Abstract
Concern has been raised in recent years that exposure to wastewater treatment effluents containing estrogenic chemicals can disrupt the endocrine functioning of riverine fish and cause permanent alterations in the structure and function of the reproductive system. Reproductive disorders may not necessarily arise as a result of estrogenic effects alone, and there is a need for a better understanding of the relative importance of endocrine disruption in relation to other forms of toxicity. Here, the integrated health effects of long-term effluent exposure are reported (reproductive, endocrine, immune, genotoxic, nephrotoxic). Early life-stage roach, Rutilus rutilus, were exposed for 300 days to treated wastewater effluent at concentrations of 0, 15.2, 34.8, and 78.7% (with dechlorinated tap water as diluent). Concentrations of treated effluents that induced feminization of male roach, measured as vitellogenin induction and histological alteration to gonads, also caused statistically significant alterations in kidney development (tubule diameter), modulated immune function (differential cell count, total number of thrombocytes), and caused genotoxic damage (micronucleus induction and single-strand breaks in gill and blood cells). Genotoxic and immunotoxic effects occurred at concentrations of wastewater effluent lower than those required to induce recognizable changes in the structure and function of the reproductive endocrine system. These findings emphasize the need for multiple biological end points in tests that assess the potential health effects of wastewater effluents. They also suggest that for some effluents, genotoxic and immune end points may be more sensitive than estrogenic (endocrine-mediated) end points as indicators of exposure in fish.
Keywords: endocrine, fish, genotoxic, health, immunotoxic, reproductive, wastewater treatment works
It has been hypothesized that a wide variety of reproductive and developmental problems observed in some wildlife populations and in humans are caused by exposure to environmental contaminants that can interfere with endocrine signaling pathways (reviewed by Vos et al. 2000). This hypothesis is supported by a growing body of evidence from laboratory studies that show that pollutants present in the environment can disrupt the endocrine functioning of wildlife species, thus causing permanent alterations in the structure and function of the endocrine system [reviewed by Tyler et al. (1998)].
There are, however, significant gaps in our knowledge of endocrine disruption in wildlife and humans, particularly in the causes and mechanisms of these phenomena. Although the interaction of pollutants with endogenous hormone receptors (e.g., the estrogen receptor) continues to dominate the literature, there are many associations between exposure to endocrine-disrupting chemicals (EDCs) and a variety of biological outcomes for which the mechanisms of action are poorly understood. This makes it difficult to distinguish between direct (hormone-receptor–mediated) and indirect effects and between primary and secondary outcomes.
Moreover, wildlife species are rarely exposed to single chemicals but instead are exposed to complex, fluctuating mixtures of contaminants that may act in various ways (Silva et al. 2002; Sumpter 2003; Thorpe et al. 2001, 2003) and that may induce combination effects (Rajapakse et al. 2002) via the same or different mechanisms. Wastewater treatment works (WwTW) effluent, for example, contains a mixture of natural and synthetic xenobiotics, household and agricultural chemicals, pharmaceuticals, hormones, and other compounds, many of which remain unidentified (Stevens et al. 2003). Studies have correlated exposure to WwTW effluent with alterations in sex steroid hormone levels in adult and juvenile fish (Folmar et al. 1996, 2001a; Hecker et al. 2002), impaired gonadal development in adults and juveniles (Hemming et al. 2001; Jobling et al. 2002; Sheahan et al. 2002), altered sexual differentiation in early life stages (Rodgers-Gray et al. 2001), and induction of the egg-yolk precursor protein vitellogenin (VTG) in adult male and juvenile fish of both sexes (Harries et al. 1999; Purdom et al. 1994; Rodgers-Gray et al. 2001). These effects have been associated with the presence of chemical contaminants in the effluents that act as estrogen receptor agonists, including natural and synthetic steroids (Desbrow et al. 1998; Routledge et al. 1998), alkylphenol polyethoxylates (Gimeno et al. 1997, 1998; Gronen et al. 1999; Jobling et al. 1996; Seki et al. 2003), and phthalates and pesticides (Ankley et al. 2001; Christiansen et al. 2000; Jobling et al. 1995; Sohoni and Sumpter 1998). These disorders, however, may not necessarily be a consequence of estrogenic effects alone.
Many EDCs are reactive chemicals that exhibit multiple mechanisms of toxicity by acting at different sites within the body. Consequently, alterations to the endocrine immune and nervous systems and damage to genetic material may each contribute toward the overall toxic impact of a particular chemical (Choi et al. 2004; Galloway and Handy 2003; Handy 2003; Roy and Liehr 1999). It is pertinent to note that a recent comprehensive literature survey of 48 EDCs revealed that 79% of these were also carcinogenic or mutagenic, 52% were also immunotoxic, and 50% were also neurotoxic (Choi et al. 2004). Essentially half of all the chemicals tested (including both natural and synthetic) cause tissue injury at high enough doses (and thus could result in disruptions in immune function and in cancers, etc.). Both 4-tert-nonylphenol (4-NP) and bisphenol A (BPA), for example, are contaminants found at appreciable concentrations in the aquatic environment that can cause endocrine disruption by interacting with both the estrogen receptor (as agonists; Gaido et al. 1997) and the androgen receptor (Sohoni and Sumpter 1998). In addition, 4-NP can disrupt steroidogenesis in the liver and can interfere with the dynamic control of follicle-stimulating hormone release from the pituitary (Harris et al. 2001). Moreover, recent studies have also shown that concentrations of 4-NP and BPA that inhibit gonadal development and reproductive function in fish can also cause damage to the kidneys (as a consequence of VTG induction) and decreased body weight and induce stressed behavior (Magliulo et al. 2002) as well as result in damage to DNA [in barnacles, Atienzar et al. (2002)] and to the immune system [in rats, Karrow et al. (2004)]. Similarly, steroid estrogens that are known to be present in WwTW effluents (Desbrow et al. 1998) and to cause feminizing effects in fish have been reported to be genotoxic in mammals both in cell lines and in vivo (Banerjee et al. 1994; Floyd et al. 1990; Han and Liehr 1994; Nutter et al. 1991, 1994).
With these issues in mind, we need a better understanding of the relative importance of the impact of endocrine disruption on the physiology of wild organisms compared with the other forms of toxicity. Furthermore, studies are also needed to help unravel how some of the health effects seen in wildlife and ascribed to endocrine disruption are actually mediated. To date, virtually nothing is known of the potential importance of interacting modes of toxicity in complex cocktails of pollutants. Several studies have documented either the immunological (Kakuta and Murachi 1997) or genotoxic (White et al. 1996) activity/effects of WwTW effluents separately, but there are no studies in which the impacts of WwTW effluents on these axes have been examined simultaneously or that have compared the relative importance and sensitivity for these disruptive/toxic effects. Our goal in this present study was to fill these knowledge gaps by examining the integrated health effects (reproductive, endocrinological, immunological, genotoxic, and nephrotoxic) of long-term (300-day) exposure to a range of concentrations of a treated WwTW effluent on the roach (Rutilus rutilus), a common European fish species in which a widespread incidence of endocrine disruption has been described and reported (Jobling et al. 1998; Nolan et al. 2001). The exposure regime adopted covered the period of embryogenesis and development during early life.
Materials and Methods
Fish and experimental protocol
The influent entering the study WwTW was predominantly domestic in origin, with an industrial component of approximately 24%. The population equivalent of the WwTW was approximately 312,700. Treatment of influent to the WwTW consisted of screens and primary settlement, followed by either a bubble-diffused air sludge treatment process (60% of the influent) or a biological phosphorus removal treatment process (40% of the influent), and both treatment components were combined before discharge from the WwTW. Final effluent was passed through a 100-μm filter before it was supplied to the tanks holding the fish. The flow rate through each of the tanks was 5 L/min, and flow rate and water temperature were monitored daily. Measured concentrations of WwTW effluent were 0 ± 0, 15.24 ± 0.23, 34.81 ± 0.31, and 78.70 ± 0.43%, diluted with dechlorinated tap water. The temperature of the effluent/diluent water fluctuated with the ambient temperature in all exposure tanks, but there were no significant differences in water temperature between the various treatments at any time point (p > 0.05). Throughout the trial the tanks were aerated to ensure sufficient oxygen supply to sustain the fish biomass.
Roach were exposed from fertilization throughout embryonic development until they were 300 days posthatch (dph) to encompass the periods of embryonic development and sexual differentiation in this species. For the collection of gametes to generate the required embryos, adult broodstock roach were induced to spawn using intraperitoneal injections of carp pituitary extract according to established protocols. Eggs were collected and pooled from five females and pooled sperm from six males was used to fertilize these eggs. Fertilized eggs were placed on raised mesh hatching trays in each 50-L glass-reinforced plastic exposure tank with flow-through conditions. The embryos were cleaned of sediment twice daily by gently pushing water over them with a Pasteur pipette. From hatching, fry were fed newly hatched Artemia until they were approximately 60 days old, and then commercial cyprinid pelleted food was introduced (Calverton Fish Farm, Nottingham, UK) and Artemia feeds were gradually phased out. For the remainder of the trial, fish were fed the pelleted food twice daily. Fish were transferred into larger 600-L mesocosm tanks at approximately 70 dph.
Chemical analysis
We performed chemical analysis on the effluent to measure steroid estrogens and some alkylphenolic chemicals that are known/suspected to induce feminizing effects in wild fish. Seven-day composite effluent samples were collected 3 times during the exposure period (on days 0–7, 27–33, and 54–60) and analyzed for the steroid estrogens 17β-estradiol, estrone, and 17α-ethinyl-estradiol and the alkylphenolic compounds octylphenol, nonylphenol, and nonylphenol mono- and diethoxylates. Analysis methods used solid-phase extraction to isolate the compounds of interest and analysis by gas chromatography–mass spectrometry (GC-MS) (described in detail by Blackburn and Waldock 1995; Kelly 2000). Briefly, the estrogenic chemicals were immobilized on a C18 silica-bonded solid-phase extraction column, eluted, and analyzed by GC-MS.
Biological sampling
After 300 days of exposure, 60 roach from each treatment were sacrificed with a lethal dose of anesthetic (benzocaine). Thirty fish from each treatment were weighed (milligrams) and their fork length measured (millimeters) for growth analyses. These fish were then placed into cryovials, frozen on dry ice, and stored at −20°C for subsequent analysis of VTG and sex steroids. Thirty fish from each treatment were fixed in Bouin’s solution for 24 hr and stored in 70% industrial methylated spirits before processing for histological analysis of gonad and kidney development. A further 20 fish from each treatment group transported live from the field to the laboratory and were processed for analysis of genetic damage and immunotoxicity.
Gonadal and kidney histology
Transverse tissue blocks of roach whole bodies (30 from each treatment) were prepared by cutting each fish trunk either side of the dorsal fin. Samples were then embedded in paraffin wax, sectioned at 5 μm, mounted and stained with hematoxylin and eosin, and analyzed by light microscopy. Any abnormalities in gonadal development were assessed (feminized reproductive ducts or developing oocytes in the testes) according to Nolan et al. (2001).
Each histological section was also assessed for any abnormalities of the structure of the kidney. In fish, injured kidney nephrons have the capacity for repair after toxicant-induced damage. Fish have also been shown to be able to produce developing nephrons (DNs) after renal damage (Reimschuessel et al. 1990, 1993; Salice et al. 2001). During the process of de novo nephron neogenesis, a basophilic cell cluster (BC) forms, which develops a cavity to form the renal vesicle. This grows and becomes first C-shaped and then S-shaped. One end of the S-shape indents farther and forms the glomerulus and Bowman’s capsule, whereas the other end grows out farther and fuses with the archinephric (collecting) duct. The tubules elongate, coil, and intertwine as the nephron develops (Reimschuessel 2001). BCs and DNs have been proposed as useful biomarkers for assessing nephrotoxicity (Cormier et al. 1995), and in this study, numbers of glomeruli, DNs, and BCs were counted over one field of view of a kidney cross-section. Kidney tubule diameter was measured for 10 nephrons in each kidney section. Measurements were taken using image analysis software (analySIS, version 3.2; Soft Imaging System, GmHB, Münster, Germany).
VTG immunohistochemistry
We used immunohistochemistry to detect the presence and assess the distribution of VTG in the body tissues of 10 male and 10 female juvenile roach from the control and 80% effluent treatments, using a carp VTG polyclonal antibody (validated for use in the roach; Tyler et al. 1996). For immunohistochemistry, glass microscope slides were treated with Vectabond (Vector Laboratories Ltd, Peterborough, UK) to aid slide attachment of paraffin sections and prevent detachment during processing. Whole-body sections of juvenile roach were cut at 5 μm and applied to treated slides at three sections per slide. Two tissue sections were incubated with the first (unlabeled) antibody (anti-carp VTG, raised in rabbit), and a solution of blocking buffer and 0.03% hydrogen peroxide was applied to the third section on each slide to demonstrate any nonspecific binding of antibody. A labeled second antibody was then applied (goat anti-rabbit IgG, conjugated with peroxidase). The horseradish peroxidase label was visualized using 3,3′-diaminobenzidine (DAB), which produces a brown precipitate (Sigma Fast DAB kit; Sigma, Poole, Dorset, UK). Sections were then counterstained with hematoxylin, dehydrated, and mounted with dibutyl phthalate polystyrene xylene mountant.
Measurement of VTG in the body
We prepared whole-body homogenates by defrosting the bodies of individual fish on ice and homogenizing with a phosphate-buffered saline, 0.05% Tween, and 1% bovine serum albumin, pH 7.4 (1 mL buffer/g of fish). After centrifugation at 15,000 rpm, the supernatant was removed and stored at −20°C. Quantification of whole-body VTG was determined using a carp VTG enzyme-linked immunosorbent assay (ELISA) (Tyler et al. 1999) until assayed in the VTG ELISA that has been validated for use in the roach (Tyler et al. 1996).
Assessment of genetic integrity
Comet assay
We used the comet assay to investigate the level of DNA damage in single cells in terms of strand breaks and alkali labile sites. The assay was performed on blood and gill cells of 30 fish from each treatment. The methodology was essentially as described by Singh et al. (1988). Gills were dissected and placed in 500 μL of phosphate buffer (50 mM sodium phosphate, pH 7.4) on ice. The gills were homogenized gently, and the cells were filtered through a 30-μm mesh to produce a single-cell suspension. Blood (5 μL) was placed into 1 mL of 4°C phosphate buffer. Two hundred micro-liters of the gill or blood cell suspension was centrifuged at 2,000 rpm at 4°C for 2–3 min, after which the supernatant was removed. Eighty-five microliters of 1% low-melting-point agarose was pipetted onto the cell pellet. The cell suspension was then carefully pipetted onto a frosted slide coated with high-melting-point agarose, sandwiching the cells between two layers of agarose gel. After the gel had set, the slides were placed into a freshly prepared lysing solution at 4°C for 1 hr [2.5 M NaCl, 100 mM Na2–EDTA, 10 mM Tris, 1% Na sarcosinate (pH 10.0), 1% Triton X-100, and 10% dimethylsulfoxide]. The lysis and following steps of the comet assay protocol were carried out in the dark and at 4°C to prevent any additional damage to the cellular DNA.
The slides were then removed from the lysis solution and placed on a horizontal gel electrophoresis tank. The tank was filled with fresh electrophoresis buffer (300 mM NaOH, 1 mM Na2–EDTA) and the DNA allowed to unwind for 40 min before electrophoresis, which was carried out at 25 V for 30 min. After electrophoresis the slides were washed for 5 min in neutralizing buffer (0.4 M Tris, pH 7.5), and the wash was repeated 3 times. The slides were stained with 20 μL of 5 μg/mL ethidium bromide solution, viewed under ultraviolet fluorescence light, and scored using Komet software (version 5.0; Kinetic Imaging Ltd. Wirral, UK). A total of 100 randomly chosen cells were scored per fish, 50 per replicate.
Micronucleus test
Micronuclei provide an index of both chromosome breakage and chromosome loss. We used blood and gill suspensions for analysis of micronucleus/micronuclei (Mn) formation as described by Das and Nanda (1986). Gills were dissected and placed in 500 μL of phosphate buffer on ice. The gills were homogenized gently, and the cells filtered through a 30-μm mesh to produce a single-cell suspension. Gill suspension (200–300 μL) or a drop of blood was smeared onto slides that had been precoated in 10% poly-l-lysine solution. The slides were left to air dry and then fixed in methanol for 15 min, followed by staining in 5% Giemsa buffer solution for 20 min, after which the slides were mounted with a coverslip. Slides were scored blind under 40× magnification, and Mn were validated under oil immersion. A total of 1,000 red blood cells and 1,000 gill cells were analyzed for the presence of Mn per fish (Countryman and Heddle 1976).
Assessment of immunotoxicity
We used a tiered approach to assess the integrated functioning of the immune system in the test fish (Galloway and Handy 2003; Zelikoff et al. 1994). A general screen of immune function included total blood cell count and histology. Functional parameters included differential blood cell count (Stolen 1995) and phagocytic activity of spleen and kidney cells. Total and differential blood cell counts were determined microscopically using a hemocytometer. The phagocytic activity of kidney and spleen cells was determined by measuring the uptake of zymosan particles (from Saccharomyces cerevisae) dyed with neutral red dye. In this method (Rickwood and Galloway 2004), particle uptake by adhered cells is estimated by absorbance at 540 nm against a standard curve prepared using zymosan particles in the range of 1.56–100 × 107/mL. The protein concentration of each hemolymph sample from the phagocytosis assay was determined using the Bradford protein assay. The viability of spleen and kidney cells was determined by measuring their ability to retain neutral red dye (Babich and Boernfreund 1994). Cells (50 μL) were incubated in triplicate in flat-bottomed microtiter plates in order to allow a monolayer of cells to adhere to the wells. After 45 min nonadhered cells were discarded and the plates washed with phosphate buffer. A solution of 0.004% neutral red dye in phosphate buffer (200 mL) was added to each well, and the cells were incubated for a further 3 hr at room temperature. The wells were washed, and an acidified solution of 1% acetic acid and 20% ethanol was added to resolubilize the dye. The plate was gently shaken for 10 min before reading the absorbance at 540 nm.
Statistical analyses
We performed all statistical analyses using Sigmastat (version 2.0; Jandel Scientific, Woking, Surry, UK) or Statgraphics (version 5; Rockville, MD, USA). Statistical significance was accepted at p < 0.05 for all comparisons. Intergroup differences were assessed using one-way analysis of variance (parametric, for normalized data) or Kruskal-Wallis test (non-parametric). Multiple comparisons tests were performed using post-hoc analyses for parametric or nonparametric data.
Results
Concentrations of steroid estrogens and alkylphenolic chemicals in the test effluent
Chemical analysis of the effluent showed that concentrations of estrone were between 6.5 and 8.6 ng/L, and of 17β-estradiol between 0.7 and 3.6 ng/L. The synthetic estrogen 17α-ethinylestradiol was not detected in the effluent at any of the sampling points (limit of detection, 0.5 ng/L). Concentrations of nonylphenol ranged between 0.62 and 0.92 μg/L, and concentrations of nonylphenol mono- and diethoxylates were between 0.79 and 2.7 μg/L. Concentrations of octylphenol were between 0.1 and 0.41 μg/L.
Effects of effluent exposure on fish growth and survival
We could not determine the rate of mortality in these experiments because the aquaria were too large to catch all the fry without damaging them. At the end of the trial, the fish in the 80% effluent treatment group were significantly larger than fish from the tap water control group or from the 40% effluent treatment group (p < 0.05) but equal in size to those in the 20% effluent treatment group. Differences in growth between treatments were not found to be related to the concentration of the effluent and may be due to stocking density and/or natural food availability.
Gonadal histopathology
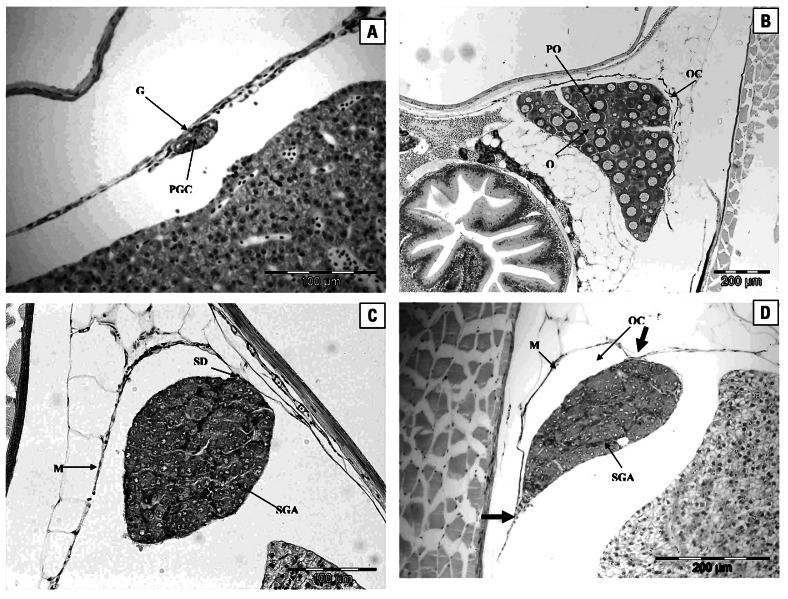
Gonad histopathology of roach at 300 dph revealed that there were four gonad phenotypes: undifferentiated, female, male, and male with female reproductive ducts (intersex; Figure 1A–D). Undifferentiated fish had no discernable reproductive duct and no obviously differentiated germ cells (Figure 1A). A greater proportion of the fish were undifferentiated in the controls compared with those in any of the other treatments. In the 80% effluent treatment, all the fish had differentiated. The frequencies of female or male fish did not differ significantly between the different treatments. Ovaries of phenotypic female fish contained primary oocytes (Figure 1B), and there were no discernable differences in the status of the ovaries in control fish compared with effluent-exposed females. The testes in phenotypic males in the control group contained spermatogonia A or both spermatogonia A and B. In some males the testes consisted of well-defined cysts of spermatogonia A and B (Figure 1C), and several individuals had very advanced testes containing spermatogonia A and B, spermatocytes, and spermatids within the cyst structures. Some male fish that had been exposed to WwTW effluent had feminized reproductive ducts. These testes contained male germ cells (spermatogonia A, and both spermatogonia A and B in more advanced fish) but were connected to the body wall by two distinct points of attachment, forming a femalelike duct or ovarian cavity (Figure 1D).
Figure 1.
Histopathology of roach. (A) Undifferentiated gonad (G) of a 300-dph roach. The gonad consists of several primordial germ cells (PGC). Scale bar = 100 μm. (B) Ovary of a 300-dph female roach reared in tap water. The ovary contains oogonia (O) and primary oocytes (PO). The plate also shows the ovarian cavity (OC). Scale bar = 200 μm. (C) Testis of a 300-dph male roach reared in tap water. The testis contains spermatogonia A (SGA) and is connected to the mesentery (M) by a single point of attachment, the sperm duct (SD). Scale bar = 100 μm. (D) Gonad of a 300-dph intersex roach reared in 80% effluent. The testis contains spermatogonia A (SGA) and is connected to the mesentery (M) by two points of attachment (large arrows), forming a femalelike ovarian cavity (OC). Scale bar = 200 μm.
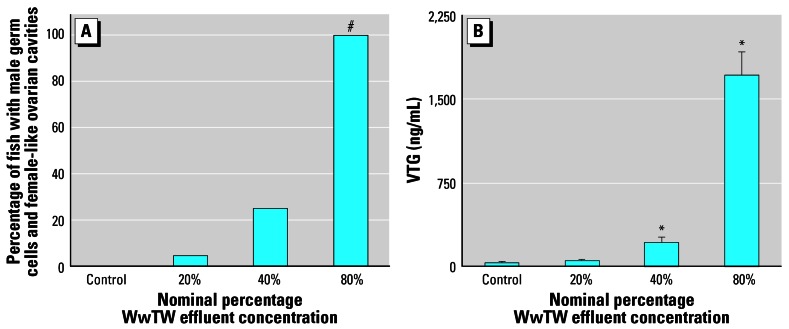
All sexually differentiated female fish had an ovarian cavity (female reproductive duct), as expected. Furthermore, in the effluent-treated tanks, there was a concentration-related response in the number of fish with obvious male germ cells and ovarian cavities (p < 0.001). The frequencies of ovarian cavities or femalelike reproductive ducts in sexually differentiated male fish are shown in Figure 2A. At 300 dph the incidences of duct feminization in fish sexually differentiated with male germ cells were 4.5, 25, and 100% for the 20, 40, and 80% effluent-exposed groups, respectively. None of the males derived from control tap water contained an ovarian cavity.
Figure 2.
(A) Percentage of fish with male germ cells that had femalelike ovarian cavities (i.e., feminized males) in each treatment group. (B) Mean whole-body VTG concentrations (n = 30; mixed sex) for all treatment groups. Error bars are SEM.
Significantly different from control: *p < 0.05, #p < 0.001.
Whole-body VTG concentrations
As with the feminization of the reproductive ducts in male fish, there was a concentration-related response for whole-body VTG with increasing concentrations of effluent (Figure 2B). Fish exposed to 80% effluent had significantly higher body VTG content (1,709 ± 211 ng/mL) compared with controls (33 ± 9 ng/mL).
Localization of VTG in body tissues
In control fish, we did not detect VTG on any of the sections in any tissue in either male or female fish (detection capability for the immunolocalization technique employed, ~ 100 ng). This corresponded with VTG measurements for whole-body homogenates and the status of sexual development in females (all were at the previtellogenic stage). However, in fish exposed to 80% effluent, VTG was detected in all sections and had permeated many tissues. VTG was detected in the liver, its site of synthesis, and particularly in hepatic blood vessels. It was also observed to accumulate in the kidneys, specifically around the tubules, and in the gonad. In the ovary and testis of effluent-exposed fish, VTG was detected in the blood vessels and between the germ cells (oocytes in the ovary and spermatogonia in the testis). No differences were apparent in amount or location of VTG in the somatic tissues between effluent-exposed males and females.
Genetic damage
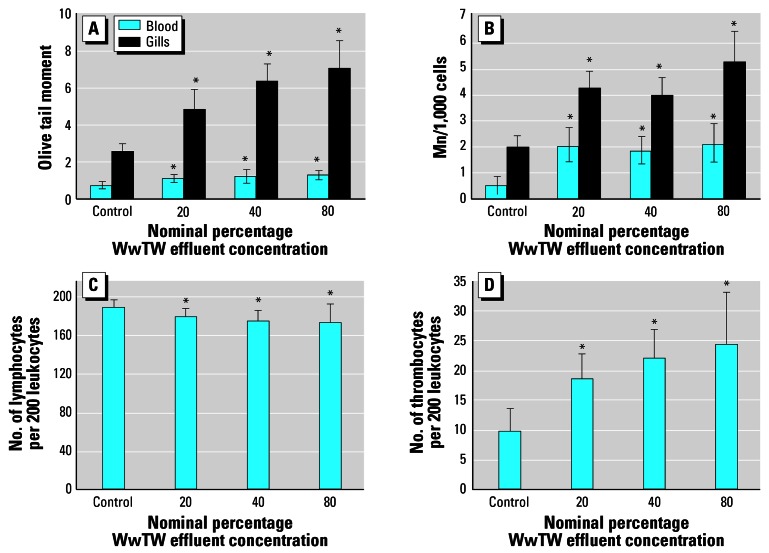
We found significant differences in the incidence of single-strand DNA breaks among treatments assessed using the Olive tail moment for blood and gill cells (Figure 3A). Olive tail moment is defined as the product of the tail length and the fraction of total DNA in the tail (intensity of DNA in the tail). Fish exposed to only 20% WwTW effluent had a significantly greater incidence of single-strand DNA breaks than the controls in both blood (p < 0.005) and gill (p < 0.001). Moreover, this level of damage was also present in fish exposed to all other concentrations of effluent. There was no dose relationship in the number of single-strand breaks found in blood cells of effluent-exposed fish, although there were differences in gill cells (p < 0.001, R2 = 0.575). The fish exposed to the 20% effluent had significantly less damage than those fish exposed to either 40 or 80% effluent. There was no difference in the degree of damage between fish exposed to 40 or 80% effluent. In addition to the incidence of single-strand breaks, there were also significant differences in the induction of Mn in the blood (p < 0.001) and gill cells (p < 0.001) between the control fish and fish exposed to all effluent concentrations (Figure 3B). Generally there was no significant difference between any of the concentrations of effluent, except for a significant difference between the Mn frequency in gill cells from fish exposed to the 40 and 80% concentrations.
Figure 3.
(A) Olive tail moment (comet assay) in roach blood cells (n = 20 fish for all treatment groups). (B) Average number of Mn per 1,000 roach cells (n = 20 fish). (C) Average number of lymphocytes per 200 leukocytes in blood of roach (n = 20 fish). (D) Average number of thrombocytes per 200 leukocytes in blood of roach (n = 20 fish). Error bars represent 2× SE.
Significantly different from control: *p < 0.001.
Immunotoxic effects
We found no significant differences in the weight or histology of the spleen (data not shown). In addition, we observed no functional differences in cell viability or phagocytic activity, and the antioxidant capacity of the cells remained unaltered. There were, however, significant changes in differential cell count. There was a decrease in the proportion of circulating lymphocytes with increasing concentrations of effluent (p < 0.05; Figure 3C) and a concomitant increase in the proportion of thrombocytes in the blood in fish with increasing concentrations of effluent (Figure 3D; p < 0.05). Statistically significant effects for both the number of lymphocytes and thrombocytes occurred for all effluent concentrations tested.
Kidney histopathology
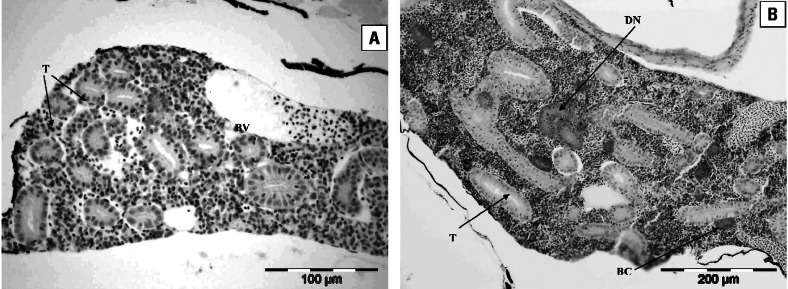
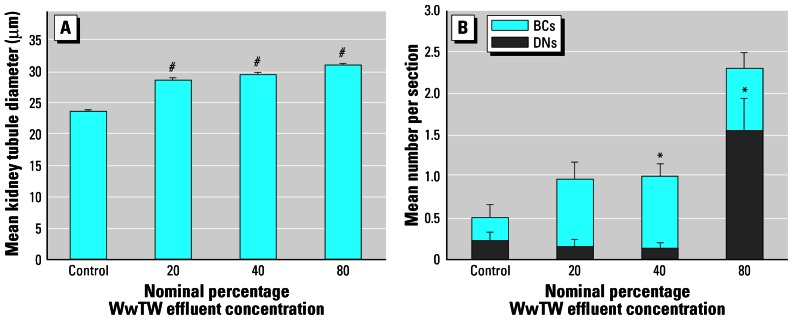
Figure 4A and B, shows typical sections of the trunk kidney of 300 dph roach exposed to tap water or to 80% WwTW effluent, respectively. Kidney tubules were surrounded by hematopoietic tissue and were normal in structure in both control and effluent-treated fish. The diameter of nephrons, however, was increased in a concentration-dependent manner with increasing effluent concentration. Tubule diameter in fish exposed to all effluent concentrations was greater than in the control fish (p < 0.001; Figure 5A). There was no evidence of any degeneration, hemorrhage, or accumulation of eosinophilic material in the tubule lumen. Glomeruli and associated Bowman’s capsules were observed in many of the trunk regions of the kidney cross-sections, and all glomeruli observed were considered to be normal and healthy. There was an apparent concentration-dependent increase in glomeruli in effluent-exposed fish, but this was not statistically significant (data not shown). There was a higher occurrence of BCs in the 40% effluent group but not in the 80% effluent-exposed fish (Figure 5B). There was also a higher occurrence of DNs in the 80% effluent-exposed fish (p < 0.05) but not in fish in the lower effluent concentrations. Within each treatment group and in the controls, no gender-related differences were seen for any of the effects.
Figure 4.
Histopathology of roach kidney. (A) Transverse section through the trunk kidney of a 300 dph roach reared in tap water. Kidney tubules (T) and a blood vessel (BV) are shown. Scale bar = 100 μm. (B) Transverse section through the trunk kidney of a 300-dph roach reared in 80% effluent. Several BCs and DNs were present at various stages of development, among mature tubules (T). Scale bar = 200 μm.
Figure 5.
Kidney histopathology parameters. (A) Kidney tubule diameter of roach (± SE). (B) Number of BCs and DNs per trunk kidney section (± SE).
Significantly different from control: *p < 0.05, #p < 0.001.
Discussion
The results of this experiment clearly demonstrate that concentrations of treated WwTW effluents that induce feminization of male roach also alter kidney development, modulate immune function, and cause genotoxic damage in exposed animals. Moreover, the genotoxic and immunotoxic effects measured appeared to occur at concentrations of WwTW effluent lower than that required to induce recognizable changes in the structure and function of the reproductive endocrine system.
Other field studies have also reported genotoxic (Raiaguru et al. 2003) and immunotoxic (Kakuta and Murachi 1997; Secombes et al. 1991) effects in fish and invertebrates living downstream of WwTW effluent discharges. In some cases, these effects manifest quite rapidly and at low concentrations of effluent. As an example, the frequency of Mn in erythrocytes in the sea bass, Dicentrarchus labrax, increased significantly at 4, 8, 16, 24, 48, and 96 hr after exposure to 0.1 and 1% secondary treated industrial/urban effluent discharged into the Aveiro coastal area, Portugal (Gravato and Santos 2003). In another case, however, no difference in Mn formation was reported in fish from a WwTW site compared with fish from a clean water site (Grisolia and Starling 2001), thus indicating that not all effluents are genotoxic. In our present study, even the lowest concentration of the WwTW effluent tested (20%) induced genotoxic effects; hence the threshold concentration for genotoxicity for this effluent could not be established.
In addition to its genotoxic and endocrine effects, the treated WwTW effluent tested induced changes to the immune system. Previous studies have indicated that adult gold-fish (Carassius auratus) exposed for 30 days to treated WwTW effluent diluted to 5% experienced significant immunosuppression (Kakuta and Murachi 1997). Both the total number and the function (phagocytic activity) of lymphocytes and granulocytes were decreased, thus leading to an increased susceptibility to infection (Kakuta and Murachi 1997). Secombes et al. (1991) found less marked immunosuppressive effects of treated WwTW effluent in dab, Limanda limanda, exposed for 12 weeks, although a small decrease in circulating thrombocytes was noted. This indicates either that different species may show different susceptibilities for immunosuppressive effects of WwTW or that different WwTW effluents have different compositions (Secombes et al. 1991). Life stage may be more important than the concentration and duration of exposure for immunosuppressive effects. A recent study showed that a short-term exposure of very early life-stage chinook salmon, Oncorhynchus tshawytscha, to organic contaminants induced long-term effects on immune competence (1 year after treatment) in the absence of any effects on growth and reproduction (Milston et al. 2003).
Both VTG and feminization of the reproductive ducts seen in this study are known to be induced in male fish after exposure to estrogenic chemicals or to WwTW effluents containing estrogenic chemicals (Gimeno et al. 1997; Rodgers-Gray et al. 2001). The concentrations of steroid estrogens and alkylphenolic chemicals in the study effluent were comparable with those measured in other WwTW effluents in the United Kingdom and worldwide, although concentrations of steroid estrogens were in the lower range of concentrations reported in other studies (Eggen et al. 2003; Lee and Peart 1998; Spengler et al. 2001; Ternes et al. 1999). Notwithstanding this, the effective effluent concentration (40%) for induction of an ovarian cavity in male roach in this study was similar to that shown for another WwTW effluent (Rodgers-Gray et al. 2001), whereas the threshold for VTG induction was higher than that previously reported. If exposed to an estrogenic stimulus, even young roach can produce VTG (Rodgers-Gray et al. 2001), and our recent studies have shown that estrogen receptors α and β are expressed in roach as early as 28 dph (Katsu Y, Iguchi T, Lange N, Tyler CR, unpublished data), well before the onset of gametogenesis (roach mature as 2- or 3-year-olds). The titer of VTG produced in the juvenile roach in this study (1,709 ng/mL) was relatively low compared with the induction seen in roach exposed to other WwTW effluents, for example, mean homogenate VTG titers of 5,684 ng/mL and 27,040 ng/mL reported in juvenile roach after exposure to a WwTW effluent (Liney et al. 2005; Rodgers-Gray et al. 2001), reflecting the somewhat lower levels of steroid estrogens in this effluent compared with others that have been studied. The magnitude of VTG induction in the juvenile roach, however, was very similar in adult roach exposed to this same effluent (Liney et al. 2005), thus confirming the previously documented sensitivity of the early life stages of fish to estrogens (van Aerle et al. 2002). Immunostaining with antibodies against VTG revealed that VTG induced in the effluent-exposed fish was detectable in many of the body tissues, including the liver (the site of its synthesis), kidney, and gonad (ovaries and testes). Ovaries in the female fish were at the previtellogenic stage of development and thus would not normally be exposed to or incorporate VTG. The VTG that had entered the ovary, however, was not incorporated into the oocytes but rather was located in the interstitial tissues. In the testes, VTG was similarly localized in the interstitial tissue. The kidney in the effluent-exposed fish was especially loaded with VTG. Eosinophilic material has been observed to accumulate in the tubules and Bowman’s capsule of fish exposed to a variety of estrogenic compounds, including estradiol (Folmar et al. 2001b; Zaroogian et al. 2001) and ethinylestradiol (Schwaiger et al. 2000; Weber et al. 2003). Ethinylestradiol has also been reported to cause severe hemorrhage within the kidney tubule lumen when administered in large doses by injection (500 μg/kg body weight) (Schwaiger et al. 2000). High levels of VTG induction have also been linked to renal failure and subsequent death of fish (Folmar et al. 2001b; Herman and Kincaid 1988). However, laboratory exposures of flounder to the common alkylphenolic compound octylphenol and to isomers of DDT (dichlorodiphenyltrichloroethane) at very high doses via injection did not induce the kidney pathology observed when fish were injected with estradiol in the same study (Mills et al. 2001; Zaroogian et al. 2001). Aquatic exposure of carp to nonylphenol at environmentally relevant concentrations also did not cause any observable renal disruption (Weber et al. 2003). Field studies have reported renal disruption and accumulation of material in kidney tubules in fish sampled from rivers (Schmidt-Posthaus et al. 2001; Schrank et al. 1997), lakes (Koponen et al. 2001), and estuaries (Simpson et al. 2000; Stentiford et al. 2003) known to be contaminated with a variety of EDCs and other contaminants.
It is important to consider that fish collected from the field in these studies had been exposed to a mixture of many aquatic pollutants, and pathology observed in these cases may be due not only to the effects of EDCs but also to any possible nephrotoxic agents present. Induction of VTG in the young roach exposed to effluent was associated with increased numbers of glomeruli, increases in size of kidney tubules, and higher numbers of BCs and DNs (reflecting the increased demand for filtration of VTG for its removal from the circulation). There was no evidence, however, of severe renal damage. Complementary studies have also shown a capacity for renal repair after exposure to effluent during early life; fish exposed as embryos to this effluent and subsequently allowed to depurate for 240 days (to 300 dph) in clean water showed no effects on kidney structure (Liney KE, Jobling S, Tyler CR, unpublished data). After nephrotoxic damage, the number of DNs has been shown to significantly increase. This effect has now been reported after exposure to a variety of renal toxicants, in many fish species at all life stages, including rainbow trout (Reimschuessel et al. 1993), goldfish (Reimschuessel et al. 1990), tilapia (Augusto et al. 1996), and zebrafish (Reimschuessel 2001). It has been proposed that BCs and DNs may be useful biomarkers for assessing nephrotoxicity (Cormier et al. 1995). Field evidence indicates that wild fish sampled from sites of known contamination have higher numbers of BCs and DNs than do fish of the same species taken from less contaminated reference sites (Cormier et al. 1995). Many toxicants are present in effluents, and this implies that VTG and other estrogen-inducible proteins are not necessarily the only contributing factors to the increased kidney tubule size and other renal effects seen in the fish in this study.
Identification of the individual causative agents of the various types of toxicity observed here was beyond the scope of this study, but it is possible to draw strong associations between the effects seen and certain chemicals present in effluent, based on findings in the published literature. The overall estrogenic potency of WwTW effluents from domestic sources is principally determined by steroid estrogens (Desbrow et al. 1998) and, to a lesser extent, by alkylphenolic compounds, and substantial evidence suggests a causal association between steroid estrogens in effluent and VTG induction in fish. Many studies have shown that steroid estrogens (and in some cases alkylphenolic chemicals) induce VTG in fish at various life stages at concentrations measured in WwTW effluents (Routledge et al. 1998). The causative agent or agents of duct disruption in the developing roach observed in this study are, however, unknown. Feminization of the reproductive duct has been induced in male fish during laboratory exposure to estrogenic chemicals commonly found in WwTW effluents, including estradiol (Gimeno et al. 1996), ethinylestradiol (van Aerle et al. 2002), and alkylphenolic compounds (Gimeno et al. 1997, 1998). The concentrations of any of these estrogenic chemicals required to do this, however, were higher than those found in the effluent tested here, which suggests that feminization of the reproductive ducts is probably due to combination effects of estrogens present in the effluents and/or to effects via other mechanisms of action.
Several environmental pollutants, including polycyclic hydrocarbons (PAHs) and heavy metals (e.g., tributyltin and lead) can cause DNA damage, by direct strand breakage, Mn formation, or metabolic conversion into reactive intermediates that form unstable DNA adducts (Ferraro et al. 2004). Heavy metals have a tendency to bind to phosphates and a wide variety of organic molecules, including base residues of DNA, which can lead to mutations by altering the primary and secondary structures of the DNA. EDCs present in treated WwTW effluents, such as steroidal estrogens [Dhillon and Dhillon 1995; reviewed by Joosten et al. (2004); Liehr 2000], xenoestrogens [nonylphenol, nonylphenol polyethoxylates (NPEOs); Harreus et al. 2002], and BPA (Suarez et al. 2000), are also capable of inducing various types of genetic lesions, including aneuploidy, chromosomal aberrations, Mn, and DNA strand breaks both in vitro (Dhillon and Dhillon 1995; Yared et al. 2002) and in vivo (Gronen et al. 1999). 17β-Estradiol has also been shown to attenuate nucleotide excision repair (Evans et al. 2003) and hence might perpetuate any damage caused by genotoxins and prolong the time taken for DNA repair. The literature would, however, suggest that the concentrations of any of these chemicals required to induce genotoxicity in vitro are higher than those found in most treated WwTW effluents. NPEO (Harreus et al. 2002) and BPA (Ochi 1999; Pfeiffer et al. 1997; Suarez et al. 2000), for example, induced DNA damage (strand breaks, Mn, aneuploidy, and/or adduct formation) at concentrations ≥ 10 μg/L (Harreus et al. 2002) and 12 ng/L, respectively. Estradiol and estrone induced increases in Mn and dose-related increases in strand breaks in human breast cancer cell lines (Yared et al. 2002) at concentrations as low as 10−9 M (~ 200 ng/L). Experiments to investigate the microsomal metabolism of estrogen in the channel catfish Ictalarus punctatus have indicated that the predominant metabolites of estradiol formed by the liver, gill, and gonad microsomes were 2-hydroxyestradiol and estrone but that exposure to the genotoxic compound benzopyrene induced the hepatic formation of the 4-hydroxyestradiol, a potentially genotoxic estrogen metabolite (Butala et al. 2004). These results suggest that highly reactive genotoxic metabolites of estradiol may form in fish when exposed to genotoxic and estrogenic compounds simultaneously.
Many of the chemical pollutants commonly found in WwTW effluents have been shown individually to affect immune function (Dean et al. 1994; Vos 1977). Although most of this work has been performed in mammals, many of the chemical pollutants capable of suppressing human immune responses have since been found to alter immune responses in fish and, in many cases, bring about similar effects and act via similar mechanisms (Galloway and Handy 2003; Zelikoff et al. 1994). This is true not only for xenobiotics such as PAHs, solvents, pesticides, aromatic amines, and heavy metals but also for endogenous hormones that can act indirectly to suppress immune function (Zelikoff et al. 1994). In a field study of feral fish populations exposed to PAH-contaminated sediments, a decrease in the activity of nonspecific cytotoxic cells (NCCs) was attributed to PAH-induced degradation of the NCC surface receptor (Faisal et al. 1991). However, chronic and acute exposures may cause the release of immunosuppressive neuroendocrine mediators, and the immunosuppression in these studies may have been due to the release of endogenous opioids (Faisal et al. 1991). This highlights the ways in which both direct and indirect (nonchemical) stress factors may contribute toward immunosuppression and hence susceptibility to disease, particularly in relation to complex mixtures such as WwTW effluents.
When taken together, the data presented demonstrate that WwTW effluents (and the chemicals therein) have a multiplicity of biological effects and can be genotoxic and immunotoxic and/or can cause endocrine disruption in fish. An unexpected finding in our study was that the damage to DNA (measured by the comet assay), increased number of Mn, and alteration in numbers of lymphocytes found in the exposed fish were more responsive to the treated effluent than VTG induction or feminization of the reproductive ducts because the effective concentrations of effluent inducing these effects were half (or even less) for the concentrations found to induce endocrine (feminizing) effects or effects on kidney development (BCs and DNs; Figure 6). These findings thus indicate the need for multiple biological end points in tests for assessing the potential health effects of WwTW effluent.
Figure 6.
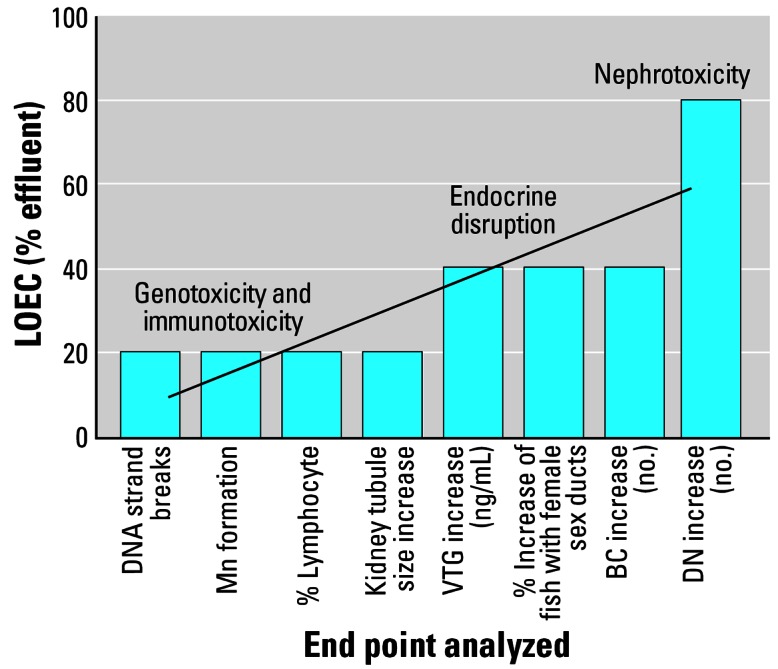
Lowest observed effect concentrations (LOEC) for different forms of developmental toxicity.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
We gratefully acknowledge the support of the participating water companies in this work and the members of the research teams at Exeter and Brunel for their help in sampling the fish. We recognize the (unpublished) work of A. Filby, who developed the basis for assessing the effects of endocrine-disrupting chemicals on kidney structure in fish. We acknowledge the contribution of E. Santos in establishment of the vitellogenin immunohistochemistry protocol. The analytical chemistry was carried out by Centre for Environment, Fisheries, and Agriculture Science, Burnham-on-Crouch, United Kingdom.
References
- Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2001;20(6):1276–1290. [PubMed] [Google Scholar]
- Atienzar FA, Billinghurst Z, Depledge MH. 4-n-Nonylphenol and 17-beta estradiol may induce common DNA effects in developing barnacle larvae. Environ Pollut. 2002;120(3):735–738. doi: 10.1016/s0269-7491(02)00184-7. [DOI] [PubMed] [Google Scholar]
- Augusto J, Smith B, Smith S, Robertson J, Reimschuessel R. Gentamicin-induced nephrotoxicity and nephroneogenesis in Oreochromis nilotica, a tilapian fish. Dis Aquat Org. 1996;26(1):49–58. [Google Scholar]
- Babich H, Boernfreund E. An in vitro assay of cytotoxicity. Appl Environ Microbiol. 1994;57:2101–2103. doi: 10.1128/aem.57.7.2101-2103.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SK, Banerjee S, Li SA, Li JJ. Induction of chromosome-aberrations in Syrian-hamster renal cortical-cells by various estrogens. Mutat Res Fundam Mol Mech Mutagen. 1994;311(2):191–197. doi: 10.1016/0027-5107(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Blackburn MA, Waldock MJ. Concentrations of alkylphenols in rivers and estuaries in England and Wales. Water Res. 1995;29(7):1623–1629. [Google Scholar]
- Butala H, Metzger C, Rimoldi J, Willett KL. Microsomal estrogen metabolism in channel catfish. Mar Environ Res. 2004;58(2–5):489–494. doi: 10.1016/j.marenvres.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Choi SM, Yoo SD, Lee BM. Toxicological characteristics of endocrine-disrupting chemicals: developmental toxicity, carcinogenicity, and mutagenicity. J Toxicol Environ Health B Crit Rev. 2004;7(1):1–32. doi: 10.1080/10937400490253229. [DOI] [PubMed] [Google Scholar]
- Christiansen LB, Pedersen KL, Pedersen SN, Korsgaard B, Bjerregaard P. In vivo comparison of xenoestrogens using rainbow trout vitellogenin induction as a screening system. Environ Toxicol Chem. 2000;19(7):1867–1874. [Google Scholar]
- Cormier SM, Neiheisel TW, Wernsing P, Racine RN, Reimschuessel R. New nephron development in fish from polluted waters—a possible biomarker. Ecotoxicology. 1995;4(3):157–168. doi: 10.1007/BF00116479. [DOI] [PubMed] [Google Scholar]
- Countryman PI, Heddle JA. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat Res. 1976;41:321–331. doi: 10.1016/0027-5107(76)90105-6. [DOI] [PubMed] [Google Scholar]
- Das RK, Nanda NK. Induction of micronuclei in peripheral erythrocytes of fish Heteropneustes-fossilis by mitomycin-C and paper-mill effluent. Mutat Res. 1986;175(2):67–71. [Google Scholar]
- Dean JH, Luster ML, Munson AE, Kimber I, eds. 1985. Immunotoxicity and Immunopharmacology, 2nd ed. New York:Raven Press.
- Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol. 1998;32(11):1549–1558. [Google Scholar]
- Dhillon VS, Dhillon IK. Genotoxicity evaluation of estradiol. Mutat Res Genet Toxicol. 1995;345(1–2):87–95. doi: 10.1016/0165-1218(95)90073-x. [DOI] [PubMed] [Google Scholar]
- Eggen RI L, Bengtsson BE, Bowmer CT, Gerritsen AAM, Gibert M, Hylland K, et al. Search for the evidence of endocrine disruption in the aquatic environment: lessons to be learned from joint biological and chemical monitoring in the European Project COMPREHEND. Pure Appl Chem. 2003;75(11–12):2445–2450. [Google Scholar]
- Evans MD, Butler JM, Nicoll K, Cooke MS, Lunec J. 17 Beta-oestradiol attenuates nucleotide excision repair. FEBS Lett. 2003;535(1–3):153–158. doi: 10.1016/s0014-5793(02)03898-x. [DOI] [PubMed] [Google Scholar]
- Faisal M, Weeks BA, Vogelbein WK, Huggett RJ. Evidence of aberration of the natural cytotoxic-cell activity in Fundulus heteroclitus (Pisces, Cyprinodontidae) from the Elizabeth River, Virginia. Vet Immunol Immunopathol. 1991;29(3–4):339–351. doi: 10.1016/0165-2427(91)90024-7. [DOI] [PubMed] [Google Scholar]
- Ferraro MVM, Fenocchio AS, Mantovani MS, Ribeiro CD, Cestari MM. Mutagenic effects of tributyltin and inorganic lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay and the piscine micronucleus and chromosome aberration tests. Genet Mol Biol. 2004;27(1):103–107. [Google Scholar]
- Floyd RA, West MS, Eneff KL, Schneider JE, Wong PK, Tingey DT, et al. Conditions influencing yield and analysis of 8-hydroxy-2’-deoxyguanosine in oxidatively damaged DNA. Anal Biochem. 1990;188(1):155–158. doi: 10.1016/0003-2697(90)90544-j. [DOI] [PubMed] [Google Scholar]
- Folmar LC, Denslow ND, Kroll K, Orlando EF, Enblom J, Marcino J, et al. Altered serum sex steroids and vitellogenin induction in walleye (Stizostedion vitreum) collected near a metropolitan sewage treatment plant. Arch Environ Contam Toxicol. 2001a;40(3):392–398. doi: 10.1007/s002440010188. [DOI] [PubMed] [Google Scholar]
- Folmar LC, Denslow ND, Rao V, Chow M, Crain DA, Enblom J, et al. Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinus carpio) captured near a major metropolitan sewage treatment plant. Environ Health Perspect. 1996;104:1096–1101. doi: 10.1289/ehp.961041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmar LC, Gardner GR, Schreibman MP, Magliulo-Cepriano L, Mills LJ, Zaroogian G, et al. Vitellogenin-induced pathology in male summer flounder (Paralichthys dentatus) Aquat Toxicol. 2001b;51(4):431–441. doi: 10.1016/s0166-445x(00)00121-1. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Leonard LS, Lovell S, Gould JC, Babai D, Portier CJ, et al. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997;143(1):205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- Galloway T, Handy R. Immunotoxicity of organophosphorous pesticides. Ecotoxicology. 2003;12(1–4):345–363. doi: 10.1023/a:1022579416322. [DOI] [PubMed] [Google Scholar]
- Gimeno S, Gerritsen A, Bowmer T, Komen H. Feminization of male carp. Nature. 1996;384(6606):221–222. doi: 10.1038/384221a0. [DOI] [PubMed] [Google Scholar]
- Gimeno S, Komen H, Gerritsen AG M, Bowmer T. Feminisation of young males of the common carp, Cyprinus carpio, exposed to 4-tert-pentylphenol during sexual differentiation. Aquat Toxicol. 1998;43(2–3):77–92. [Google Scholar]
- Gimeno S, Komen H, Venderbosch PWM, Bowmer T. Disruption of sexual differentiation in genetic male common carp (Cyprinus carpio) exposed to an alkylphenol during different life stages. Environ Sci Technol. 1997;31(10):2884–2890. [Google Scholar]
- Gravato C, Santos MA. Dicentrarchus labrax biotransformation and genotoxicity responses after exposure to a secondary treated industrial/urban effluent. Ecotoxicol Environ Saf. 2003;55(3):300–306. doi: 10.1016/s0147-6513(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Grisolia CK, Starling F. Micronuclei monitoring of fishes from Lake Paranoa, under influence of sewage treatment plant discharges. Mutat Res Genet Toxicol Environ Mutagen. 2001;491(1–2):39–44. doi: 10.1016/s1383-5718(00)00168-6. [DOI] [PubMed] [Google Scholar]
- Gronen S, Denslow N, Manning S, Barnes S, Barnes D, Brouwer M. Serum vitellogenin levels and reproductive impairment of male Japanese medaka (Oryzias latipes) exposed to 4-tert-octylphenol. Environ Health Perspect. 1999;107:385–390. doi: 10.1289/ehp.99107385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han XL, Liehr JG. 8-Hydroxylation of guanine bases in kidney and liver DNA of hamsters treated with estradiol—role of free radicals in estrogen-induced carcinogenesis. Cancer Res. 1994;54(21):5515–5517. [PubMed] [Google Scholar]
- Handy RD. Chronic effects of copper exposure versus endocrine toxicity: two sides of the same toxicological process? Comp Biochem Physiol A Mol Integr Physiol. 2003;135(1):25–38. doi: 10.1016/s1095-6433(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Harreus UA, Wallner BC, Kastenbauer ER, Kleinsasser NH. Genotoxicity and cytotoxicity of 4-nonylphenol ethoxylate on lymphocytes as assessed by the comet assay. Int J Environ Anal Chem. 2002;82(6):395–401. [Google Scholar]
- Harries JE, Janbakhsh A, Jobling S, Matthiessen P, Sumpter JP, Tyler CR. Estrogenic potency of effluent from two sewage treatment works in the United Kingdom. Environ Toxicol Chem. 1999;18(5):932–937. [Google Scholar]
- Harris CA, Santos EM, Janbakhsh A, Pottinger TG, Tyler CR, Sumpter JP. Nonylphenol affects gonadotropin levels in the pituitary gland and plasma of female rainbow trout. Environ Sci Technol. 2001;35(14):2909–2916. doi: 10.1021/es0002619. [DOI] [PubMed] [Google Scholar]
- Hecker M, Tyler CR, Hoffmann M, Maddix S, Karbe L. Plasma biomarkers in fish provide evidence for endocrine modulation in the Elbe River, Germany. Environ Sci Technol. 2002;36(11):2311–2321. doi: 10.1021/es010186h. [DOI] [PubMed] [Google Scholar]
- Hemming JM, Waller WT, Chow MC, Denslow ND, Venables B. Assessment of the estrogenicity and toxicity of a domestic wastewater effluent flowing through a constructed wetland system using biomarkers in male fathead minnows (Pimephales promelas Rafinesque, 1820) Environ Toxicol Chem. 2001;20(10):2268–2275. doi: 10.1897/1551-5028(2001)020<2268:aoteat>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Herman RL, Kincaid HL. Pathological effects of orally-administered estradiol to rainbow-trout. Aquaculture. 1988;72(1–2):165–172. [Google Scholar]
- Jobling S, Beresford N, Nolan M, Rodgers-Gray T, Brighty GC, Sumpter JP, et al. Altered sexual maturation and gamete production in wild roach (Rutilus rutilus) living in rivers that receive treated sewage effluents. Biol Reprod. 2002;66(2):272–281. doi: 10.1095/biolreprod66.2.272. [DOI] [PubMed] [Google Scholar]
- Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environ Sci Technol. 1998;32(17):2498–2506. [Google Scholar]
- Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995;103:582–587. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobling S, Sheahan D, Osborne JA, Matthiessen P, Sumpter JP. Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ Toxicol Chem. 1996;15(2):194–202. [Google Scholar]
- Joosten HF P, van Acker EA, van den Dobbelsteen DJ, Horbach G, Krajnc EI. Genotoxicity of hormonal steroids. Toxicol Lett. 2004;151(1):113–134. doi: 10.1016/j.toxlet.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Kakuta I, Murachi S. Physiological response of carp, Cyprinus carpio, exposed to raw sewage containing fish processing wastewater. Environ Toxicol Water Qual. 1997;12(1):1–9. [Google Scholar]
- Karrow NA, Guo TL, Delclos KB, Newbold RR, Weis C, Germolec DR, et al. Nonylphenol alters the activity of splenic NK cells and the numbers of leukocyte subpopulations in Sprague-Dawley rats: a two-generation feeding study. Toxicology. 2004;196(3):237–245. doi: 10.1016/j.tox.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Kelly C. Analysis of steroids in environmental water samples using solid-phase extraction and ion-trap gas chromatography-mass spectrometry and gas chromatography-tandem mass spectrometry. J Chromatogr A. 2000;872(1–2):309–314. doi: 10.1016/s0021-9673(99)01261-3. [DOI] [PubMed] [Google Scholar]
- Koponen K, Myers MS, Ritola O, Huuskonen SE, Lindstrom-Seppa P. Histopathology of feral fish from a PCB-contaminated freshwater lake. Ambio. 2001;30(3):122–126. doi: 10.1579/0044-7447-30.3.122. [DOI] [PubMed] [Google Scholar]
- Lee HB, Peart TE. Determination of 17 beta-estradiol and its metabolites in sewage effluent by solid-phase extraction and gas chromatography mass spectrometry. J AOAC Int. 1998;81(6):1209–1216. [PubMed] [Google Scholar]
- Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000;21(1):40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- Liney KE, Jobling S, Shears JA, Simpson P, Tyler CR. Assessing the sensitivity of different life stages for sexual disruption in roach (Rutilus rutilus) exposed to effluents from wastewater treatment works. Environ Health Perspect. 2005;113:1299–1307. doi: 10.1289/ehp.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magliulo L, Schreibman MP, Cepriano J, Ling J. Endocrine disruption caused by two common pollutants at “acceptable” concentrations. Neurotoxicol Teratol. 2002;24(1):71–79. doi: 10.1016/s0892-0362(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Mills LJ, Gutjahr-Gobell RE, Haebler RA, Horowitz DJB, Jayaraman S, Pruell RJ, et al. Effects of estrogenic (o,p’-DDT; octylphenol) and anti-androgenic (p,p’-DDE) chemicals on indicators of endocrine status in juvenile male summer flounder (Paralichthys dentatus) Aquat Toxicol. 2001;52(2):157–176. doi: 10.1016/s0166-445x(00)00139-9. [DOI] [PubMed] [Google Scholar]
- Milston RH, Fitzpatrick MS, Vella AT, Clements S, Gundersen D, Feist G, et al. Short-term exposure of chinook salmon (Oncorhynchus tshawytscha) to o,p’-DDE or DMSO during early life-history stages causes long-term humoral immunosuppression. Environ Health Perspect. 2003;111:1601–1607. doi: 10.1289/ehp.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan M, Jobling S, Brighty G, Sumpter JP, Tyler CR. A histological description of intersexuality in the roach. J Fish Biol. 2001;58(1):160–176. [Google Scholar]
- Nutter LM, Ngo EO, Abulhajj YJ. Characterization of DNA damage induced by 3,4-estrone-ortho-quinone in human-cells. J Biol Chem. 1991;266(25):16380–16386. [PubMed] [Google Scholar]
- Nutter LM, Wu YY, Ngo EO, Sierra EE, Gutierrez PL, Abulhajj YJ. An O-quinone form of estrogen produces free radicals in human breast-cancer cells—correlation with DNA-damage. Chem Res Toxicol. 1994;7(1):23–28. doi: 10.1021/tx00037a004. [DOI] [PubMed] [Google Scholar]
- Ochi T. Induction of multiple microtubule-organizing centers, multipolar spindles and multipolar division in cultured V79 cells exposed to diethylstilbestrol, estradiol-17 beta and bisphenol A. Mutat Res Fundam Mol Mech Mutagen. 1999;431(1):105–121. doi: 10.1016/s0027-5107(99)00190-6. [DOI] [PubMed] [Google Scholar]
- Pfeiffer E, Rosenberg B, Deuschel S, Metzler M. Interference with microtubules and induction of micronuclei in vitro by various bisphenols. Mutat Res Genet Toxicol Environ Mutagen. 1997;390(1–2):21–31. doi: 10.1016/s0165-1218(96)00161-9. [DOI] [PubMed] [Google Scholar]
- Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP. Estrogenic effects of effluents from sewage treatment works. Chem Ecol. 1994;8:275–285. [Google Scholar]
- Raiaguru P, Suba S, Palanivel M, Kalaiselvi K. Genotoxicity of a polluted river system measured using the alkaline comet assay on fish and earthworm tissues. Environ Mol Mutagen. 2003;41(2):85–91. doi: 10.1002/em.10134. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimschuessel R. A fish model of renal regeneration and development. ILAR J. 2001;42:285–291. doi: 10.1093/ilar.42.4.285. [DOI] [PubMed] [Google Scholar]
- Reimschuessel R, Bennett RO, May EB, Lipsky MM. Development of newly formed nephrons in the goldfish kidney following hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol. 1990;18(1):32–38. doi: 10.1177/019262339001800105. [DOI] [PubMed] [Google Scholar]
- Reimschuessel R, Bennett RO, May EB, Lipsky MM. Pathological alterations and new nephron development in rainbow-trout (Oncorhynchus mykiss) following tetra-chloroethylene contamination. J Zoo Wildl Med. 1993;24(4):503–507. [Google Scholar]
- Rickwood CJ, Galloway TS. Acetylcholinesterase inhibition as a biomarker of adverse effect—a study of Mytilus edulis exposed to the priority pollutant chlorfenvinphos. Aquat Toxicol. 2004;67(1):45–56. doi: 10.1016/j.aquatox.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Rodgers-Gray TP, Jobling S, Kelly C, Morris S, Brighty G, Waldock MJ, et al. Exposure of juvenile roach (Rutilus rutilus) to treated sewage effluent induces dose-dependent, and persistent disruption in gonadal duct development. Environ Sci Technol. 2001;35(3):462–470. doi: 10.1021/es001225c. [DOI] [PubMed] [Google Scholar]
- Routledge EJ, Sheahan D, Desbrow C, Brighty GC, Waldock M, Sumpter JP. Identification of estrogenic chemicals in STW effluent. 2. In vivo responses in trout and roach. Environ Sci Technol. 1998;32(11):1559–1565. [Google Scholar]
- Roy D, Liehr JG. Estrogen, DNA damage, and mutations. Mutat Res Fundam Mol Mech Mutagen. 1999;424(1–2):107–115. doi: 10.1016/s0027-5107(99)00012-3. [DOI] [PubMed] [Google Scholar]
- Salice CJ, Rokous JS, Kane AS, Reimschuessel R. New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med. 2001;51(1):56–59. [PubMed] [Google Scholar]
- Schmidt-Posthaus H, Bernet D, Wahli T, Burkhardt-Holm P. Morphological organ alterations and infections diseases in brown trout Salmo trutta and rainbow trout Oncorhynchus mykiss exposed to polluted river water. Dis Aquat Org. 2001;44(3):161–170. doi: 10.3354/dao044161. [DOI] [PubMed] [Google Scholar]
- Schrank CS, Cormier SM, Blazer VS. Contaminant exposure, biochemical, and histopathological biomarkers in white suckers from contaminated and reference sites in the Sheboygan River, Wisconsin. J Great Lakes Res. 1997;23(2):119–130. [Google Scholar]
- Schwaiger J, Spieser OH, Bauer C, Ferling H, Mallow U, Kalbfus W, et al. Chronic toxicity of nonylphenol and ethinylestradiol: haematological and histopathological effects in juvenile Common carp (Cyprinus carpio) Aquat Toxicol. 2000;51(1):69–78. doi: 10.1016/s0166-445x(00)00098-9. [DOI] [PubMed] [Google Scholar]
- Secombes CJ, Fletcher TC, Oflynn JA, Costello MJ, Stagg R, Houlihan DF. Immunocompetence as a measure of the biological effects of sewage-sludge pollution in fish. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1991;100(1–2):133–136. doi: 10.1016/0742-8413(91)90139-k. [DOI] [PubMed] [Google Scholar]
- Seki M, Yokota H, Maeda M, Tadokoro H, Kobayashi K. Effects of 4-nonylphenol and 4-tert-octylphenol on sex differentiation and vitellogenin induction in medaka (Oryzias latipes) Environ Toxicol Chem. 2003;22(7):1507–1516. [PubMed] [Google Scholar]
- Sheahan DA, Brighty GC, Daniel M, Kirby SJ, Hurst MR, Kennedy J, et al. Estrogenic activity measured in a sewage treatment works treating industrial inputs containing high concentrations of alkylphenolic compounds—a case study. Environ Toxicol Chem. 2002;21(3):507–514. [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”—eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol. 2002;36(8):1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Simpson MG, Parry M, Kleinkauf A, Swarbreck D, Walker P, Leah RT. Pathology of the liver, kidney and gonad of flounder (Platichthys flesus) from a UK estuary impacted by endocrine disrupting chemicals. Mar Environ Res. 2000;50(1–5):283–287. doi: 10.1016/s0141-1136(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low-levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158(3):327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Spengler P, Korner W, Metzger JW. Substances with estrogenic activity in effluents of sewage treatment plants in southwestern Germany. 1. Chemical analysis. Environ Toxicol Chem. 2001;20(10):2133–2141. [PubMed] [Google Scholar]
- Stentiford GD, Longshaw M, Lyons BP, Jones G, Green M, Feist SW. Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar Environ Res. 2003;55(2):137–159. doi: 10.1016/s0141-1136(02)00212-x. [DOI] [PubMed] [Google Scholar]
- Stevens JL, Northcott GL, Stern GA, Tomy GT, Jones KC. PAHs, PCBs, PCNs, organochlorine pesticides, synthetic musks, and polychlorinated n-alkanes in UK sewage sludge: survey results and implications. Environ Sci Technol. 2003;37(3):462–467. doi: 10.1021/es020161y. [DOI] [PubMed] [Google Scholar]
- Stolen JS, Fletcher TC, Anderson DP, Roberson BS, van Muiswinkel WB. 1990. Techniques in Fish Immunology. Fair Haven, NJ:SOS Publications.
- Suarez S, Sueiro RA, Garrido J. Genotoxicity of the coating lacquer on food cans, bisphenol A diglycidyl ether (BADGE), its hydrolysis products and a chlorohydrin of BADGE. Mutat Res Genet Toxicol Environ Mutagen. 2000;470(2):221–228. doi: 10.1016/s1383-5718(00)00109-1. [DOI] [PubMed] [Google Scholar]
- Sumpter JR. Endocrine disruption in wildlife: the future? Pure Appl Chem. 2003;75(11–12):2355–2360. [Google Scholar]
- Ternes TA, Stumpf M, Mueller J, Haberer K, Wilken RD, Servos M. Behavior and occurrence of estrogens in municipal sewage treatment plants. I. Investigations in Germany, Canada and Brazil. Sci Total Environ. 1999;225(1–2):81–90. doi: 10.1016/s0048-9697(98)00334-9. [DOI] [PubMed] [Google Scholar]
- Thorpe KL, Cummings RI, Hutchinson TH, Scholze M, Brighty G, Sumpter JP, et al. Relative potencies and combination effects of steroidal estrogens in fish. Environ Sci Technol. 2003;37(6):1142–1149. doi: 10.1021/es0201348. [DOI] [PubMed] [Google Scholar]
- Thorpe KL, Hutchinson TH, Hetheridge MJ, Scholze M, Sumpter JP, Tyler CR. Assessing the biological potency of binary mixtures of environmental estrogens using vitellogenin induction in juvenile rainbow trout (Oncorhynchus mykiss) Environ Sci Technol. 2001;35(12):2476–2481. doi: 10.1021/es001767u. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Jobling S, Sumpter JP. Endocrine disruption in wildlife: a critical review of the evidence. Crit Rev Toxicol. 1998;28(4):319–361. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- Tyler CR, van Aerle R, Hutchinson TH, Maddix S, Trip H. An in vivo testing system for endocrine disrupters in fish early life stages using induction of vitellogenin. Environ Toxicol Chem. 1999;18(2):337–347. [Google Scholar]
- Tyler CR, vanderEerden B, Jobling S, Panter G, Sumpter JP. Measurement of vitellogenin, a biomarker for exposure to oestrogenic chemicals, in a wide variety of cyprinid fish. J Comp Physiol B. 1996;166(7):418–426. [Google Scholar]
- van Aerle R, Pounds N, Hutchinson TH, Maddix S, Tyler CR. Window of sensitivity for the estrogenic effects of ethinylestradiol in early life-stages of fathead minnow, Pimephales promelas. Ecotoxicology. 2002;11(6):423–434. doi: 10.1023/a:1021053217513. [DOI] [PubMed] [Google Scholar]
- Vos JG. Immunosuppression as related to toxicology. CRC Crit Rev Toxicol. 1977;5:67–101. doi: 10.3109/10408447709101342. [DOI] [PubMed] [Google Scholar]
- Vos JG, Dybing E, Greim HA, Ladefoged O, Lambre C, Tarazona JV, et al. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit Rev Toxicol. 2000;30(1):71–133. doi: 10.1080/10408440091159176. [DOI] [PubMed] [Google Scholar]
- Weber LP, Hill RL, Janz DM. Developmental estrogenic exposure in zebrafish (Danio rerio): II. Histological evaluation of gametogenesis and organ toxicity. Aquat Toxicol. 2003;63(4):431–446. doi: 10.1016/s0166-445x(02)00208-4. [DOI] [PubMed] [Google Scholar]
- White PA, Rasmussen JB, Blaise C. Comparing the presence, potency, and potential hazard of genotoxins extracted from a broad range of industrial effluents. Environ Mol Mutagen. 1996;27(2):116–139. doi: 10.1002/(SICI)1098-2280(1996)27:2<116::AID-EM7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Yared E, McMillan TJ, Martin FL. Genotoxic effects of oestrogens in breast cells detected by the micronucleus assay and the comet assay. Mutagenesis. 2002;17(4):345–352. doi: 10.1093/mutage/17.4.345. [DOI] [PubMed] [Google Scholar]
- Zaroogian G, Gardner G, Horowitz DB, Gutjahr-Gobell R, Haebler R, Mills L. Effect of 17 beta-estradiol, o,p’-DDT, octylphenol and p,p’-DDE on gonadal development and liver and kidney pathology in juvenile male summer flounder (Paralichthys dentatus) Aquat Toxicol. 2001;54(1–2):101–112. doi: 10.1016/s0166-445x(00)00177-6. [DOI] [PubMed] [Google Scholar]
- Zelikoff JT, Smialowicz R, Bigazzi PE, Goyer RA, Lawrence DA, Maibach HI, et al. Immunomodulation by metals. Fundam Appl Toxicol. 1994;22(1):1–7. doi: 10.1006/faat.1994.1001. [DOI] [PubMed] [Google Scholar]