Abstract
Marine mammals are susceptible to the effects of anthropogenic contaminants. Here we examine the effect of different polychlorinated biphenyl (PCB) accumulation scenarios on potential population growth rates using, as an example, data obtained for the population of bottlenose dolphins from Sarasota Bay, Florida. To achieve this goal, we developed an individual-based model framework that simulates the accumulation of PCBs in the population and modifies first-year calf survival based on maternal blubber PCB levels. In our example the current estimated annual PCB accumulation rate for the Sarasota Bay dolphin population might be depressing the potential population growth rate. However, our predictions are limited both by model naivety and parameter uncertainty. We emphasize the need for more data collection on the relationship between maternal blubber PCB levels and calf survivorship, the annual accumulation of PCBs in the blubber of females, and the transfer of PCBs to the calf through the placenta and during lactation. Such data require continued efforts directed toward long-term studies of known individuals in wild and semi-wild populations.
Keywords: calf survival, endocrine disruption, risk assessment, Tursiops truncatus
Marine mammals, as top predators in the marine ecosystem, are affected by persistent organic pollutants. Because the concentration of this group of chemicals is biomagnified in the food web and they are highly lipophilic, recalcitrant compounds (Hutchinson and Simmonds 1994), they can accumulate at very high levels in the blubber of pinnipeds and cetaceans (Boon et al. 1994). There is also a growing body of data that relates the exposure and uptake of these compounds [such as polychlorinated biphenyls (PCBs), dichlorodiphenyltrichloroethanes, and polybrominated diphenyl ethers] to endocrine-disrupting effects, including effects on reproduction (Addison 1989; Reijnders 1986; Wells et al. 2005) and immunity (de Swart et al. 1994; Lahvis et al. 1995; Ross et al. 1995). Links with circulating levels of thyroid and reproductive hormones have also been reported (De Guise et al. 1995; Skaare et al. 2001; Subramanian et al. 1987). Although many of these studies have been conducted on seals, increasing associative evidence indicates similar effects on cetaceans (Beland et al. 1993; Jepson et al. 1999; Kuiken et al. 1994). In addition, cetacean populations worldwide continue to be exposed to these compounds despite regulatory control on their production and use (Aguilar et al. 2002). Blubber concentrations of PCBs in some populations still exceed those estimated to be a threshold level for observed effects on reproduction and immunity (between 17 and 20 mg/kg lipid weight; Kannan et al. 2000; Ross et al. 2000).
The effects of contaminants on individual marine mammals have received much greater attention than their effects on populations. One of the reasons for this lack of attention to populations is the need for long-term monitoring studies of wild populations, which follow the reproductive success and health of individuals in relation to their contaminant burden. However, the long-term study of bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida, has provided an excellent opportunity for investigation of such population responses. Here we use the data collected for this population as an example of how an individual-based model approach can estimate and predict potential effects at the population level. Although this model could be generalized and parameterized with data from many different sources, we have chosen to focus on one population to illustrate the applicability of the approach and to maintain, as far as possible, internal data consistency.
Research on the year-round resident dolphin population in Sarasota Bay has been ongoing since 1970, and about 140 identifiable individual dolphins are currently being studied. Using tagging, tracking, and photographic techniques (Scott et al. 1990), we have identified individual home ranges, monitored female reproductive histories, determined birth order, and measured reproductive success (Wells 2003). Photo identification methods have also been used in mark-recapture studies to determine vital population rates such as survival and fecundity (Wells and Scott 1990). Indeed, such methods, now used in many cetacean species worldwide, were pioneered in this population. Since the 1980s, a capture-release program has allowed detailed health assessments and contaminant exposure studies to be conducted (Lahvis et al. 1995; Vedder 1996; Wells et al. 2004).
Previous risk assessment studies that investigated the potential effect of contaminant exposure on bottlenose dolphin populations have used data from laboratory studies in mink that demonstrate likely effects on offspring survival (Schwacke et al. 2002). This approach is useful in the absence of data on marine mammals, but it may have drawbacks because responses may differ between species. A recently published study (Reddy et al. 2001) found a similar negative relationship between the concentration of PCBs in the blubber of captive reproductive females and the survival of their calves. They reported PCB levels in the blubber of the females whose calves did not survive 12 days postpartum compared with levels in females whose calves survived beyond 6 months. Although this study was not able to control for the differences in the length of lactation, which will have an effect on maternal blubber concentrations, it is the only data set that indicates potential responses in the species of interest and is therefore used here. Additional observations from the Sarasota population alone (Wells et al. 2005) and from other studies of bottlenose dolphins (Cockcroft et al. 1989) suggest a similar response in the wild populations. Previous observational studies have documented disproportionately high first-calf mortality rates (Wells 2003), which is consistent with the risk assessment model of Schwacke et al. (2002). Here we combine an estimated dose–response relationship from the data of Reddy et al. (2001) with data on the level of PCBs in the blubber of the Sarasota bottlenose dolphins (Wells et al. 2005) and the vital rates for this population (age-specific fecundity and survival from mark-recapture studies, Wells and Scott 1990) to develop a predictive model framework.
To quantify the effects of PCBs on potential population growth rate, we have adopted an individual-based model (IB model) approach (Carlsen et al. 2004; Stow and Carpenter 1994). In this preliminary model we use maternal contaminant concentrations to modify calf survival. The remaining population vital parameters are from studies on the Sarasota Bay dolphin population described above. The states (live/dead, age, parturition, and contaminant concentration) of individuals are simulated through time. Because IB models incorporate stochasticity, multiple simulations produce a range of potential growth rates from which confidence intervals may be calculated. We refer to the growth rates as “potential” because there are no available data to incorporate density dependence into the model.
Thus, there are two aims to this study. The first is to develop an IB model framework that may be refined and used to estimate population level effects with associated confidence intervals that reflect uncertainty in the model parameters. The second is to examine the effect of different contaminant accumulation rates on potential population growth rates in the example Sarasota Bay bottlenose dolphin population. This process will allow us to determine where future data collection should be prioritized to reduce prediction error.
Methods
A model was constructed using R (R Development Core Team 2004) to simulate the fate of individual female dolphins, using fecundity and survivorship data derived from Wells and Scott (1990). The model also simulates the accumulation of PCBs through transplacental transfer, suckling, and prey and loss of PCBs through female lactation (depuration). Maternal PCB concentrations then affect first-year calf survival in a dose-dependent manner. Details of blubber biopsy sampling and analysis are given by Wells et al. (2005). All PCB concentrations are expressed as milligrams per kilogram lipid weight in blubber. First, we describe the model parameters and their values and, second, the model framework and operation.
Fecundity and survivorship
The following fecundity (f ) and survival (s) probabilities are from Wells and Scott (1990):
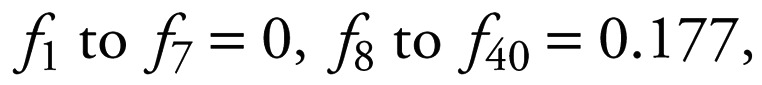 |
where ft is the probability that a female gives birth immediately before time t + 1, given that it survives to t + 1. The fecundity value of 0.177 was modified to exclude the effect of first-year calf mortality (i.e., 0.144/0.811).
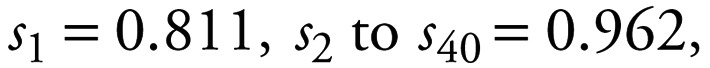 |
where st is the probability that a female survives from time t to time t + 1. In our model, death of the mother within the lactation period results in the death of the calf. If a female survives its 40th year, it is retained in age class 40. There is no reproductive senescence, and animals in this maximum age class are still able to reproduce. The length of lactation is 2 years. The manner in which s1 is modified is outlined below.
Accumulation
To estimate the annual accumulation of PCBs from prey by the dolphins, we fitted a linear model to the relationship between blubber PCB concentrations and age in male Sarasota Bay dolphins. We restricted this to males because they do not depurate their contaminant load and they continue to accumulate contaminants in the blubber throughout their lifetime. All the data were collected during June 2000 and June 2001, which avoided confounding because of season. These data were collected and analyzed as part of the International Whaling Commission Pollution 2000+ project (Reijnders et al. 1999) and the sums of 22 PCB congeners are reported (Wells et al. 2005). An outlier (a 43-year-old male with a PCB level of 868 mg/kg lipid weight) was excluded.
Depuration
Maternal depuration is the proportion of PCB concentration transferred from a female to the calf via lactation. The value of 0.77 was from Cockcroft et al. (1989). In the case where the calf (of either sex) dies within its first year, we do not know or predict exactly when this death occurs. Therefore, we assume that when death occurs it is at 6 months into the year, and thus the maternal depuration for that year is halved to 0.38. Otherwise, the birth of male calves is ignored in the model. To estimate the PCB concentration in newborn calves through transplacental transfer, we used a neonate-to-mother transfer ratio of 0.6. This is a mean estimate obtained from three studies (Cockcroft et al. 1989; Salata et al. 1995; Tanabe et al. 1982) in different odontocete species because only one value for bottlenose dolphins has been reported.
Reduced calf survivorship
In this model we consider only one consequence of maternal PCB exposure—first-year survivorship. Data published by Reddy et al. (2001) and by Wells et al. (2005) were pooled and used to estimate a potential dose-response linking maternal PCB blubber concentration to calf survival. The caveats associated with this approach are highlighted in “Discussion.” However, before using these data, we investigated the relationship between the number of calves produced by a female and the survival of the offspring because this factor may be just as important as her blubber PCB level. For example, primiparous females may have a lower calf survival because of inexperience. Therefore additional information provided by Reddy et al. (2001) on the number of calves produced by each female allowed us to test whether the number of calves was indeed a more important predictor of calf survival than was maternal blubber PCBs. In a generalized linear model (with a binomial family and a logit link function, and using stepwise regression with Akaike’s Information Criterion to determine the best-fitting model (Burnham and Anderson 1998), the model including only maternal blubber PCBs as the independent variable was a better fit to the data than one including the number of calves and an interaction term (to determine whether the relationship between maternal PCBs and survival varied significantly by the number of calves). Therefore we can conclude there is no evidence that the number of calves alone is a better predictor of survival and that experience is not a confounding factor in this data set.
We then fitted a similar generalized linear model with a logit link function to the pooled data sets (Reddy et al. 2001; Wells et al. 2005) to obtain probability estimates for calf survival in relation to maternal blubber PCBs (p = 0.0395, Figure 1). Uncertainty in this relationship was then estimated by resampling with replacement from the original data and recalculating the regression equation. This procedure was repeated 500 times, and the resulting regressions are also shown in Figure 1. We then used this relationship, and its uncertainty, to estimate first-year survival in the individual-based model, with the assumption that the risk of mortality during the first year is primarily within the first 6 months of life.
Figure 1.
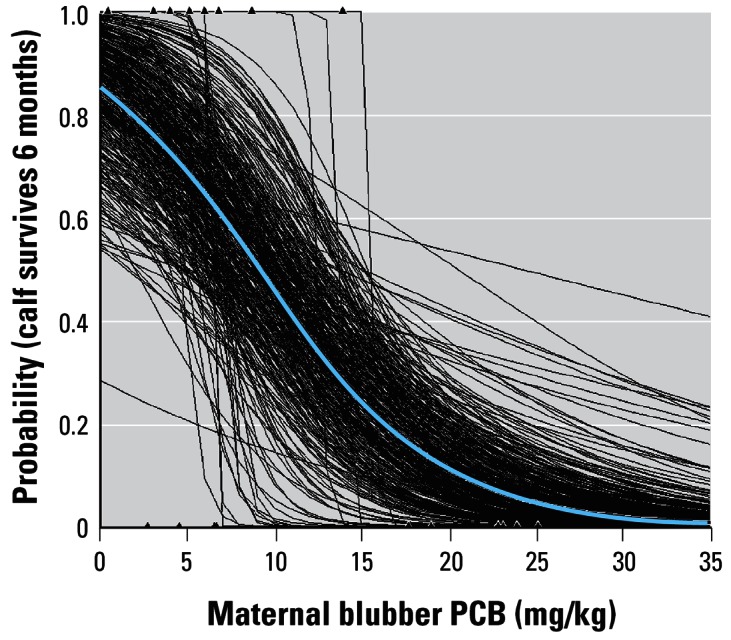
Logistic regression model that predicts the probability of 6-month calf survival in relation to the mother’s blubber PCB concentration (blue line). Black lines show 500 predictions obtained from resampling the data.
Model structure
Each individual female in the model had a state variable of alive (1) or dead (0), age (1 to ≥ 40 years) and blubber PCB concentration (milligrams per kilogram). All blubber concentrations were assumed to be lipid normalized. Survival and birth outcomes were determined by whether a random number (drawn from a uniform distribution between zero and one) was less than or equal to the probability associated with that event. The random number generator was continuously reseeded. The model assumed that population breeding dates are synchronized to a single day in the year and population predictions were made for the following day (postbreeding census). Thus, age class 1 refers to newborn calves. We also assume that the sex ratio at birth is 1:1. Forty age classes were considered, the last contained all females ≥ 40 years of age.
Each model simulation covered a period of 100 years. An arbitrary starting abundance of 300 dolphins was chosen to ensure that sufficient dolphins were still alive in the population after 100 years, when the population growth rate was less than unity. For any given set of fecundity and survivorship values, we calculated the stable age structure (SAS) by multiplying an arbitrary seed age structure by the appropriate Leslie matrix 100 times. The SAS was used to apply age structure to the initial population of 300 animals. Twenty-five simulations were performed for each parameter set combination, initially only using the best-fit regression of calf survivorship on maternal PCBs. At first, each dolphin was assigned an arbitrary zero PCB level. After the first year, animals were then allocated an appropriate level of PCBs, depending on their age class and reproductive status. After approximately the 40th simulation year, the relationship between PCB levels and age stabilized. From the population trajectories after the first 40 years, we calculated the mean potential annual growth rates. The 2.5 and 97.5 percentile growth rates were then estimated from the ranked individual growth rates. The sensitivity of annual growth rates to variability in the annual PCB accumulation was assessed with multiple runs of the model, with different accumulation rates. The entire process was then repeated, but this time incorporating the uncertainty in the dose–response relationship. For each 100-year simulation the model chose at random a regression from the set of 500 regressions calculated by data resampling.
Results
Accumulation
The relationship between male blubber PCB concentrations and age is shown in Figure 2. The least-squares linear regression equation was as follows:
Figure 2.
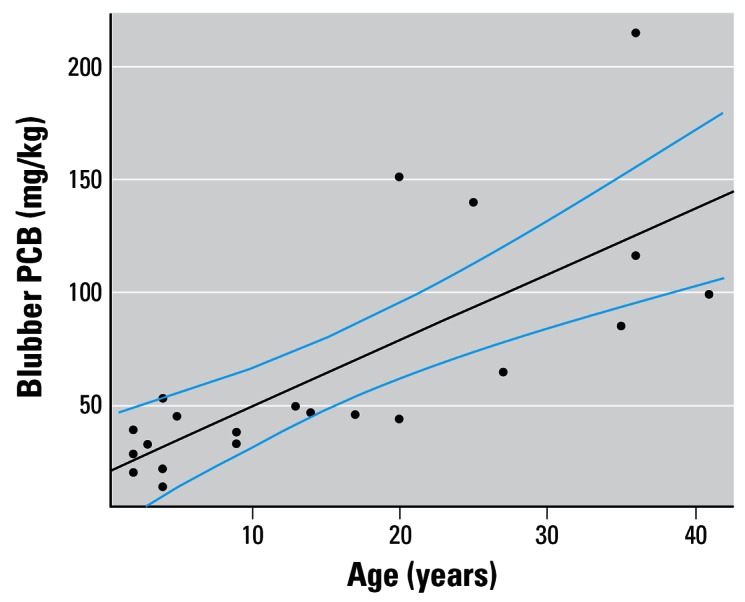
Accumulation of PCBs with age in male dolphins. Fitted regression line and 95% CI values are also shown (data from Wells et al. 2005).
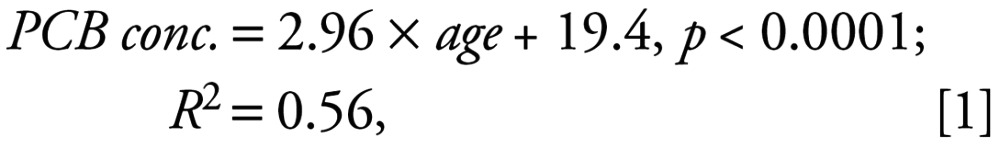 |
where PCB conc. is in units of milligrams per kilogram and age is in years. The SE of the slope was 0.6.
Reduced calf survivorship
The result of the logistic regression of probability of first-year survivorship on maternal contaminant burden is shown in Figure 1. The best fit regression equation was as follows:
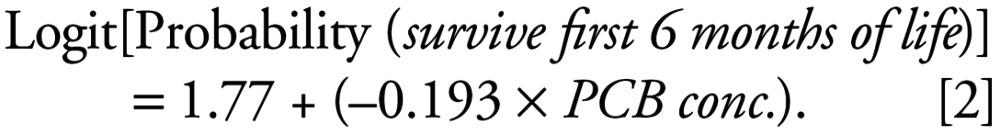 |
Figure 1 also shows the 500 alternative regressions obtained by resampling the data.
Model output
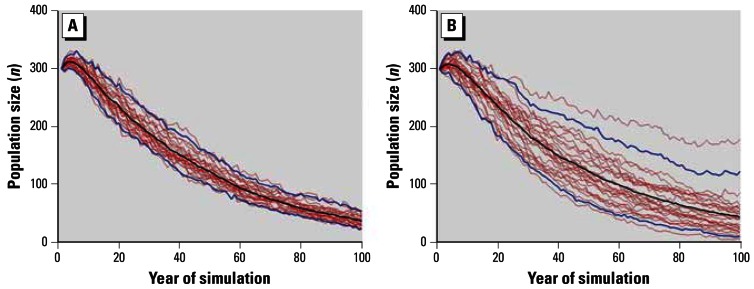
Figure 3A shows the estimated effect of PCBs on the individual potential annual growth rate when the annual rate of PCB accumulation was set at 2.96 mg/kg (i.e., the coefficient from Equation 1). The mean potential population growth was 0.977 [95% confidence interval (CI), 0.967–0.984]. The blue lines connect the 95% CI range of population size for each year. The initial positive growth rate in Figure 3A was caused by the time lag required for a stable PCB level for each age class to form. Figure 3B shows the effect of PCBs on potential population growth rate, but this time uncertainty in the relationship between calf survival and maternal PCBs is incorporated. The mean potential population growth rate (0.980) was similar to the mean without this uncertainty but the 95% CI values (95% CI, 0.966 – 0.990) were 41% greater.
Figure 3.
(A) Twenty-five model simulations (red lines) showing the negative potential population growth over 100 years for the Sarasota bottlenose dolphin population using current estimates for vital rates, PCB accumulation rate (2.96 mg/kg/year), and potential effect of maternal PCB levels on calf survival. (B) Same as A but incorporating uncertainty into the effect of maternal PCB levels on calf survival. Black line connects the mean of the population size for each year, and blue lines connect the 95% CI of the population size for each year.
The effect of changing the rate of PCB accumulation on potential annual growth rate is shown in Figure 4. The potential annual growth rate for a clean population is 1.014. At the estimated annual PCB accumulation rate for the Sarasota Bay dolphins (2.96 mg/kg), the potential annual growth rate is decreased by 3.6% to 0.977. At the higher accumulation rates (> ~ 6 mg/kg/year), the growth rate decrease stabilizes at about 5%. In addition, Figure 4 shows the effect of incorporating the extra uncertainty into the relationship between calf survival and maternal PCBs. The additional variability had a greater impact at low accumulation rates (e.g., 54% greater at 1 mg/kg compared with 15% greater at 9 mg/kg). This effect was probably caused by a greater range of calf survival probabilities at lower maternal blubber PCB concentrations, as illustrated in Figure 1.
Figure 4.
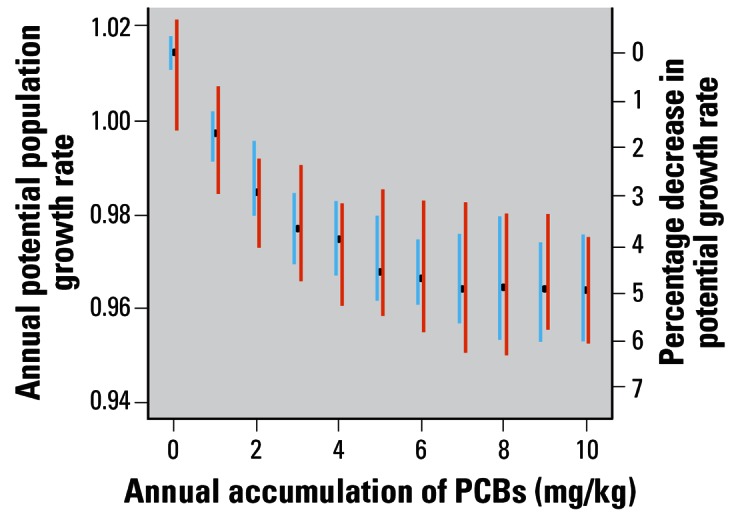
The effect of changing the rate of PCB accumulation on potential population annual growth rate. Blue lines show the 95% CI intervals without uncertainty in the dose–response relationship. Red lines include this uncertainty.
Figure 5 shows the PCB concentrations in individual females in the final 20 years of a model run where PCB accumulation was set at 2.96 mg/kg/year. The PCB concentrations are plotted against age. There is an initial decrease where neonates with high PCB concentrations have a higher probability of first-year mortality. Thereafter, young continue to increase PCB concentrations until at 8 years of age they may recruit into the breeding population. The consequent depuration is evident from 9 years of age onward. Because the model is stochastic, not all females give birth in year 8. In fact, in this model run, one female reached 20 years of age and accumulated PCBs at a concentration of 78 mg/kg before first giving birth. The average PCB concentration of all females ≥ 9 years of age was 23.8 mg/kg.
Figure 5.
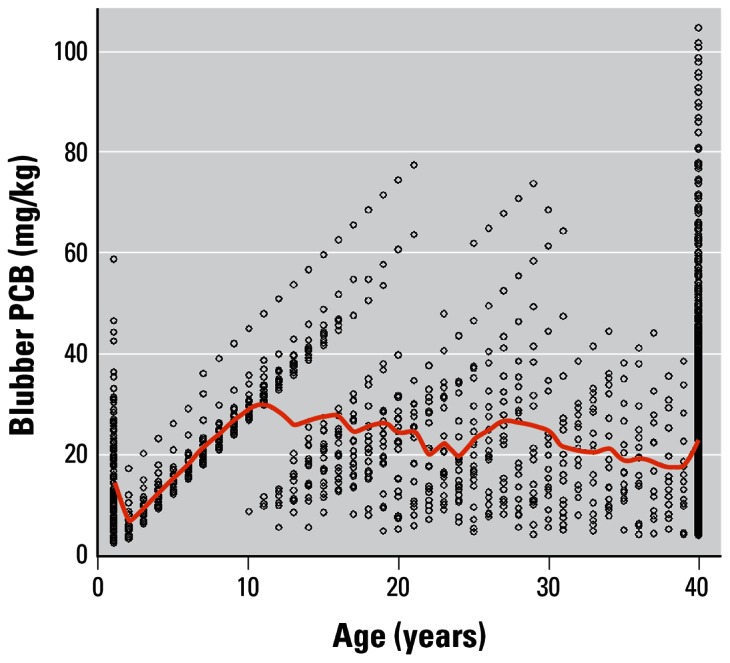
The PCB concentrations of individual females in the final 20 years of a model run where mean annual PCB accumulation was set at 2.96 mg/kg. The PCB concentrations are plotted against age. The red line shows the mean blubber PCB concentrations in each age class.
Discussion
We describe an individual-based model framework to predict potential population-level effects of PCB exposure on cetaceans. We illustrate this approach using, as much as possible, data from a single population—the bottlenose dolphins in Sarasota Bay. However, where necessary we used data from other sources. We suggest that the current estimated annual PCB accumulation rate for this population is depressing the potential annual growth rate by 3.6% compared with a zero accumulation rate. We also suggest that higher accumulation rates would produce a maximum depression of about 5% in potential growth rate. This latter depression represents a calf survivorship of zero. We do, however, wish to emphasize uncertainty in the model parameters and also in the biological assumptions implicit in this model.
Vital population parameters used in the model were derived from previous studies of the Sarasota Bay bottlenose dolphins [annual survival age 1 year = 0.811, SD 0.0644; ≥ age 2 years = 0.962, SD 0.0076; fecundity = 0.177, SD 0.035 (Wells and Scott 1990)]. However, we have not incorporated the uncertainty surrounding these parameters into the potential annual growth predictions. Also, these published data were limited to those compiled during the 1980s; subsequent research is leading to a multidecade data set based on more complete knowledge of the population, which allows for more refined population parameters and matched contaminant load and vital rate analyses. In addition we have not incorporated density dependence into our model because no appropriate data are available. Therefore, we accept that our growth rate predictions may be an inaccurate reflection of reality. For this reason we refer to growth rates as “potential,” and we emphazise their relative rather than their absolute values.
When we ran the model with an annual accumulation rate of 2.96 mg/kg (the estimated rate for the Sarasota Bay population), we predicted that the mean PCB level of females ≥ 9 years of age would be 23.8 mg/kg. However, the mean PCB level measured in blubber biopsies from Sarasota females ≥ 9 years of age was 8 mg/kg, SD 6.8 (Wells et al. 2005). Furthermore, the model simulations suggested that obtaining the measured mean levels required an annual accumulation rate of 0.9 mg/kg/year rather than the 2.96 estimated from the male data. This reduced rate resulted in an annual population growth rate of 0.998 (95% CI, 0.990–1.005) (Figure 4).
Uncertainty in the dose–response relationship had a significant impact on the model predictions (Figures 3 and 4). In our data the EC50 (median effective concentration) from the best fit regression relationship is approximately 10 mg/kg, which is much lower than the 33 mg/kg estimated from effects on mink used as a surrogate species in the risk assessment study by Schwacke et al. (2002) on bottlenose dolphins. Mink are particularly susceptible to the effects of PCB exposure, and if we substitute the dose–response relationship used in Schwacke et al. (2002) in our model (with an annual accumulation of 2.96 mg/kg), the potential population growth rate is 1.005 (95% CI, 1.00–1.01), an increase of 2.8%. However, it should be noted that the mink data refer to the excess offspring mortality risk from maternal PCBs after accounting for mortality from other causes. Although the bottlenose dolphin dose–response data include both natural and contaminant-mediated effects, the mortality is still less than the threshold level for effects in pinnipeds reported by Ross et al. (2000) and Kannan et al. (2000) of between 17 and 20 mg/kg. Clearly, more data from cetaceans in general and from bottlenose dolphins in particular would not only reduce the prediction confidence intervals but would also provide greater power in distinguishing the effect of being a “firstborn” calf per se on survivorship. We do not know the mechanism for the relationship between calf survival and maternal blubber PCB concentrations. Other studies have shown this group of compounds to be endocrine disruptors and have demonstrated links between perinatal exposure and brain cell development (Yang et al. 2003), thyroid hormone disruption (Bansal et al. 2005), and immune function effects (Grasman and Whitacre 2001). Some or all of these end points may be the reason for decreased survival in perinatally exposed bottlenose dolphin calves, but further species-specific molecular and endocrine toxicological studies are required. In addition, we have not included multiple system effects (such as on growth and development and immune function) or concomitant effects on other reproductive parameters, such as fecundity.
We have used a depuration estimate of 0.77 from the studies by Cockcroft et al. (1989). However, we have not incorporated any uncertainty into this parameter. Higher maternal transfer of PCBs will decrease the survival probability of the immediate offspring but will increase the survival probability of subsequent offspring. Seasonal variation also was not included at this stage. Seasonal variations in blubber thickness will have an impact on PCB levels because concentrations increase as the total amount of body fat decreases (Kleivane et al. 2004). This factor would be an additional aspect to incorporate into the model in future.
Many of the persistent ocean contaminants co-occur, and highly significant positive correlations have been reported among concentrations of different classes of organic compounds measured in the blubber of marine mammals (Kajiwara et al. 2004; Law et al. 1989; Wells et al. 2005). Although we have focused on one class of compounds, it must be remembered that some effects may be caused by the mixture of chemicals to which these animals are exposed in the wild.
In this study we have constructed an individual-based model and incorporated into it the best available data on PCB accumulation and life history parameters to assess the consequences of PCB concentrations on potential population growth trajectories in an example population of bottlenose dolphins. Some, but not all, of the uncertainty in these parameters is incorporated in trajectory uncertainty. We emphasize this uncertainty in the current model output and caution against the naïve use of our results. Indeed, the observed population increase for the Sarasota Bay population from the mid-1990s of approximately 40% (Wells RW, unpublished data), which equates to an annual growth rate of approximately 1.03, lies outside the 95% confidence intervals of our best potential growth rate estimate.
Nevertheless, we see our current model as a valid starting point for the development of more biologically appropriate models as more is learned about the ecology and toxicology of bottlenose dolphins. More important, the process of model construction has forced us to highlight both the level of sufficiency of published data and the prioritization of future data collection. For a long-lived species, such as the bottlenose dolphin, which for logistical and ethical reasons cannot be subjected to conventional toxicological studies, such key data require continued efforts into long-term studies of known individuals in wild and semiwild populations.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
The long-term data and sample collection represented in this study reflect the work of many dedicated Sarasota Dolphin Research Program staff members and volunteers. We especially thank L. Fulford, J. Greene, K. Hull, B. Irvine, S. Nowacek, M. Scott, J. Sweeney, F. Townsend, and K. Urian. While the ages of most animals considered in this article were known from observation, some ages were determined through examination of growth layer groups by A. Hohn. We are grateful to P. Reijnders, G. Donovan, and the International Whaling Commission’s Pollution 2000+ Programme, who provided some of the basic support for the data referred to in this study. The NOAA Marine Mammal Health and Stranding Response Program, the National Marine Fisheries Service, and Dolphin Quest provided additional crucial sample collection and analyses. Earthwatch Institute, the Chicago Zoological Society, and Mote Marine Laboratory provided support for the long-term monitoring of the Sarasota Bay dolphins.
Research was conducted under the National Marine Fisheries Service Scientific Research Permit No. 522-1569 issued to R.S. Wells. All research was conducted under Mote Marine Laboratory’s Institutional Animal Care and Use Committee approval.
References
- Addison RF. Organochlorines and marine mammal reproduction. Can J Fish and Aquat Sci. 1989;46:360–368. [Google Scholar]
- Aguilar A, Borrell A, Reijnders PJH. Geographical and temporal variation in levels of organochlorine contaminants in marine mammals. Mar Environ Res. 2002;53:425–452. doi: 10.1016/s0141-1136(01)00128-3. [DOI] [PubMed] [Google Scholar]
- Bansal R, You SH, Herzig CT, Zoeller RT. Maternal thyroid hormone increases HES expression in the fetal rat brain: an effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs) Brain Res Dev Brain Res. 2005;156:13–22. doi: 10.1016/j.devbrainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Beland P, Deguise S, Girard C, Lagace A, Martineau D, Michaud R, et al. Toxic compounds and health and reproductive effects in St. Lawrence beluga whales. J Great Lakes Res. 1993;19:766–775. [Google Scholar]
- Boon JP, Oostingh I, Van de Meer J, Hillebrand MTJ. A model for the bioaccumulation of chlorobiphenyl congeners in marine mammals. Eur J Pharmacol. 1994;270:237–251. doi: 10.1016/0926-6917(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Burnham K, Anderson D. 1998. Model Selection and Inference. A Practical Information-Theoretic Approach. New York: Springer-Verlag
- Carlsen TM, Coty JD, Kercher JR. The spatial extent of contaminants and the landscape scale: an analysis of the wildlife, conservation biology, and population modeling literature. Environ Toxicol Chem. 2004;23:798–811. doi: 10.1897/02-202. [DOI] [PubMed] [Google Scholar]
- Cockcroft VG, De Kock AC, Lord DA, Ross GJB. Organochlorines in bottlenose dolphins (Tursiops truncatus) from the East coast of South Africa. S Afr J Mar Sci. 1989;8:207–217. [Google Scholar]
- De Guise S, Martineau D, Béland P, Fournier M. Possible mechanisms of action of environmental contaminants on St. Lawrence beluga whales (Delphinapterus leucas) Environ Health Perspect. 1995;103(suppl 4):73–77. doi: 10.1289/ehp.95103s473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Swart RL, Ross PS, Vedder LJ, Timmerman HH, Heisterkamp S, Van Loveren H, et al. Impairment of immune function in harbour seals (Phoca vitulina) feeding on fish from polluted waters. Ambio. 1994;23:155–159. [Google Scholar]
- Grasman KA, Whitacre LL. Effects of PCB 126 on thymocyte surface marker expression and immune organ development in chicken embryos. J Toxicol Environ Health A. 2001;62:191–206. doi: 10.1080/009841001458307. [DOI] [PubMed] [Google Scholar]
- Hutchinson JD, Simmonds MP. Organochlorine contamination in pinnipeds. Rev Environ Contam Toxicol. 1994;136:123–167. doi: 10.1007/978-1-4612-2656-7_4. [DOI] [PubMed] [Google Scholar]
- Jepson PD, Bennett PM, Allchin CR, Law RJ, Kuiken T, Baker JR, et al. Investigating potential associations between chronic exposure to polychlorinated biphenyls and infectious disease mortality in harbour porpoises from England and Wales. Sci Total Environ. 1999;243/244:339–348. doi: 10.1016/s0048-9697(99)00417-9. [DOI] [PubMed] [Google Scholar]
- Kajiwara N, Matsuoka S, Iwata H, Tanabe S, Rosas FC, Fillmann G, et al. Contamination by persistent organochlorines in cetaceans incidentally caught along Brazilian coastal waters. Arch Environ Contam Toxicol. 2004;46:124–134. doi: 10.1007/s00244-003-2239-y. [DOI] [PubMed] [Google Scholar]
- Kannan K, Blankenship AL, Jones PD, Giesy JP. Toxicity reference values for the toxic effects of polychlorinated biphenyls to aquatic mammals. Hum Ecol Risk Assess. 2000;6:181–201. [Google Scholar]
- Kleivane L, Severinsen T, Lydersen C, Berg V, Skaare JU. Total blubber burden of organochlorine pollutants in phocid seals; methods and suggested standardization. Sci Tot Environ. 2004;320:109–119. doi: 10.1016/j.scitotenv.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Kuiken T, Bennett PM, Allchin CR, Kirkwood JK, Baker JR, Lockyer CH, et al. PCBs, cause of death, and body condition in harbour porpoises (Phocoena phocoena) from British waters. Aquat Toxicol. 1994;28:13–28. [Google Scholar]
- Lahvis GP, Wells RS, Kuehl DW, Stewart JL, Rhinehart HL, Via CS. Decreased lymphocyte responses in free-ranging bottlenose dolphins (Tursiops truncatus) are associated with increased concentrations of PCB’s and DDT in peripheral blood. Environ Health Perspect. 1995;103:67–72. doi: 10.1289/ehp.95103s467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R, Allchin C, Harwood J. Concentrations of organochlorine compounds in the blubber of seals from eastern and north-eastern England, 1988. Mar Poll Bull. 1989;20:110–115. [Google Scholar]
- R Development Core Team. 2004. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
- Reddy ML, Reif JS, Bachand A, Ridgway SH. Opportunities for using Navy marine mammals to explore associations between organochlorine contaminants and unfavorable effects on reproduction. Sci Total Environ. 2001;274:171–82. doi: 10.1016/s0048-9697(01)00741-0. [DOI] [PubMed] [Google Scholar]
- Reijnders PJH. Reproductive failure in common seals feeding on fish from polluted coastal waters. Nature. 1986;324:456–457. doi: 10.1038/324456a0. [published erratum Nature 324:418] [DOI] [PubMed] [Google Scholar]
- Reijnders PJH, Aguilar A, Donovan GP, eds. 1999. Chemical Pollutants and Cetaceans. Cambridge, UK:International Whaling Commission.
- Ross PS, de Swart RL, Reijnders PJH, Van Loveren H, Vos JG, Osterhaus ADME. Contaminant-related suppression of delayed-type hypersensitivity and antibody responses in harbor seals fed herring from the Baltic Sea. Environ Health Perspect. 1995;103:162–167. doi: 10.1289/ehp.95103162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PS, Ellis GM, Ikonomou MG, Barrett-Lennard LG, Addison RF. High PCB concentrations in free-ranging Pacific killer whales, Orcinus orca: effects of age, sex and dietary preference. Mar Poll Bull. 2000;40:504–515. [Google Scholar]
- Salata GG, Wade TL, Sericano JL, Davis JW, Brooks JM. Analysis of Gulf of Mexico bottlenose dolphins for organo-chlorine pesticides and PCBs. Environ Pollut. 1995;88:283–292. doi: 10.1016/0269-7491(95)91441-m. [DOI] [PubMed] [Google Scholar]
- Schwacke LH, Voit EO, Hansen LJ, Wells RS, Mitchum GB, Hohn AA, et al. Probabilistic risk assessment of reproductive effects of polychlorinated biphenyls on bottlenose dolphins (Tursiops truncatus) from the southeast United States coast. Environ Toxicol Chem. 2002;21:2752–2764. [PubMed] [Google Scholar]
- Scott MD, Wells RS, Irvine AB. 1990. A long-term study of bottlenose dolphins on the west coast of Florida. In: The Bottlenose Dolphin (Leatherwood S, Reeves RR, eds). San Diego:Academic Press, 235–244.
- Skaare JU, Bernhoft A, Wiig O, Norum KR, Haug E, Eide DM, et al. Relationships between plasma levels of organochlorines, retinol, and thyroid hormones from polar bears (Ursus maritimus) at Svalbard. J Toxicol Environ Health A. 2001;62:227–241. doi: 10.1080/009841001459397. [DOI] [PubMed] [Google Scholar]
- Stow CA, Carpenter SR. PCB accumulation in Lake Michigan coho and chinook salmon: individual-based models using allometric relationships. Environ Sci Technol. 1994;28:1543–1549. doi: 10.1021/es00057a026. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tanabe S, Tatsukawa R, Saito S, Miyazaki N. Reduction in the testosterone levels by PCBs and DDE in Dall’s porpoises of northwestern North Pacific. Mar Poll Bull. 1987;18:643–646. [Google Scholar]
- Tanabe S, Tatsukawa R, Maruyama K, Miyazaki N. Transplacental transfer of PCBs and chlorinated hydrocarbon pesticides from the pregnant striped dolphin (Stenella coeruleoalba) to her fetus. Agric Biol Chem. 1982;42:1249–1254. [Google Scholar]
- Vedder J. 1996. Levels of Organochlorine Contaminants in Milk Relative to Health of Bottlenose Dolphins (Tursiops truncatus) from Sarasota, Florida [MSc Thesis]. Santa Cruz, CA:University of California.
- Wells RS. 2003. Dolphin social complexity: lessons from long-term study and life history. In: Animal Social Complexity: Intelligence, Culture, and Individualized Societies (de Waal FBM, Tyack PL, eds). Cambridge, MA:Harvard University Press, 35–56.
- Wells RS, Rhinehart HL, Hansen LJ, Sweeney JC, Townsend FI, Stone R, et al. Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system. EcoHealth. 2004;1:246–254. [Google Scholar]
- Wells RS, Scott MD. 1990. Estimating bottlenose dolphin population parameters from individual identification and capture-release techniques. In: Individual Recognition of Cetaceans: Use of Photo-Identification and other Techniques to Estimate Population Parameters (Hammond PS, Mizroch SA, Donovan GP, eds). Cambridge, UK:International Whaling Commission, 407–415.
- Wells RS, Tornero V, Borrell A, Aguilar A, Rowles TK, Rhinehart HL, et al. Integrating life history and reproductive success data to examine potential relationships with organochlorine compounds for bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Sci Tot Environ. 2005;349:106–119. doi: 10.1016/j.scitotenv.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Yang JH, Derr-Yellin EC, Kodavanti PR. Alterations in brain protein kinase C isoforms following developmental exposure to a polychlorinated biphenyl mixture. Brain Res Mol Brain Res. 2003;111:123–135. doi: 10.1016/s0169-328x(02)00697-6. [DOI] [PubMed] [Google Scholar]