chain (HC) and class II proteins DRα and DMα, triggering their degradation by proteasomes. Effects of US2 on class II proteins were originally characterized in HCMV- or adenovirus vector-infected U373 astroglioma cells. Here, we have extended characterization of US2-mediated degradation of class II DRα to two other cell lines, including biologically relevant epithelial cells. Comparison of the effects of US2 in cells expressing both class I and II proteins demonstrated only a slight preference for class I HC. Moreover, US2 caused degradation of DRα and DMα when these proteins were expressed by transfection without DRβ, invariant chain (Ii), or DMβ. Therefore, US2 binds to α chains of DR and DM and triggers endoplasmic reticulum degradation without formation of class II DR αβ/Ii or DM αβ complexes. Similar levels of degradation of class II α were observed in cells expressing vastly different amounts of class II, suggesting that cellular factors, other than class II, were limiting. We concluded that US2 has broad effects in a variety of cells that express both class I and II proteins and is relevant to HCMV infection in vivo.
Human cytomegalovirus (HCMV) is a ubiquitous β-herpesvirus that causes lifelong infections characterized by low-level persistence, latency, and bouts of reactivation (6). HCMV expresses a plethora of proteins that allow escape from host immunity, and these likely play a major role in persistence. The HCMV genome contains a cassette of genes in the US2 to US11 region that encode eight homologous glycoproteins. Five of these, US2, US3, US6, US10, and US11, inhibit the major histocompatibility complex (MHC) class I pathway and antigen presentation to CD8+ T lymphocytes (1, 2, 17, 24, 33, 34, 36, 52, 53). We have proposed that these and other HCMV immune evasion strategies function during a specific time of viral life cycle, or in specific cells, creating a window of opportunity for transient virus replication and spread (reviewed in references 22, 30, and 31).
HCMV not only blocks MHC class I-mediated presentation of viral antigens to CD8+ T cells but also inhibits the class II antigen presentation pathway, allowing the virus to avoid detection by CD4+ T cells (reviewed in references 22 and 31). MHC class II proteins are normally expressed by professional antigen-presenting cells (APCs) like B cells, macrophages, and dendritic cells (DCs). HCMV may infect certain APCs, especially monocyte-macrophages, but only at a specific stage of activation and often abortively or inefficiently (reviewed in reference 29). HCMV also replicates in epithelial cells of alimentary and upper respiratory tracts, vascular endothelial cells, and glial cells (reviewed in reference 29), cells that express MHC class II naturally, especially after induction by gamma interferon (IFN-γ). HCMV inhibits IFN-γ signaling and induction of class II promoters, reducing the inducible expression of class II genes (7, 37, 39). However, even before this inhibition of class II transcription, HCMV early glycoproteins US2 and US3 inhibit class II antigen presentation by destroying or abolishing the functions of class II proteins (8, 9, 23, 50). US2 binds to class II DR and causes rapid and efficient proteosome-mediated degradation of only the α chain of the class II DR αβ complex (8, 9, 50). US2 also causes degradation of the α chain of DM (50), an MHC class II complex required for loading of antigenic peptides onto class II DR complexes (reviewed in reference 42). HCMV US3 binds to class II DR αβ heterodimers, inhibiting binding of the invariant chain (Ii), leading to intracellular mislocalization and reduced peptide loading of DR complexes (23).
Our laboratory's previous characterization of the effects of US2 on class II proteins involved U373 astroglioma cells expressing the human class II transactivator (CIITA) and infected with either HCMV or with replication-defective adenovirus (Ad) vectors (8, 9, 23, 50). Recent studies involving bacterially produced, in vitro-translated, or Ad-expressed US2 lead to suggestions that US2 might not cause degradation of class II proteins in vitro or in DCs (19, 20, 43). These authors also suggested that US2 might not generally affect class II in other cells and that effects might be limited to U373 cells, and they suggested that glial cells do not normally express class II. In fact, glial cells and other cell types that HCMV infects frequently in vivo, including epithelial and endothelial cells, express class II proteins, although generally lower levels are produced compared with DCs and expression is often upregulated by IFN-γ (reviewed in reference 22). DCs and other so-called professional APCs such as monocyte-macrophages are probably much less relevant in terms of the effects of HCMV US2 and US3. These cells are poorly infected by HCMV, expression of HCMV gene products is often relatively low, and class II proteins are expressed at high levels (29). Rehm et al. (43) found insufficient levels of US2 were expressed by using Ad vectors, so that neither class I or class II proteins were substantially affected. It is not clear whether US2 might have caused degradation of class II in DCs had enough US2 been delivered.
To characterize whether US2 has general effects on MHC class II proteins in a variety of cells, we characterized US2-mediated degradation of class I and II proteins in several different cell lines, including two human epithelial cell lines. US2 showed only a slight preference for class I compared with class II proteins. US2 triggered efficient degradation of the class II DRα and DMα proteins when these were expressed without the DRβ or DMβ chains. Cells with substantial differences in levels of class II proteins displayed similar degradation at any given level of US2, supporting the hypothesis that cellular factors apart from MHC proteins are limiting in US2-induced endoplasmic reticulum (ER) degradation.
MATERIALS AND METHODS
Cells.
All cell lines were propagated in media supplemented with 10% fetal bovine serum (HyClone, Logan, Utah), 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin (Biowhittaker, Walkersville, Md.)/ml, unless otherwise stated. HeLa, U373-MG (American Type Culture Collection, Manassas, Va.), and 293 (Microbix, Toronto, Ontario, Canada) cells were propagated in Dulbecco's modified Eagle's medium (DMEM; Mediatec, Herndon, Va.). His16 and Neo6 cells were derived by stable transfection of the human astroglioma cells U373-MG with the human class II transactivator CIITA and pSV2His or pSV2Neo, respectively (23, 50). His16 cells were propagated in DMEM lacking histidine (University of California San Francisco Cell Culture Facility, San Francisco, Calif.) and supplemented with 0.5 mM histidinol (Sigma, St. Louis, Mo.). Neo6 cells were grown in DMEM supplemented with 200 μg of G418 sulfate (GIBCO)/ml. HeLa-CIITA cells (48) were a gift from Philip Benaroch, Institut Curie, Paris, France, and were grown in DMEM supplemented with 300 μg of hygromycin (Sigma)/ml. 2E12 cells (5), which are AGS human gastric carcinoma cells stably transfected with CIITA, were a gift from Lindsey Hutt-Fletcher, University of Missouri, Kansas City, and were propagated in Ham's F12 medium supplemented with 0.4 μg of puromycin/ml.
Recombinant Ad's.
Replication-defective (E1−) Ad vectors expressing HCMV US2, US3, US7, and US11 have been described previously (23, 26, 50). Briefly, the relevant genes were amplified by PCR from HCMV strain AD169 and inserted into plasmid pΔE1sp1Btet (47). 293 cells were cotransfected with pJM17, a plasmid containing the Ad type 5 genome (38), and each of the above plasmids. Recombinant Ad vectors were screened by PCR. The resulting viruses, AdtetUS2, AdtetUS3, AdtetUS7, and AdtetUS11, were plaque purified twice to eliminate wild-type virus. For expression of the glycoproteins, cells were coinfected with these viruses and a second virus, Adtet-trans, using 20% of the amount of AdtetUS virus. Infections were carried out in the absence of tetracycline, which represses the tet-trans promoter. AdBHG10T7Rβgal (Adβgal [49]) was obtained from Frank Graham, McMaster University, Hamilton, Ontario, Canada. Adβgal was constructed by recombination between a shuttle plasmid containing the T7 promoter, β-galactosidase gene, and simian virus 40 poly(A) sequences and the circular Ad5 genomic plasmid pBHG10, as described elsewhere (49). Other recombinant Ad's (E1−, E3−) encoding US11 and US2 (43) were obtained from Armin Rehm, Max Delbruck Center for Molecular Medicine, Berlin, Germany. Briefly, the genes were coupled to the HCMV immediate-early promoter and bovine growth hormone poly(A) signal in a shuttle vector. Recombination with the viral vector was performed in Escherichia coli for US11 and in 293 cells for US2 (43). We refer to these viruses as AdUS2 and AdUS11, as opposed to our tetracycline transactivator-regulated AdtetUS viruses. All Ad vectors were propagated and titers were determined on 293 cells as described previously (25).
Plasmids and antibodies.
Eukaryotic expression constructs encoding human leukocyte antigen (HLA)-DRα or HLA-DRβ (14) were gifts from Elizabeth Mellins, Stanford University, Palo Alto, Calif. Rabbit antiserum to the N-terminal peptide of US2 has been described previously (50). The monoclonal antibody (MAb) HC10, specific for class I heavy chain (HC) (46), was obtained from Hidde Ploegh, Harvard Medical School, Boston, Mass. MAb's to HLA-DRα (DA6.147) (21) and HLA-DRβ (HB10A) (10) were gifts from Peter Cresswell, Yale University, New Haven, Conn. The MAb to HLA-DMα (45) was a gift from John Trowsdale, Cambridge University, Cambridge, United Kingdom. The MAb (B3/25) to human transferrin receptor was obtained from Roche Molecular Corp., Indianapolis, Ind.
Transfections.
For transient expression of class II proteins, HeLa cells were transfected with plasmid DNA containing relevant genes by using the Geneporter 2 transfection reagent, according to the manufacturer's recommendations (Gene Therapy Systems, San Diego, Calif.). Briefly, 106 cells were seeded into 60-mm Falcon tissue culture dishes (Becton Dickinson, Franklin Lakes, N.J.) and transfected the next day with 4 to 6 μg of DNA in serum-free Opti-MEM (GIBCO). For expression of HCMV glycoproteins, the transfected cells were infected with Ad vectors 48 to 60 h posttransfection after the cells had reached 4 × 106 cells/dish.
Metabolic labeling of cells and immunoprecipitation of proteins.
Cells were radiolabeled after 18 to 20 h of infection with recombinant Ad vectors. In general, cells were plated in 150-mm Nunclon tissue culture dishes (Nalge Nunc International, Rochester, N.Y.) at a density of 6 × 106 (His16, Neo6, or 2E12) or 15 × 106 (HeLa-CIITA) cells per dish and infected the next day. Infected or control cells were washed in DMEM lacking methionine and cysteine (GIBCO) and then incubated for 1 h in the same medium containing 1% dialyzed fetal bovine serum (starvation medium). Cells were labeled for various time periods in starvation medium supplemented with [35S]methionine-cysteine (Promix; Amersham Pharmacia Biotech, Piscataway, N.J.). For longer periods, e.g., 3 h, adherent cells were labeled in tissue culture dishes with 50 to 100 μCi of [35S]methionine-cysteine/ml. For short pulse labels, cells were trypsinized, washed twice in DMEM lacking methionine and cysteine, and labeled in suspension with 500 μCi of [35S]methionine-cysteine/ml. To chase the label, cells were incubated in medium containing 10-fold excess methionine and cysteine. The cells were then washed with cold phosphate-buffered saline, and cell extracts were made using Nonidet P-40-deoxycholate (NP-40-DOC) lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1% NP-40, 0.5% DOC, 1 mg of bovine serum albumin [BSA]/ml, and a cocktail of protease inhibitors). For denaturation of protein complexes, cell extracts were made in small volumes with sodium dodecyl sulfate (SDS) lysis buffer comprised of 50 mM Tris (pH 7.4), 100 mM NaCl (Tris-saline), 1% SDS, 1 mg of BSA/ml, and a cocktail of protease inhibitors and boiled for 10 min. SDS was then diluted 10-fold by addition of Tris-saline containing 1% Triton X-100, 1 mg of BSA/ml, and a cocktail of protease inhibitors. Proteins of interest were immunoprecipitated by incubating cell extracts with the appropriate antibody for 2 h and subsequently with protein-A agarose beads for 2 h, as described previously (8, 9, 23, 50).
Electrophoresis and autoradiography.
Immunoprecipitated proteins were eluted by boiling in 2% SDS and then subjected to electrophoresis using 10 to 12% polyacrylamide gels, as described elsewhere (8, 9, 23, 50). The gels were fixed in 30% acetic acid and 10% methanol and treated with Enlightning (New England Nuclear, Beverly, Mass.), dried, and exposed to X-ray film (Eastman Kodak Company, Rochester, N.Y.) or PhosphorImager screens (Molecular Dynamics, Sunnyvale, Calif.). Protein bands were quantified by using the PhosphorImager BAS2500 system (Molecular Dynamics).
RESULTS
Class II complexes are lost in a dose-dependent manner in US2-expressing epithelial and glial cells.
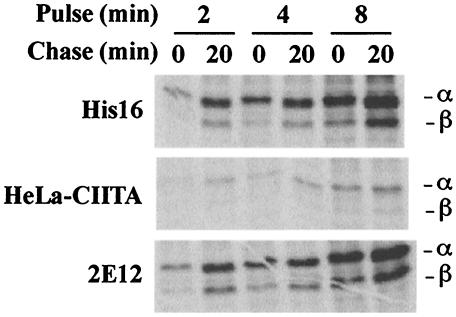
His16 cells are U373 cells transfected with CIITA and express substantial quantities of class II α, β, and Ii, and they efficiently present antigens to CD4+ T cells (23, 50). To extend our observations beyond His16 cells, we obtained HeLa cervical carcinoma and AGS gastric carcinoma cells that had both been stably transfected with CIITA (5, 48). Initially, the levels of expression of class II proteins were examined in a pulse-chase labeling format. His16, HeLa-CIITA, and 2E12 (AGS-CIITA) cells were labeled in suspension for 2, 4, or 8 min, and the label was chased for 0 or 20 min. All three cell types expressed class II proteins, although at lower levels in the HeLa-CIITA cells (Fig. 1). In longer labeling periods and in chase samples, the β chain was coprecipitated with α chains by MAb DA6.147, an antibody that recognizes the cytoplasmic domain of the class II α chain but that can also precipitate αβ complexes. With all the cell lines, the detection of class II proteins increased with longer periods of labeling or chase (Fig. 1), due in part to acquisition of antibody epitopes (8, 9, 23, 50). Normalization of class II expression to cell number indicated that HeLa-CIITA and 2E12 cells express ≈10% and ≈60% of class II proteins compared to His16 cells. The respective parental cell lines expressed much lower levels of class II proteins (data not shown), although it should be noted that U373 cells (without CIITA transfection) less efficiently present antigens to CD4+ T cells (49).
FIG. 1.
Expression of class II proteins by various cell lines. His16, HeLa-CIITA, and 2E12 cells were labeled in suspension for 2, 4, or 8 min with [35S]methionine-cysteine, and the label was chased for 0 or 20 min. NP-40-DOC cell extracts derived from 6 × 106 (His16, 2E12) or 15 × 106 (HeLa-CIITA) cells were mixed with anti-DRα MAb DA6.147, and MHC class II complexes immunoprecipitated.
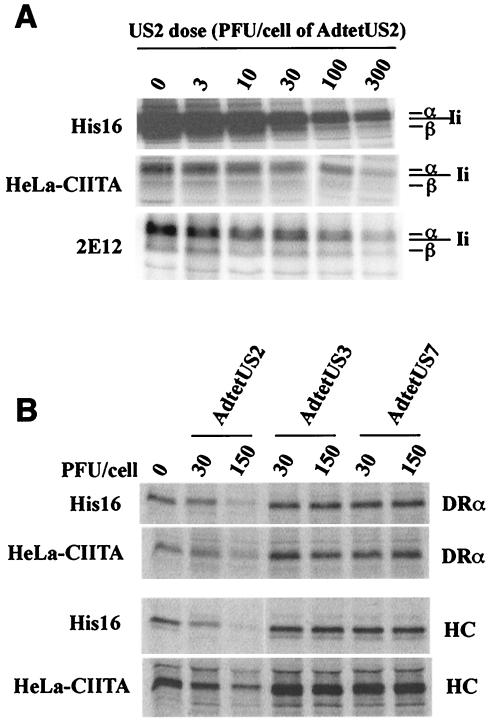
Replication-defective Ad vectors have been used to study the biochemical effects of HCMV proteins in U373 cells (8, 9, 23, 26, 50) because infection of U373 cells with HCMV is frequently inefficient. Here, we have used an Ad vector to deliver US2 into HeLa-CIITA and 2E12 epithelial cells, as well as His16 glial cells, and in every case class II was lost in a US2 dose-dependent manner (Fig. 2A). The control Ad vector, Adtet-trans, slightly increased the amount of class II in a dose-dependent fashion (data not shown), similar to previous observations from our laboratory (8, 9). Since it has been suggested that the effects of US2 were related to overexpression of a glycoprotein that accumulated in the ER or to consequences of CIITA expression (43), we compared the effects of US2 to HCMV US3 and US7, homologous glycoproteins that do not cause degradation of MHC proteins (23). There was no obvious effect of expressing US3 or US7 in either His16 or HeLa-CIITA cells (Fig. 2B). Similar results were also obtained with Neo6 and 2E12 cells (data not shown). Therefore, US2 causes degradation of class II α with similar doses in several cell types.
FIG. 2.
US2 causes loss of class I and II proteins. (A) His16, HeLa-CIITA, or 2E12 cells were left uninfected (0 PFU/cell) or were infected with AdtetUS2 and Adtet-trans using 3 and 0.6, 10 and 2, 30 and 6, 100 and 20, or 300 and 60 PFU/cell, respectively, for 18 h. Infected cells were labeled with [35S]methionine-cysteine for 3 h, and cell extracts were made using NP-40-DOC lysis buffer. Class II complexes were immunoprecipitated from 6 × 106 (His16, 2E12) or 15 × 106 (HeLa-CIITA) cells using MAb DA6.147. (B) His16 (6 × 106) and HeLa-CIITA (15 × 106) cells were left uninfected (0 PFU/cell) or were infected with AdtetUS2, AdtetUS3, or AdtetUS7, in each case with Adtet-trans using 30 and 6 PFU/cell or 150 and 30 PFU/cell, respectively, for 18 h. Infected cells were labeled with [35S]methionine-cysteine for 1 min, and the label was chased for 20 min. Cell extracts were made with 1% SDS lysis buffer and denatured at 100°C, and the SDS was diluted 10-fold with 1% Triton X-100 buffer. Lysates were divided equally into two, and class I HC or class II α chain (DRα) was immunoprecipitated with the MAb's HC10 or DA6.147, respectively.
Degradation of class II α chains in cells expressing only DRα or DMα.
US2 triggers degradation of the class II α chain shortly after its synthesis while not affecting β and Ii chains (50). However, it is not clear whether US2 binds preferentially to αβ or αβ/Ii complexes or whether β or Ii are required for degradation. To analyze this further, we transiently transfected HeLa cells with plasmids encoding just DRα, DRβ, or DMα chains and then delivered various doses of US2. When expressed alone in HeLa cells, DRα was degraded in a dose-dependent manner but DRβ was not affected by US2 expression (Fig. 3). When both α and β were cotransfected into HeLa cells (HeLa/DRα/DRβ), both the α and β chains disappeared because an antibody specific to the α chain was used and the α chain was lost. US2 also caused degradation of DMα when DMα was expressed without DMβ in HeLa cells (Fig. 3, lower panel). Therefore, US2 can bind to and promote degradation of DRα and DMα without other components of these class II complexes.
FIG. 3.
US2 causes degradation of class II α when expressed without the β chain. HeLa cells were transiently transfected with plasmids containing either the class II α (HeLa/DRα) or β genes (HeLa/DRβ), or both plasmids (HeLa-DRα/DRβ), or one containing the DMα gene (HeLa-DMα). After 48 h, these transfected HeLa cells (4 × 106) and His16 cells (1 × 106) were left uninfected (0 PFU/cell) or infected with 3 and 0.6, 10 and 2, 30 and 6, 100 and 20, or 300 and 60 PFU/cell of AdtetUS2 and Adtet-trans, respectively, for 18 h. The cells were then labeled with [35S]methionine-cysteine for 3 h. Class II α was immunoprecipitated using MAb DA6.147, and class II β was immunoprecipitated with MAb HB10A. αβ complexes were precipitated from HeLa cells transfected with both DRα and DRβ plasmids with MAb DA6.147, and DMα was precipitated with MAb 5C1. Note that fewer His16 cells were used, and extracts of these cells were made using SDS buffer, as in Fig. 2, allowing selective precipitation of DRα but reducing binding of MAb DA6.147 compared with extracts of transfected HeLa cells made using NP-40-DOC buffer (see Fig. 4A).
Evidence for a limiting cellular factor.
Previous studies from our laboratory, involving mutant forms of US2, demonstrated that binding of US2 to MHC class I and II proteins was not sufficient for their degradation (8). This and other lines of research (18) have suggested that cellular factors or events beyond binding of US2 to MHC proteins are required for the degradation process. This is an important area of investigation because US2- and US11-mediated degradation of MHC proteins is a model for ER degradation in mammalian cells (54). His16 and 2E12 cells expressed substantially higher levels of class II proteins than HeLa cells transfected with the DRα gene. Therefore, we reasoned that US2 might cause much more rapid degradation in transfected HeLa cells, unless some cellular factor was limiting.
First, to quantify the levels of expression of DRα in His16 cells and transiently transfected HeLa cells, cells were labeled for 3 h and DRα was immunoprecipitated using the α-specific antibody DA6.147. In the left panel of Fig. 4A, class II α/β/Ii complexes were precipitated using MAb DA6.147 from His16 cell extracts made using NP-40-DOC lysis buffer. Under these conditions, the α and Ii chain bands form a tight doublet. In the middle lane, class II complexes were first denatured with SDS to separate Ii and α chains and then immunoprecipitated with MAb DA6.147. Clearly, DA6.147 much less efficiently precipitated denatured DRα under these conditions, but the band indicates the position of the α chain in this gel. In the right lane, DRα was precipitated from transfected HeLa extracts made using NP-40-DOC buffer (there is no β or Ii in these cells). To compare the amount of α chain in the left and right lanes, we used a PhosphorImager to quantify the entire α/Ii band from His16 cells and then calculated the contribution of the α chain to the band intensity, based on the relative quantities of methionine and cysteine. This difference, combined with the fact that fourfold more HeLa-DRα cells were used, established that His16 cells expressed 20- to 40-fold more class II DRα than was the case in transfected HeLa cells. In Fig. 1 we established that 2E12 cells express approximately 60% the class II DRα that is expressed in His16 cells.
FIG. 4.
Similar degradation in cells with different amounts of class II proteins. (A) His16 cells (1 × 106) or DRα-transfected HeLa cells (4 × 106) were labeled with [35S]methionine-cysteine for 3 h. His 16 cells were lysed in NP-40-DOC buffer (left lane denoted α/β/Ii)) or in buffer containing 1% SDS, and the extract was denatured at 100°C before the SDS was diluted with 1% Triton X-100 buffer (middle lane, denoted α). Transfected HeLa cells (HeLa-DRα) were lysed in NP-40-DOC buffer. All samples were immunoprecipitated with anti-DRα MAb DA6.147. (B) His16 or 2E12 cells (1 × 106) or transfected HeLa cells (4 × 106) were left uninfected (0 PFU/cell) or infected with 3 and 0.6, 10 and 2, 30 and 6, 100 and 20, or 300 and 60 PFU of AdtetUS2 and Adtet-trans/cell, respectively, for 18 h. Infected cells were labeled with [35S]methionine-cysteine for 3 h, extracts were made in every case with 1% SDS lysis buffer, proteins were denatured, and the SDS was diluted with 1% Triton X-100 buffer. Class II α or β chains were immunoprecipitated from the cell extracts with anti-α MAb DA6.147 or anti-β MAb HB10A, respectively. The intensities of protein bands were quantified by phosphorimager analyses. The data are the averages of three independent experiments, and the density of protein bands obtained from cells left uninfected (no AdtetUS2) was considered 100%. Data points without error bars are due to standard deviations that are too small for depiction. (C) Cells were labeled as in panel B, and US2 was immunoprecipitated with rabbit polyclonal serum to an N-terminal peptide of US2. The amount of US2 was quantified by phosphorimager analyses from the three experiments depicted in panel A. (D) The effects of US2 on class II DMα were analyzed as for panel B, except that HeLa cells were transfected with a plasmid containing the DMα gene and compared to His16 cells that also express DM. Immunoprecipitation was performed with anti-DMα MAb 5C1.
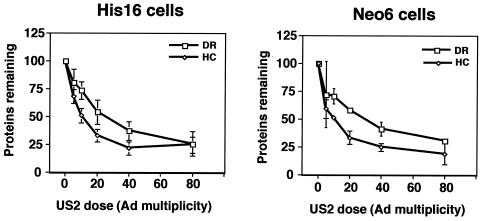
Different doses of US2 were delivered into 2E12, His16, or HeLa cells transfected with DRα or DRβ. The loss of DRα was similar in the three different cells (Fig. 4B), even though there were 10- to 40-fold more class II proteins expressed in 2E12 and His16 cells. No loss of DRβ was observed. Expression of US2 was very similar in His16 and transfected HeLa cells at any dose of AdtetUS2 (Fig. 4C), and similar levels of US2 were observed in 2E12 cells (data not shown). Further, in His16 cells and HeLa cells transfected with the DMα gene, degradation of class II DMα was similar or identical at any given dose of US2 (Fig. 4D). Thus, degradation of both DRα and DMα occurred with a similar US2 dose response, even though substantially different amounts of the MHC class II proteins were present in cells. This supports the existence of cellular factors other than MHC proteins and US2 that are involved and limiting in US2-induced ER degradation of MHC proteins.
Relative effects of US2 on class I HC versus class II α chain.
Based on in vitro experiments involving recombinant forms of US2 (19, 20), it was suggested that US2 only affects MHC class I HC and has little or no effect on class II α. In mammalian cells, however, our group has found that US2 affects both proteins (8, 9, 23, 50). The relative effects of US2 on MHC class I and II proteins have not been compared in cells that express both and where different doses of US2 could be delivered. His16 cells and Neo6 cells, a second CIITA-transfected U373 cell line that expresses approximately half the class II expressed in His16 cells (23), were analyzed. When these cells were infected with low to intermediate doses of AdtetUS2 (10 to 40 PFU/cell), more class I HC was degraded than class II DRα in both His16 and Neo6 cells (Fig. 5). However, the preference for HC was only 1.5- to 2-fold greater than that for class II α. At higher doses of US2 (80 PFU/cell), both proteins were efficiently degraded.
FIG. 5.
US2 shows preference for class I HC over class II α chain. His16 or Neo6 cells (6 × 106) were left uninfected (0 PFU/cell) or infected with 5 and 1, 10 and 2, 20 and 4, 40 and 8, or 80 and 16 PFU/cell of AdtetUS2 and Adtet-trans, respectively, for 18 h. Cells in suspension were labeled for 1 min, the label was chased for 20 min, cell extracts were prepared using SDS lysis buffer, proteins were denatured, and the SDS was diluted as described in the legend for Fig. 4. Extracts were then divided equally into two, and class II α (DRα) or class I HC was immunoprecipitated with MAbs DA6.147 or HC10, respectively. The amount of α or β that remained after expression of each dose of US2 expression was quantified by phosphorimager analyses and compared to that in uninfected cells (100%). The data represent the averages of three independent experiments. Data points without error bars are due to standard deviations that are too small for depiction.
Comparison of Ad vectors expressing US2.
The report of Rehm et al. (43) suggested that an Ad vector expressing US2 did not affect the stability of class II DR proteins in DCs. In preliminary experiments, we were unable to substantially express US2 or US3 by using Ad vectors in monocyte-macrophages or DCs cultured from blood (R. Tomazin and N. Hegde, unpublished data). Low levels of US2 were expressed in these cells, but there was no obvious effect on MHC proteins or antigen presentation (Tomazin and Hegde, unpublished). Our Ad vectors, e.g., AdtetUS2 and AdtetUS11, rely on the use of the HCMV immediate-early (IE) promoter that is upregulated by coinfecting cells with a second Ad vector expressing a tetracycline transactivator protein (8, 26, 50). Rehm et al. constructed AdUS2 and AdUS11 that rely on constitutive activity of the HCMV IE promoter (43). We found that Ad vectors utilizing constitutive promoters coupled to HCMV US2 to US11 genes produced poor yields, apparently because these ER-retained US2 to US11 proteins were toxic to 293 cells used to produce the Ad viruses (reference 26 and data not shown).
To compare our Ad vectors with those of Rehm et al., His16 cells were infected with AdtetUS2 or AdUS2 of Rehm et al. (43). Both US2-expressing Ad vectors caused degradation of class II DRα and class I HC in these cells (Fig. 6A). However, AdUS11 (43) also reduced DRα in these cells, whereas our AdtetUS11 did not. Since AdUS11 grew to significantly lower titers, larger amounts of virus stock were required in order to obtain similar levels of US11 expression (data not shown). Moreover, AdUS11 inhibited expression of an unrelated cellular protein, the transferrin receptor, but this was not the case with AdtetUS11 (Fig. 6B; compare to Ad-βgal lanes). US2 delivered by using AdUS2 also markedly reduced the stability of class II DRα in HeLa-CIITA and 2E12 epithelial cells (data not shown). We concluded that US2 delivered by both Ad vectors caused degradation of both class I and II proteins in a variety of cells, and this cannot be the reason for any discrepancies.
FIG. 6.
Different Ad vectors expressing US2 cause degradation of MHC proteins. His16 cells (6 × 106) were infected with 50 PFU/cell of Ad viruses AdUS2 and AdUS11 (described by Rehm et al. [43]) or Adβ-gal (described by Tomanin et al. [49]), or AdtetUS2, AdtetUS3, or AdtetUS11 (described elsewhere by our group [23, 50]). The cells were labeled in suspension with [35S]methionine-cysteine for 1 min, and the label was chased for 20 min. Cell extracts were made using SDS lysis buffer, proteins were denatured, and the SDS was diluted as described in the legend for Fig. 4. Extracts were divided into three, and class II α chain (DRα), class I HC (panel A), or transferrin receptor (TfR; panel B) were immunoprecipitated with MAbs DA6.147, HC10, or B3/25, respectively.
DISCUSSION
The results presented here demonstrate that US2 causes degradation of MHC class II α in several different cells: gastric epithelial cells, cervical carcinoma cells, and two different clones of astroglioma cells. The extent of degradation of class II α was similar or identical with each cell type at any given dose of US2. In cells expressing both class I and II proteins, there was a 1.5- to 2-fold preference for class I HC over class II α. The half-life of class II α was reduced from ≈45 to 60 min down to 2 to 5 min by expressing intermediate levels of US2 (data not shown). Other homologous HCMV glycoproteins, US3 and US7, had no effect on the stability of MHC proteins. Therefore, US2 causes degradation of class II proteins in several relevant cells and does not show a high degree of preference for MHC class I versus class II.
The second important set of observations involved US2-mediated degradation of class II DRα and DMα in cells expressing these α chains alone, i.e., without β and Ii chains. There was no detectable effect on the DRβ chain in cells expressing DRβ alone, providing an important negative control for nonspecific effects of US2. These results have important implications for our understanding of how US2 functions. Normally, DRα and DRβ chains associate rapidly in the ER and fold extensively to form αβ complexes (reviewed in reference 42). Obviously, US2 binding does not depend upon protein surfaces that evolve after αβ complex formation. Instead, US2 can bind directly to free α chains. This highlights the rapid nature of US2-DRα interactions in the ER. These results also ruled out the suggestion that U373 cells transfected with CIITA express disproportionate amounts of class II proteins, leading to induction of unfolded protein response and ER degradation (43), because the transiently transfected HeLa cells do not express CIITA.
Cells that contained substantially different amounts of class II α exhibited similar levels of degradation of α chain at any given concentration of US2. These observations support the hypothesis that US2 triggers processes leading to ER-associated degradation by recruiting cellular proteins onto MHC proteins. The cellular factors limiting in US2-mediated degradation are unlikely to include components of the Sec61 protein translocon because US2 expression at high levels does not apparently affect Sec61-mediated forward translocation of proteins into the ER. The cytoplasmic tail of US2 is required for degradation of MHC proteins (8, 51), and our group recently showed that swapping this domain in place of the US3 cytoplasmic tail produced a chimera that could cause degradation of MHC proteins (9). Unlike wild-type US3, US2/US3 chimeras capable of degrading MHC proteins bound p97 adenosine triphosphatase (ATPase) (9), a protein involved in the extraction of ER proteins into the cytoplasm (54). Therefore, p97 ATPase may be a candidate cellular protein that is limiting in the US2-provoked retrotranslocation and degradation pathway, but others are also likely to be involved.
One major question that prompted these studies was whether or not US2 affects class II generally in any cell, in a subset of cells, or not at all. Based on extrapolation of structural analyses of the binding of US2 to class I HC, Gewurz et al. (19) predicted that US2 should bind to the class II β chain, rather than the α chain, and this group reported that US2 failed to bind to or cause degradation of class II complexes in vitro (20, 43). However, in these in vitro studies, US2 also failed to destabilize certain class I proteins that are known to be degraded in US2-expressing mammalian cells (20). Thus, these in vitro and structural studies failed to reproduce several effects of US2 previously observed in mammalian cells. In the studies of Rehm et al. (43), relatively large input doses (500 PFU/cell) of an Ad vector expressing US2 were used in attempts to deliver US2 into DCs. There was little or no effect on the stability of MHC class I HC, compared with control cells, although band intensities were not quantified (see Fig. 4C in reference 43). Given the well-established effects of US2 on class I HC in other cells, it appears that inadequate amounts of US2 were delivered, and any conclusions about whether or not US2 can inhibit class II in DCs or generally in other cell types are not warranted. Alternatively, DCs may lack factors required by US2 to cause class I and II degradation.
We did not pursue efforts to test US2's effects in DCs here for several reasons. First, DCs and monocyte-macrophages are inefficiently infected by HCMV, especially when these cells are cultured from blood and infected by laboratory strains of HCMV passaged on fibroblasts (29, 44). Moreover, Ad vectors often do not infect DCs well (40, 41). For example, the human immunodeficiency virus nef protein can reduce cell surface expression of MHC class I molecules in a variety of cells (reviewed in reference 16) but did not affect class I when expressed by Ad vectors in DCs (13). Our preliminary efforts to transduce monocyte-macrophages and DCs cultured with Ad vectors lead to the conclusion that HCMV proteins could not be delivered effectively (R. A. Tomazin and N. R. Hegde, unpublished results). Therefore, we do not know whether US2 functions in DCs or macrophages to inhibit class II antigen presentation.
The question of whether US2 functions in professional APCs is likely irrelevant for at least two reasons. First, the high levels of MHC proteins expressed in APCs, coupled with low levels of viral gene expression, make it difficult to imagine that US2, US3, and other HCMV immune evasion proteins could be effective in these cells in vivo. Second, US2 and US3 are intracellular membrane proteins that function only in HCMV-infected cells. In vivo, only a small fraction of APCs are infected (reviewed in reference 29), and other, uninfected APCs are free to take up HCMV antigens and effectively prime or initiate immune responses. Consistent with this, there are robust and sustained anti-HCMV CD4+ T-cell responses in humans (reference 4 and references therein). Therefore, it is highly unlikely that HCMV benefits from inhibiting the class II pathway in APCs.
Rather, we believe that US2 and US3 function to prevent class II-mediated presentation of endogenous viral antigens in cells such as endothelial, glial, or epithelial cells or other class II-expressing cells that are important hosts for HCMV replication in vivo. Herpesviruses extensively target their structural proteins to endosomal compartments (reviewed in reference 32), where peptides destined to be loaded onto class II are generated (reviewed in references 22 and 31). We have evidence for highly efficient loading of peptides derived from endogenous HCMV antigens, e.g., gB, onto class II proteins (C. Dunn, D. M. Lewinsohn, and D. C. Johnson, unpublished results). Such presentation will cause endothelial or epithelial cells to be recognized by anti-HCMV CD4+ T cells that can lyse the cells or produce antiviral cytokines. Therefore, we believe that the real function of US2 and US3 is to prevent presentation of endogenous HCMV antigens via the class II pathway and recognition of virus-producing cells, rather than presentation of exogenous antigens by professional APCs (reviewed in references 22 and 31).
The observations that US2 targets several distinct MHC proteins (3, 33, 50) is not novel. US3 affects classical and nonclassical class I proteins, as well as class II proteins (23, 34, 35). HCMV UL16 affects MIC-B, UL16-binding protein-1 (ULBP-1), and ULBP-2 (15). Human immunodeficiency virus nef targets CD4 and class I molecules for demise in lysosomes (16) and alters sorting and peptide loading of class II proteins (48). The Kaposi's sarcoma herpesvirus K3 and K5 proteins target MHC class I proteins HLA-A, -B, -C, and -E for internalization from the cell surface followed by degradation (11, 28), and K5 also downregulates B7-2 and ICAM-1 (12, 27). Therefore, many viral immunomodulatory proteins have apparently evolved to affect multiple molecules that exhibit only limited homology but with shared structural features.
Acknowledgments
We are thankful to Peter Cresswell, Klaus Früh, John Trowsdale, and Hidde Ploegh for antibodies and to Betsy Mellins for plasmids. We are grateful to Armin Rehm for providing recombinant Ad vectors. We are also indebted to Lindsey-Hutt Fletcher and Philip Benaroch for gifts of 2E12 and HeLa-CIITA cells.
This work was supported by National Institutes of Health grants EY11245 and CA73996.
REFERENCES
- 1.Ahn, K., A. Angulo, P. Ghazal, P. A. Peterson, Y. Yang, and K. Fruh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. L. Ploegh, P. A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Arieh, S. V., B. Zimerman, N. I. Smorodinsky, M. Yaacubovicz, C. Schechter, I. Bacik, J. Gibbs, J. R. Bennink, J. W. Yewdell, J. E. Coligan, H. Firat, F. Lemonnier, and R. Ehrlich. 2001. Human cytomegalovirus protein US2 interferes with the expression of human HFE, a nonclassical class I major histocompatibility complex molecule that regulates iron homeostasis. J. Virol. 75:10557-10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitmansour, A. D., S. L. Waldrop, C. J. Pitcher, E. Khatamzas, F. Kern, V. C. Maino, and L. J. Picker. 2001. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J. Immunol. 167:1151-1163. [DOI] [PubMed] [Google Scholar]
- 5.Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. [DOI] [PubMed] [Google Scholar]
- 6.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2524. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Baltimore, Md.
- 7.Cebulla, C. M., D. M. Miller, Y. Zhang, B. M. Rahill, P. Zimmerman, J. M. Robinson, and D. D. Sedmak. 2002. Human cytomegalovirus disrupts constitutive MHC class II expression. J. Immunol. 169:167-176. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier, M. S., G. M. Daniels, and D. C. Johnson. 2002. Binding of human cytomegalovirus US2 to major histocompatibility complex class I and II proteins is not sufficient for their degradation. J. Virol. 76:8265-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevalier, M. S., and D. C. Johnson. 2003. Human cytomegalovirus US3 chimeras containing US2 cytosolic residues acquire major histocompatibility complex class I and II protein degradation properties. J. Virol. 77:4731-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark, E. A., and R. Yokoshi. 1984. Leukocyte typing. Springer-Verlag, Berlin, Germany.
- 11.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coscoy, L., and D. Ganem. 2001. A viral protein that selectively downregulates ICAM-1 and B7-2 and modulates T cell costimulation. J. Clin. Investig. 107:1599-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer, L. A., and J. A. Frelinger. 2001. Dendritic cells transduced with HIV Nef express normal levels of HLA-A and HLA-B class I molecules. J. Acquir. Immune Defic. Syndr. 27:417-425. [DOI] [PubMed] [Google Scholar]
- 14.Doebele, R. C., R. Busch, H. M. Scott, A. Pashine, and E. D. Mellins. 2000. Determination of the HLA-DM interaction site on HLA-DR molecules. Immunity 13:517-527. [DOI] [PubMed] [Google Scholar]
- 15.Dunn, C., N. J. Chalupny, C. L. Sutherland, S. Dosch, P. V. Sivakumar, D. C. Johnson, and D. Cosman. 2003. Human cytomegalovirus glycoprotein UL16 causes intracellular sequestration of NKG2D ligands, protecting against NK cell cytotoxicity. J. Exp. Med. 197:1427-1439. [DOI] [PMC free article] [PubMed]
- 16.Fackler, O. T., and A. S. Baur. 2002. Live and let die: Nef functions beyond HIV replication. Immunity 16:493-497. [DOI] [PubMed] [Google Scholar]
- 17.Furman, M. H., N. Dey, D. Tortorella, and H. L. Ploegh. 2002. The human cytomegalovirus US10 gene product delays trafficking of major histocompatibility complex class I molecules. J. Virol. 76:11753-11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furman, M. H., H. L. Ploegh, and D. Tortorella. 2002. Membrane-specific, host-derived factors are required for US2- and US11-mediated degradation of major histocompatibility complex class I molecules. J. Biol. Chem. 277:3258-3267. [DOI] [PubMed] [Google Scholar]
- 19.Gewurz, B. E., R. Gaudet, D. Tortorella, E. W. Wang, H. L. Ploegh, and D. C. Wiley. 2001. Antigen presentation subverted: structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc. Natl. Acad. Sci. USA 98:6794-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gewurz, B. E., E. W. Wang, D. Tortorella, D. J. Schust, and H. L. Ploegh. 2001. Human cytomegalovirus US2 endoplasmic reticulum-lumenal domain dictates association with major histocompatibility complex class I in a locus-specific manner. J. Virol. 75:5197-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy, K., V. Van Heyningen, B. B. Cohen, D. L. Deane, and C. M. Steel. 1982. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur. J. Immunol. 12:942-948. [DOI] [PubMed] [Google Scholar]
- 22.Hegde, N. R., M. S. Chevalier, and D. C. Johnson. 2003. Viral inhibition of MHC class II antigen presentation. Trends Immunol. 24:278-285. [DOI] [PubMed] [Google Scholar]
- 23.Hegde, N. R., R. A. Tomazin, T. W. Wisner, C. Dunn, J. M. Boname, D. M. Lewinsohn, and D. C. Johnson. 2002. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation. J. Virol. 76:10929-10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hengel, H., J. O. Koopmann, T. Flohr, W. Muranyi, E. Goulmy, G. J. Hammerling, U. H. Koszinowski, and F. Momburg. 1997. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6:623-632. [DOI] [PubMed] [Google Scholar]
- 25.Hitt, M. M., and F. L. Graham. 2000. Adenovirus vectors for human gene therapy. Adv. Virus Res. 55:479-505. [DOI] [PubMed] [Google Scholar]
- 26.Huber, M. T., R. Tomazin, T. Wisner, J. Boname, and D. C. Johnson. 2002. Human cytomegalovirus US7, US8, US9, and US10 are cytoplasmic glycoproteins, not found at cell surfaces, and US9 does not mediate cell-to-cell spread. J. Virol. 76:5748-5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishido, S., J. K. Choi, B. S. Lee, C. Wang, M. DeMaria, R. P. Johnson, G. B. Cohen, and J. U. Jung. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13:365-374. [DOI] [PubMed] [Google Scholar]
- 28.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarvis, M. A., and J. A. Nelson. 2002. Human cytomegalovirus persistence and latency in endothelial cells and macrophages. Curr. Opin. Microbiol. 5:403-407. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, D. C., and G. McFadden. 2002. Viral immune evasion, p. 357-377. In S. H. E. Kaufmann, A. Sher, and R. Ahmed (ed.), Immunology of infectious diseases. American Society for Microbiology, Washington, D.C.
- 31.Johnson, D. C., and N. R. Hegde. 2002. Inhibition of the MHC class II antigen presentation pathway by human cytomegalovirus. Curr. Top. Microbiol. Immunol. 269:101-115. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones, T. R., L. K. Hanson, L. Sun, J. S. Slater, R. M. Stenberg, and A. E. Campbell. 1995. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J. Virol. 69:4830-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, T. R., E. J. Wiertz, L. Sun, K. N. Fish, J. A. Nelson, and H. L. Ploegh. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. USA 93:11327-11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jun, Y., E. Kim, M. Jin, H. C. Sung, H. Han, D. E. Geraghty, and K. Ahn. 2000. Human cytomegalovirus gene products US3 and US6 down-regulate trophoblast class I MHC molecules. J. Immunol. 164:805-811. [DOI] [PubMed] [Google Scholar]
- 36.Lehner, P. J., J. T. Karttunen, G. W. Wilkinson, and P. Cresswell. 1997. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. USA 94:6904-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Le Roy, E., A. Muhlethaler-Mottet, C. Davrinche, B. Mach, and J. L. Davignon. 1999. Escape of human cytomegalovirus from HLA-DR-restricted CD4+ T-cell response is mediated by repression of gamma interferon-induced class II transactivator expression. J. Virol. 73:6582-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGrory, W. J., D. S. Bautista, and F. L. Graham. 1988. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology 163:614-617. [DOI] [PubMed] [Google Scholar]
- 39.Miller, D. M., B. M. Rahill, J. M. Boss, M. D. Lairmore, J. E. Durbin, J. W. Waldman, and D. D. Sedmak. 1998. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J. Exp. Med. 187:675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura, N., Y. Nishioka, T. Shinohara, H. Ogawa, S. Yamamoto, K. Tani, and S. Sone. 2001. Novel centrifugal method for simple and highly efficient adenovirus-mediated green fluorescence protein gene transduction into human monocyte-derived dendritic cells. J. Immunol. Methods 253:113-124. [DOI] [PubMed] [Google Scholar]
- 41.Pereboev, A. V., C. K. Asiedu, Y. Kawakami, S. S. Dong, J. L. Blackwell, E. A. Kashentseva, P. L. Triozzi, W. A. Aldrich, D. T. Curiel, J. M. Thomas, and I. P. Dmitriev. 2002. Coxsackievirus-adenovirus receptor genetically fused to anti-human CD40 scFv enhances adenoviral transduction of dendritic cells. Gene Ther. 9:1189-1193. [DOI] [PubMed] [Google Scholar]
- 42.Pieters, J. 2000. MHC class II restricted antigen processing and presentation. Adv. Immunol. 75:159-208. [DOI] [PubMed] [Google Scholar]
- 43.Rehm, A., A. Engelsberg, D. Tortorella, I. J. Korner, I. Lehmann, H. L. Ploegh, and U. E. Hopken. 2002. Human cytomegalovirus gene products US2 and US11 differ in their ability to attack major histocompatibility class I heavy chains in dendritic cells. J. Virol. 76:5043-5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riegler, S., H. Hebart, H. Einsele, P. Brossart, G. Jahn, and C. Sinzger. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393-399. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson, F., C. Thomas, J. Neefjes, and J. Trowsdale. 1996. Association between HLA-DM and HLA-DR in vivo. Immunity 4:87-96. [DOI] [PubMed] [Google Scholar]
- 46.Stam, N. J., T. M. Vroom, P. J. Peters, E. B. Pastoors, and H. L. Ploegh. 1990. HLA-A- and HLA-B-specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin-fixed, paraffin-embedded tissue sections and in cryo-immuno-electron microscopy. Int. Immunol. 2:113-125. [DOI] [PubMed] [Google Scholar]
- 47.Streblow, D. N, C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 48.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomanin, R., A. J. Bett, L. Picci, M. Scarpa, and F. L. Graham. 1997. Development and characterization of a binary gene expression system based on bacteriophage T7 components in adenovirus vectors. Gene 193:129-140. [DOI] [PubMed] [Google Scholar]
- 50.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 5:1039-1043. [DOI] [PubMed] [Google Scholar]
- 51.Tortorella, D., C. M. Story, J. B. Huppa, E. J. Wiertz, T. R. Jones, I. Bacik, J. R. Bennink, J. W. Yewdell, and H. L. Ploegh. 1998. Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J. Cell Biol. 142:365-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiertz, E. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769-779. [DOI] [PubMed] [Google Scholar]
- 53.Wiertz, E. J., D. Tortorella, M. Bogyo, J. Yu, W. Mothes, T. R. Jones, T. A. Rapoport, and H. L. Ploegh. 1996. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384:432-438. [DOI] [PubMed] [Google Scholar]
- 54.Ye, Y., H. H. Meyer, and T. A. Rapoport. 2001. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature 414:652-656. [DOI] [PubMed] [Google Scholar]