Abstract
We histopathologically examined gonads and chemically determined organotin compounds in tissues of the ivory shell, Babylonia japonica. Imposex (a superimposition of male-type genital organs on females) occurred in approximately 80–90% of B. japonica specimens that we examined, with the penis and vas deferens both well developed. No oviduct blockage by vas deferens formation was observed. Ovarian spermatogenesis and suppressed ovarian maturation were observed in the females that exhibited imposex, although no histopathological abnormalities were found in males. Tissue distributions of organotin compounds [tributyltin (TBT), triphenyltin (TPhT), and their metabolites] were different for butyltins and phenyltins; a remarkably high accumulation of TBT was observed in the ctenidium, osphradium, and heart, whereas high concentrations of TPhT were detected in the ovary and digestive gland. More than one-third of TBT accumulated in the digestive glands of both males and females, followed by the testis, ctenidium, muscle, and heart tissues in males and in the muscle, ovary, ctenidium, and head tissues (including the central nervous system ganglia) in females. In both males and females, more than half of total TPhT accumulated in the digestive glands, followed by the gonads. The next highest values were in the muscle, ctenidium, and heart tissues in males and in the muscle, oviduct, and head tissues in females. Both TBT and TPhT concentrations in the gonads were positively correlated with penis length in females. Our findings strongly suggest that reproductive failure in adult females accompanied by imposex, possibly induced by TBT and TPhT from antifouling paints, may have caused the marked decline of B. japonica populations in Japan.
Keywords: imposex, ovarian spermatogenesis, population decline, reproductive failure, suppressed ovarian maturation, tributyltin, triphenyltin
The term “imposex” was coined by Smith (1971) to describe the syndrome of a superimposition of male genital organs such as the penis and vas deferens on female gastropods. Imposex is thought to be irreversible (Bryan et al. 1986). Reproductive failure may occur in females with severe imposex, resulting in population decline or even mass extinction (Gibbs and Bryan 1986, 1996). In some species, imposex is typically induced by tributyltin (TBT) and triphenyltin (TPhT), chemicals released from antifouling paints used on ships and fishing nets (Bryan et al. 1987, 1988; Gibbs et al. 1987; Horiguchi et al. 1995, 1997a).
TBT and TPhT compounds (TBTs and TPhTs) have been used worldwide in antifouling paints for ships and fishing nets since the mid-1960s, although much lower amounts of TPhTs have been used (Goldberg 1986; Horiguchi et al. 1994). In Japan, the production, importation, and use of TBTs and TPhTs have been strictly regulated by legislation and government administrative guidance since 1990. These activities were reported to have completely stopped by 1997, although evidence suggests illegal TBT use in antifouling paints in some areas (Horiguchi 2000; Horiguchi et al. 1994; Horiguchi T, Kojima M, Kaya M, Matsuo T, Shiraishi H, Adachi Y, Morita M, unpublished data). The International Convention on the Control of Harmful Anti-fouling Systems on Ships was adopted to enforce a worldwide ban on TBT and TPhT at the meeting of the International Maritime Organization (IMO) in October 2001 (IMO 2001), although it has not come into effect yet.
As of July 2004, approximately 150 gastropod species have been reported to be affected by imposex worldwide (Bech 2002a, 2002b; Fioroni et al. 1991; Horiguchi et al. 1997b; Marshall and Rajkumar 2003; Sole et al. 1998; ten Hallers-Tjabbes et al. 2003; Terlizzi et al. 2004). Many of these species belong to the families Muricidae (e.g., Nucella lapillus, Ocenebra erinacea, Thais clavigera, Urosalpinx cinerea), Buccinidae (e.g., Babylonia japonica, Buccinum undatum, Neptunea arthritica arthritica), Conidae (e.g., Conus marmoreus bandanus, Virroconus ebraeus), and Nassariidae (e.g., Ilyanassa obsoleta, Nassarius reticulatus) of the Neogastropoda. (Fioroni et al. 1991; Horiguchi et al. 1997b). Regarding Japanese gastropods, 39 species (seven mesogastropods and 32 neogastropods) have been found to be affected by imposex (Horiguchi et al. 1997b). Numerous studies have examined the incidence or severity of imposex, investigated the use of certain gastropod species as biological indicators of TBT contamination, and surveyed TBT contamination also using gastropods. Only a few reports, however, have presented evidence for population-level effects of reproductive failure due to imposex. Such evidence has been based on either morphological or histological methods (Bryan et al. 1986; Gibbs and Bryan 1986; Gibbs et al. 1988, 1990, 1991; Horiguchi 2000; Horiguchi et al. 1994, 2000a; Oehlmann et al. 1996; Schulte-Oehlmann et al. 1997).
The ivory shell Babylonia japonica, (Neogastropoda: Buccinidae) inhabits sandy or muddy bottoms in shallow waters (approximately 10–20 m in depth) from the south of Hokkaido to Kyushu, Japan. This species is a scavenger in the inshore ecosystem and traditionally has been a target species of commercial fisheries in Japan. Imposex has been observed in B. japonica since the 1970s, and the total catch drastically decreased throughout Japan in the late 1970s and early 1980s (Horiguchi and Shimizu 1992).
Much effort has been invested in trying to enhance B. japonica stocks, including seed production using adult B. japonica reared at a hatchery and consecutive release of seeds or juveniles into the sea. Most seeds or juveniles (~ 90% of total production in Japan) of B. japonica released into the sea have been produced at the prefectural hatchery in Tomari, Tottori Prefecture, in western Japan (Figure 1). The total catch has drastically decreased since 1984, 2 years after the first observation of B. japonica females that exhibited imposex. Studies have shown an increase in both the percentage occurrence of imposex and in mean penis length in females (Hamada et al. 1988, 1989; Horiguchi 1998; Kajikawa 1984; Kajikawa et al. 1983). The number of egg capsules spawned by adult B. japonica at the hatchery and the number of seeds and juveniles artificially released into the sea have also decreased since the mid-1980s (Horiguchi 1998). Introduction of adult B. japonica from Niigata Prefecture since 1992 to compensate for insufficient offspring also resulted in failure for the release of seeds and juveniles into the sea because of their high mortality at the hatchery before the release (Horiguchi 1998). In spite of such efforts to enhance B. japonica stocks, recovery back to the original levels of the total catch has not been achieved (Horiguchi 1998). The B. japonica hatchery for stock enhancement in Tottori was closed in 1996 (Horiguchi 1998).
Figure 1.

Sampling sites of the ivory shell, B. japonica, specimens.
Our objectives in the present study were to examine the incidence of reproductive failure accompanied with imposex in B. japonica on the basis of histopathological observation of gonads and to investigate the relationship between organotin compounds and imposex in B. japonica on the basis of chemical determination of organotin concentrations in tissues. We discuss whether the marked decline of B. japonica populations in Japan could have been brought about by reproductive failure accompanied with imposex induced by TBT and TPhT from antifouling paints.
Materials and Methods
Histopathological examination of gonads in the ivory shell
Adult B. japonica reared in the hatchery of the Tottori Prefectural Sea Farming Association were sacrificed monthly from December 1988 to November 1989, when the number of egg capsules spawned by adult B. japonica at the hatchery had reached a minimum (Horiguchi 1998). Gonad samples of 10–15 B. japonica specimens were fixed monthly in Bouin’s fluid, embedded in paraffin, and stained with hematoxylin–eosin for histopathological examination under a light microscope. A total of 135 B. japonica specimens were examined (43 males and 92 females consisting of 16 normal females and 76 imposex-exhibiting individuals).
To quantitatively evaluate the gonadal maturation of B. japonica, we scored female and male reproductive cells on the basis of developmental stages similar to those described by Takamaru and Fuji (1981) and Horiguchi et al. (2000b). Female reproductive cells were categorized according to five developmental stages: oogonium, primary oocyte, first yolk globule–accumulating oocyte, second yolk globule–accumulating oocyte, and matured oocyte (Figure 2). An oogonium is an oval cell having a nucleus with small granules stained by hematoxylin and less-stained cytoplasm; the nucleus has a few nucleoli stained by eosin (Figure 2A). A primary oocyte is larger than an oogonium, and its shape is irregular. The nuclei of primary oocytes are variable, ranging from no nucleolus (chromonemata are spreading in the nucleus) to a large nucleolus (chromonemata are scattered in or located at the outskirts of the nucleolus) intensively stained by eosin (Figure 2B). No yolk globule-accumulation is observed in the first two stages. A first yolk globule–accumulating oocyte is larger than a primary oocyte, with a few yolk globules stained by eosin (Figure 2C). A second yolk globule–accumulating oocyte is a large pear-shaped cell with several yolk globules observed in the cytoplasm. The edge of a second yolk globule–accumulating oocyte is adjacent to the epithelium of the ovarian lobule, which consists of the ovary (Figure 2D). A matured oocyte is a large oval cell with plentiful yolk globule and is separating from the epithelium of the ovarian lobule; its nucleus is intensively stained by hematoxylin (Figure 2E).
Figure 2.
Reproductive cells in the ovaries of B. japonica: (A) oogonium; (B) primary oocyte; (C) first yolk globule–accumulating oocyte; (D) second yolk globule–accumulating oocyte; (E) matured oocyte. Abbreviations: Fyo, first yolk globule–accumulating oocyte; Mo, matured oocyte; Nc, nucleolus; Nu, nucleus; Oo, oogonium; Ovl, ovarian lobule; Po, primary oocyte; Syo, second yolk globule–accumulating oocyte; Yg, yolk globule.
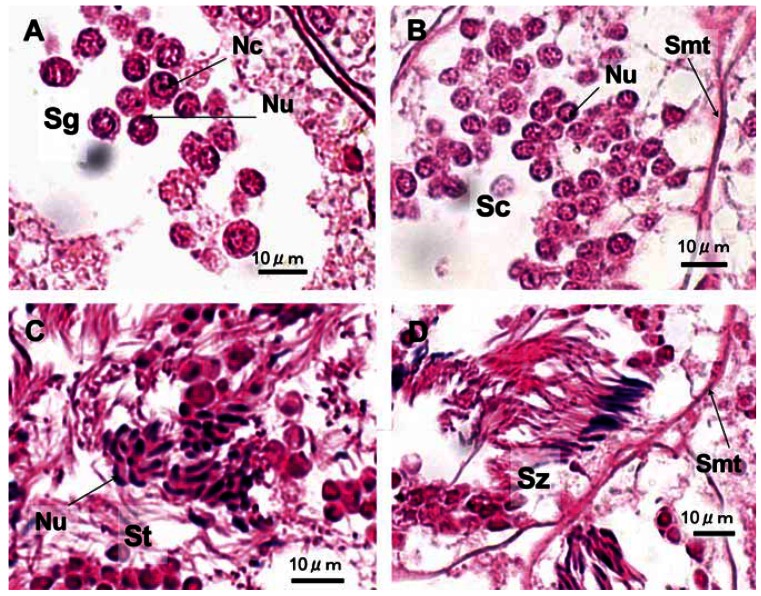
Male reproductive cells were categorized into four developmental stages: spermatogonium, spermatocyte, spermatid, and spermatozoon (Figure 3). A spermatogonium is an oval cell having a nucleus with small granules stained by hematoxylin; the nucleus also has a few nucleoli stained by eosin (Figure 3A). A spermatocyte is a round cell having a nucleus with granules intensively stained by hematoxylin; the shape of the nucleus varies, possibly suggesting primary and secondary spermatocytes (Figure 3B). A spermatid is a crescent-shaped cell whose nucleus is located at one pole of the cell and is intensively stained by hematoxylin; the shape of some spermatids resembles an oval or rod, with a reduced volume of cytoplasm (Figure 3C). A spermatozoon is a threadshaped cell, intensively stained by hematoxylin (Figure 3D). When no or few reproductive cells were observed in gonads after spawning, a score of 0 was assigned to both ovaries and testes.
Figure 3.
Reproductive cells in the testes of B. japonica: (A) spermatogonium; (B) spermatocyte; (C) spermatid; (D) spermatozoon. Abbreviations: Nc, nucleolus; Nu, nucleus; Sc, spermatocyte; Sg, spermatogonium; Smt, seminiferous tubule; St, spermatid; Sz, spermatozoon.
The individual reproductive developmental score was the mean value of these scores for the reproductive cells of each B. japonica. The population reproductive developmental score was the monthly mean value of the individual reproductive developmental scores (Horiguchi et al. 2000b).
Chemical determination of organotin compounds in tissues of the ivory shell
Adult B. japonica specimens collected at Yodoe, Tottori Prefecture, in June 1991 were used for chemical determination of organotin (butyltin and phenyltin) compounds. The specimens were dissected for sex determination based on the existence of female accessory sex organs, such as the capsule gland, and then females were examined for imposex. An imposex individual was defined as one that has either a penis or a vas deferens with female accessory sex organs (e.g., capsule gland; Horiguchi 1993). A total of 52 B. japonica specimens were used for chemical analyses (25 males and 27 females, consisting of three normal females and 24 individuals that exhibited imposex).
Chemical determination of butyltin and phenyltin compounds in tissues (muscle [foot], head with tentacle, radula with sac, esophagus with crop, stomach, digestive gland, kidney, rectum, ovary or testis, oviduct, siphon, ctenidium, heart, osphradium, and mantle) of B. japonica specimens was conducted with composite samples using the method described by Horiguchi et al. (1994). Briefly, tissues were extracted with 0.1% tropolone/benzene and 1N hydrobromic acid/ethanol by ultrasonication, derivatized with propylmagnesium bromide, cleaned by silica gel column chromatography, and quantified by gas chromatography with flame photometric detection (GC-FPD). The detection limit of the instrument was 50 pg, and certified reference material from Japanese sea bass, Lateolabrax japonicus, for TBT and TPhT analysis (prepared by the National Institute for Environmental Studies; NIES CRM no. 11) was used for quality assurance and quality control. The analytical conditions are described in more detail by Horiguchi et al. (1994).
Results and Discussion
The percentages of occurrence of imposex were 82.6 and 88.9% in B. japonica specimens collected from December 1988 to November 1989 and in June 1991, respectively. Both the penis and the vas deferens were well developed in females that exhibited imposex (Figure 4). No oviduct blockage (i.e., occlusion of the vulva) by vas deferens formation was observed in female B. japonica that exhibited imposex. This finding differs from the imposex symptoms observed in N. lapillus, Ocinebrina aciculata, and T. clavigera (Gibbs and Bryan 1986; Gibbs et al. 1987; Horiguchi et al. 1994; Oehlmann et al. 1996) Figure 4).
Figure 4.

Morphology of the ivory shell, B. japonica. Abbreviations: cg, capsule gland; e, eye; f, foot; gp, genital papilla; k, kidney; m, mantle; p, penis; r, rectum; s, siphon; t, tentacle; vd, vas deferens.
Temporal variations in the reproductive developmental score of the B. japonica population differed between females (including females that exhibited imposex) and males (Figure 5). Although the spawning season for B. japonica is late June to early August (Kajikawa et al. 1983), ovarian maturation seemed to be suppressed in females, compared to testicular maturation in males (Figure 5), which is probably because of the presence of immature females throughout the spawning season. During the spawning season, clearer ovarian maturation and spawning of many more egg capsules were observed in B. japonica females in a population from Teradomari, Niigata Prefecture, Japan, compared with those from Tottori (Hamada and Inoue 1993, 1994, 1995). Testicular maturation in males from Tottori was clear in July and August, the spawning season for B. japonica (Figure 5). Thus, the reproductive cycle was unclear in females, but it was clearly observed in males (Figure 5). This suppressed ovarian maturation during the spawning season could be the direct reason for the decreased number of egg capsules spawned by adult B. japonica at the hatchery and might accompany imposex in B. japonica (Gibbs et al. 1988).
Figure 5.
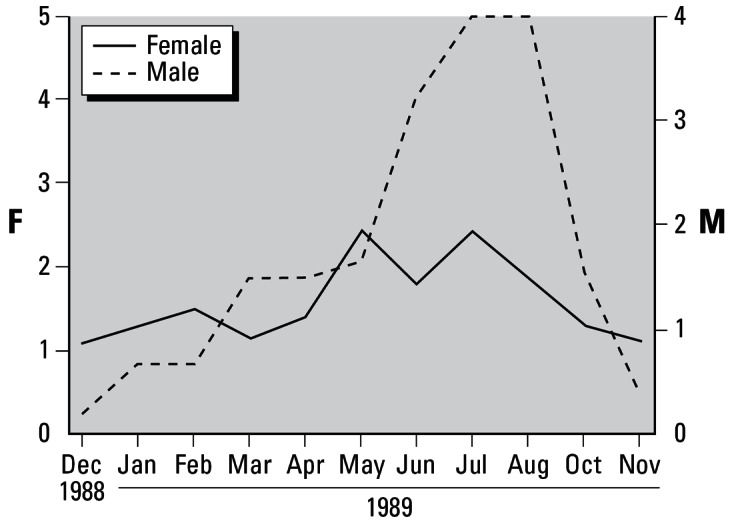
Reproductive cycle of the ivory shell, B. japonica, represented by the population reproductive developmental scores. Female (F) reproductive cells were scored based on five categories (Figure 2), and those of males (M) were based on four categories (Figure 3). The female curve includes females that exhibited imposex.
Ovarian spermatogenesis (i.e., an ovotestis) was observed in 6 (1 normal female and 5 imposex individuals) of 92 female or imposex B. japonica specimens examined, for a frequency of about 6.5% (Figure 6). It is well known that most prosobranchs (including B. japonica) are dioecious although there are relatively few hermaphroditic prosobranchs in which the gonad produces eggs and sperm simultaneously (Fretter 1984; Uki 1989). Ovarian spermatogenesis has been observed in neogastropods (e.g., N. lapillus, O. aciculata, and T. clavigera) and archaeogastropods (e.g., Haliotis madaka and H. gigantea) exposed to TBT or TPhT, although no penis formation (i.e., masculinization) is involved in spermatogenesis in ovaries of female abalone (Gibbs et al. 1988; Horiguchi and Shimizu 1992; Horiguchi et al. 2000b, 2002, 2005; Oehlmann et al. 1996). Ovarian spermatogenesis was even observed in a normal female B. japonica without any penis or vas deferens formation although the frequency was low (one of six, 16.7%). The development of male-type genital organs (penis and vas deferens) and ovarian spermatogenesis in females exposed to TBT or TPhT might be controlled through different physiological pathways (see below). This ovarian spermatogenesis may be one of the reasons why the spawning ability of female B. japonica decreased.
Figure 6.
Spermatogenesis in the ovary of a normal female B. japonica (i.e., without penis and vas deferens). Abbreviations: Dg, digestive gland; Ov, ovary; Smt, seminiferous tubule; Sz, spermatozoon; Te, testis. Testicular (A) and ovarian (B) tissues (i.e., ova–testis) were observed in the gonad of a female B. japonica, which was classified originally as a female because of the presence of female accessory sex organs (e.g., a capsule gland) with neither penis nor vas deferens. Spermatogenesis was also observed in seminiferous tubules of the ovo–testis (C).
Sex steroid hormones such as testosterone and 17β-estradiol are important physiologically in the development of sex organs and maturation of gonads (i.e., oogenesis and spermatogenesis) in vertebrates. Thus, similar sex steroid hormones might also regulate the reproduction of invertebrates such as gastropods (LeBlanc et al. 1999). After castration of the hermaphroditic organ, oogenesis and spermatogenesis were observed, respectively, in gonads of females of the slug Limax marginatus treated with 17β-estradiol and in males of that slug treated with testosterone. Egg-laying was also induced by 17β-estradiol in female slugs, thereby implying the existence of vertebrate-type sex steroid hormones in this species (Takeda 1979, 1983). In vitro metabolism of androstenedione and identification of endogenous steroids (androsterone, dehydroepiandrosterone, androstenedione, 3α-androstanediol, estrone, 17β-estradiol, and estriol) by gas chromatography–mass spectrometry (GC-MS) were reported for Helix aspersa (Le Guellec et al. 1987). Several steroids (androsterone, estrone, 17β-estradiol, ethinylestradiol, and testosterone) were also identified by GC-MS in gonads of B. japonica. The identification of the synthetic estrogen ethinylestradiol in the gonads indicated that contamination of the habitat of B. japonica had occurred (Lu et al. 2001). It has been suggested that increased androgen (testosterone) levels due to reduced activity of aromatase induced by TBT might lead to imposex in gastropods (Bettin et al. 1996; Matthiessen and Gibbs 1998; Spooner et al. 1991). However, there is also contradictory evidence of the relationship between reduced aromatase-like activity and progressed imposex symptoms in gastropods (Morcillo and Porte 1999).
It is important to consider the possible activation of androgen receptor–mediated responses caused by TBT or TPhT in gastropods, as the enhancement of androgen-dependent transcription and cell proliferation by TBT and TPhT has been reported in human prostate cancer cells (Yamabe et al. 2000). On the basis of a study of fully sequenced invertebrate genomes, however, homologs of estrogen receptor (ER) and androgen receptor (AR) have not been found in invertebrates (Escriva et al. 1997). Thus, it is unclear whether gastropods have AR and ER. ER-like cDNA was isolated from Aplysia californica (Gastropoda: Opisthobranchia), but it could not bind to estrogen and was a constitutively activated transcription factor (Thornton et al. 2003). Therefore, further studies are necessary to examine steroid receptors and the function of steroids in gastropods.
Several neuropeptides released from visceral ganglia, cerebral ganglia, or the prostate gland of gastropods (e.g., A. californica and Lymnaea stagnalis) are egg-laying, ovulation, or egg-releasing hormones (Chiu et al. 1979; Ebberink et al. 1985). Féral and Le Gall (1983) suggested that TBT-induced imposex in O. erinacea might be related to the release of neural morphogenic controlling factors. Their study used in vitro tissue cultures derived from the presumptive penis-forming area of immature slipper limpets, Crepidula fornicata, and the isolated nervous systems of male or female O. erinacea in the presence/absence of TBT (0.2 μg/L). The accumulation of TBT or TPhT in the central nervous systems of H. gigantea (Horiguchi et al. 2002), N. lapillus (Bryan et al. 1993), and T. clavigera (Horiguchi et al. 2003) indicates the potential for toxic effects of TBT and TPhT on neuroendocrine systems. APGWamide, a neuropeptide released from the cerebral ganglia of gastropods such as L. stagnalis, significantly promoted the development of imposex in female I. obsoleta (Oberdörster and McClellan-Green 2000, 2002). Thus, in addition to possible steroid modulation caused by TBT or TPhT in gastropods, it might be important to consider the possible effects of neuropeptides released from ganglia as well.
We determined tissue concentrations of organotin compounds such as butyltins and phenyltins using GC-FPD and observed different tissue distributions (Figure 7). A marked accumulation of TBT was observed in the ctenidium, osphradium, and heart in both males and females, whereas the highest concentrations of TPhT were detected in the ovaries of females and in the digestive glands of males (Figure 7). Based on the total body burden of TBT in B. japonica, more than one-third of total TBT was accumulated in the digestive glands of both males and females. The testis, ctenidium, muscle, and heart in males and the muscle, ovary, ctenidium, and head (including the central nervous system ganglia) in females followed in decreasing order of TBT accumulation (Figures 8A, C). Based on the total body burden of TPhT, approximately three-quarters and more than one-half of total TPhT accumulated in the digestive glands of males and females, respectively. The second highest tissue burden of TPhT was observed in the gonads of both males and females, followed by the muscle, ctenidium, and heart in males and the muscle, oviduct, and head in females (Figure 8B, D).
Figure 7.
Tissue distribution of organotin compounds in the ivory shell (B. japonica) from Yodoe, Tottori, Japan (June 1991): (A) butyltins in males; (B) phenyltins in males; (C) butyltins in females (including imposex individuals); (D) phenyltins in females (including imposex individuals). Abbreviations: DBT, dibutyltin; DPhT, diphenyltin; MBT, monobutyltin; MPhT, monophenyltin; TBT, tributyltin; TPhT, triphenyltin.
Figure 8.
Total body burden (ng) of organotin compounds in the ivory shell, B. japonica, from Yodoe, Tottori, Japan (June 1991): (A) tributyltin in males; (B) triphenyltin in males; (C) tributyltin in females (including imposex individuals); (D) triphenyltin in females (including imposex individuals).
The large accumulation of TBT and TPhT in the digestive glands may indicate that those organotins are metabolized thereby several enzymes (Bock 1981; Lee 1985; Matsuda et al. 1993; Suzuki et al. 1999). The relative amounts of butyltin and phenyltin species in the digestive gland might be taken as a measure of the ability of B. japonica to metabolize TBT and TPhT (Tanabe et al. 1998). The ratio of TBT to total butyltins in the digestive gland was higher in B. japonica than it was in T. clavigera, possibly suggesting lower ability of B. japonica to metabolize TBT (Horiguchi et al. 2003). The predominant phenyltin species was TPhT in the digestive gland of B. japonica, the same as for T. clavigera (Horiguchi et al. 2003). The ability to metabolize TBT differs among species, whereas the ability to metabolize TPhT seems to be generally low in many kinds of organisms (Bock 1981; Bryan et al. 1987, 1993; Horiguchi et al. 2003; Lee 1985; Matsuda et al. 1993; Suzuki et al. 1999). Biological and ecological half-lives of TBT and TPhT were estimated as 22 days and 347 days, respectively, in T. clavigera (Horiguchi et al. 1995). The biological half-life of TBT was estimated to be between about 50 days and > 100 days in N. lapillus, depending on the conditions (Bryan et al. 1987). Relatively high tissue burdens of TBT and TPhT were observed in the reproductive organs (ovary, oviduct, testis) and head (including the central nervous system ganglia), as well as in the muscle and tissues adjacent to the mantle, such as the ctenidium, siphon, and heart (Figure 8).
A similar accumulation pattern was also observed in T. clavigera (Horiguchi et al. 2003). However, a slightly different pattern was found in O. erinacea, in which approximately half of the total body TBT burden accumulated in the capsule gland (Gibbs et al. 1990), which possibly suggests a difference in organotin accumulation patterns among species. Although concentrations of TBT and TPhT in ganglia were quite high in T. clavigera, the total tissue burden for those organotins was not high because of the relatively small ganglia tissue in that species (Horiguchi et al. 2003). This explanation may also be the case for B. japonica in this study. Similar concentrations of TBT and TPhT were also detected in ganglia of B. undatum (Mensink et al. 1997).
Nishikawa et al. (2004) discussed a possible mode of action of TBT or TPhT on the development of imposex in gastropods. They reported that organotins (both TBT and TPhT) bind the human retinoid X receptors (hRXRs) with high affinity and that injection of 9-cis retinoic acid (RA), the natural ligand of hRXRs, into female rock shells (T. clavigera) induced the development of imposex. Cloning of the RXR homolog from T. clavigera revealed that the ligand-binding domain of rock shell RXR was very similar to that of vertebrate RXR and that it was bound to both 9-cis RA and organotins (Nishikawa et al. 2004). These findings suggest that RXR is important in inducing the development of imposex, namely, the differentiation and growth of male genital organs in female gastropods. Preliminary immunohistochemical staining results, using anti-RXR antibody that had a cross-reactivity with the rock shell RXR protein (as shown by the Western blotting test), suggested that the RXR protein existed in the ganglia of T. clavigera (Ohta Y and Horiguchi T, unpublished data), where high concentrations of organotins accumulated (Horiguchi et al. 2003). Possible accumulation of organotin compounds in ganglia is also suggested in N. lapillus (Bryan et al. 1993) and B. undatum (Mensink et al. 1997), and in B. japonica in the present study. Further studies with molecular biological and immunohistochemical techniques are needed to clarify the entire mode of action of TBT or TPhT on the development of imposex in gastropods.
Mortality of larvae and seeds or juveniles might be due to the accumulation of TPhT and TBT in ovaries as well as by the contamination of seawater with TPhT or TBT (Coelho et al. 2001; Inoue et al. 2004; Lapota et al. 1993; Li et al. 1997; Nakayama et al. 2005; Ruiz et al. 1995; Treuner AB, Horiguchi T, Takiguchi N, Imai T, Morita M, unpublished data). Based on a survey of imposex and organotin concentrations in tissues of T. clavigera (Horiguchi et al. 1994), contamination with TBT and TPhT was relatively serious along the coast of Tottori Prefecture, especially in Miho Bay, where the B. japonica specimens used in this study were collected.
Concentrations of TBT and TPhT were relatively high in the ovaries of females (Figure 7). Both TBT and TPhT concentrations in gonads were positively correlated with penis length in females (Figure 9), as was the case with T. clavigera (Horiguchi et al. 1994; Shim et al. 2000). Laboratory experiments revealed that both TBT and TPhT induced or promoted the development of imposex in T. clavigera (Horiguchi et al. 1995, 1997a); therefore, imposex could be caused by TBT or TPhT in B. japonica as well. However, it is difficult to estimate the threshold concentration of TBT and/or TPhT that induces the development of imposex in B. japonica. Laboratory flow-through exposure experiments with B. japonica, using TBT and TPhT, are needed to estimate the threshold concentration for the development of imposex. The estimated threshold concentration of TBT (in the whole body) that can induce the development of imposex was reported to be approximately 20 ng Sn/g dry weight (corresponding to approximately 10–12.5 ng TBT/g wet weight, assuming that the concentration on a dry weight basis is 4–5 times that on a wet weight basis) for N. lapillus (Gibbs et al. 1987), and the estimated threshold concentration of TBT was similarily reported to be 10–20 ng/g wet weight for T. clavigera (Horiguchi et al. 1994). However, because of limited experimental and analytical data, it is difficult to compare the sensitivity to TBT and TPhT of B. japonica to that of other gastropod species such as N. lapillus, O. erinacea, U. cinerea, and T. clavigera (Bryan et al. 1987; Gibbs et al. 1987, 1990, 1991; Horiguchi et al. 1994, 1995).
Figure 9.

Relationship between triorganotin (the sum of tributyltin and triphenyltin) concentrations in gonads and penis length in female B. japonica.
The planktonic stage of B. japonica is estimated to last approximately 4–5 days (Hamada et al. 1988, 1989). Thus, the recruitment of veliger larvae from other populations that inhabit remote, less contaminated areas is unlikely. Reproductive failure accompanied by imposex in females could result in extirpation of the B. japonica population within several years because the number of offspring produced by adult B. japonica in the population is likely to continue to decrease. The existence and duration of a free-swimming phase during larval development is one of the important factors in determining the linkage of impaired reproductive ability caused by imposex to population decline (Bryan et al. 1986; Gibbs and Bryan 1986; Gibbs et al. 1988, 1990, 1991).
Conclusions
Our findings suggest that reproductive failure (suppressed ovarian maturation and ovarian spermatogenesis) in adult females accompanied with imposex, possibly induced by TBT or TPhT from antifouling paints, could have brought about the marked decline in B. japonica populations that has been observed.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
This study was partially supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
We are grateful to H. Hirose, Nihon University, Japan, for her cooperation and helpful suggestions on histopathological examination of gonads.
References
- Bech M. A survey of imposex in muricids from 1996 to 2000 and identification of optimal indicators of tributyltin contamination along the east coast of Phuket Island, Thailand. Mar Pollut Bull. 2002a;44:887–896. doi: 10.1016/s0025-326x(02)00115-7. [DOI] [PubMed] [Google Scholar]
- Bech M. Imposex and tributyltin contamination as a consequence of the establishment of a marina and increasing yachting activities at Phuket Island, Thailand. Environ Pollut. 2002b;117:421–429. doi: 10.1016/s0269-7491(01)00191-9. [DOI] [PubMed] [Google Scholar]
- Bettin C, Oehlmann J, Stroben E. TBT-induced imposex in marine neogastropods is mediated by an increasing androgen level. Helgol Meeresunters. 1996;50:299–317. [Google Scholar]
- Bock R. 1981. Pharmacology and toxicology of phenyltin compounds: behavior in warm-blood animals of “triphenyltin compounds and their degradation products.” In: Residue Reviews (Gunter FA, Gunter JD, eds). Vol 79. New York:Springer-Verlag, 31–61.
- Bryan GW, Gibbs PE, Hummerstone LG, Burt GR. The decline of the gastropod Nucella lapillus around south-west England: evidence for the effect of tributyltin from antifouling paints. J Mar Biol Assoc UK. 1986;66:611–640. [Google Scholar]
- Bryan GW, Gibbs PE, Burt GR, Hummerstone LG. The effects of tributyltin (TBT) accumulation on adult dog-whelks, Nucella lapillus: long-term field and laboratory experiments. J Mar Biol Assoc UK. 1987;67:525–544. [Google Scholar]
- Bryan GW, Gibbs PE, Burt GR. A comparison of the effectiveness of tri-n-butyltin chloride and five other organotin compounds in promoting the development of imposex in the dog-whelk, Nucella lapillus. J Mar Biol Assoc UK. 1988;68:733–744. [Google Scholar]
- Bryan GW, Bright DA, Hummerstone LG, Burt GR. Uptake, tissue distribution and metabolism of 14C-labelled tributyltin (TBT) in the dog-whelk, Nucella lapillus. J Mar Biol Assoc UK. 1993;73:889–912. [Google Scholar]
- Chiu AY, Hunkapiller MW, Heller E, Stuart DK, Hood LE, Strumwasser F. Purification and primary structure of neuropeptide egg-laying hormone of Aplysia californica. Proc Natl Acad Sci USA. 1979;76:6656–6660. doi: 10.1073/pnas.76.12.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho MR, Fuentes S, Bebianno MJ. TBT effects on the larvae of Ruditapes decussates. J Mar Biol Assoc UK. 2001;81:259–265. [Google Scholar]
- Ebberink RHM, Loenhout H, van Geraerts WPM, Joosse J. Purification and amino acid sequence of the ovulation neuro-hormone of Lymnaea stagnalis. Proc Natl Acad Sci USA. 1985;82:7767–7771. doi: 10.1073/pnas.82.22.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriva H, Safi R, Hänni C, Langlois M-C, Saumitou-Laprade P, Stehelin D, et al. Ligand binding was acquired during evolution of nuclear receptors. Proc Natl Acad Sci USA. 1997;94:6803–6808. doi: 10.1073/pnas.94.13.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Féral C, Le Gall S. 1983. The influence of a pollutant factor (TBT) on the neurosecretory mechanism responsible for the occurrence of a penis in the females of Ocenebra erinacea In: Molluscan Neuro-endocrinology (Lever J, Boer HH, eds). Amsterdam:North Holland Publishing, 173–175.
- Fioroni P, Oehlmann J, Stroben E. The pseudohermaphroditism of prosobranchs; morphological aspects. Zool Anz. 1991;226:1–26. [Google Scholar]
- Fretter V. 1984. Prosobranchs. In: The Mollusca (Wilbur KM, ed). Vol 7. Reproduction (Tompa AS, Verdonk NH, van den Biggelaar JAM, eds). Orlando, FL:Academic Press, 1–45.
- Gibbs PE, Bryan GW. Reproductive failure in populations of the dog-whelk, Nucella lapillus, caused by imposex induced by tributyltin from antifouling paints. J Mar Biol Assoc UK. 1986;66:767–777. [Google Scholar]
- Gibbs PE, Bryan GW. 1996. TBT-induced imposex in neogastropod snails: masculinization to mass extinction. In: Tributyltin: Case Study of an Environmental Contaminant (de Mora SJ, ed). Cambridge, UK:Cambridge University Press, 212–236.
- Gibbs PE, Bryan GW, Pascoe PL, Burt GR. The use of the dog-whelk, Nucella lapillus, as an indicator of tributyltin (TBT) contamination. J Mar Biol Assoc UK. 1987;67:507–523. [Google Scholar]
- Gibbs PE, Pascoe PL, Burt GR. Sex change in the female dog-whelk, Nucella lapillus, induced by tributyltin from antifouling paints. J Mar Biol Assoc UK. 1988;68:715–731. [Google Scholar]
- Gibbs PE, Bryan GW, Pascoe PL, Burt GR. Reproductive abnormalities in female Ocenebra erinacea (Gastropoda) resulting from tributyltin-induced imposex. J Mar Biol Assoc UK. 1990;70:639–656. [Google Scholar]
- Gibbs PE, Spencer BE, Pascoe PL. The American oyster drill, Urosalpinx cinerea (Gastropoda): evidence of decline in an imposex affected population (R. Blackwater, Essex) J Mar Biol Assoc UK. 1991;71:827–838. [Google Scholar]
- Goldberg ED. TBT: an environmental dilemma. Environment. 1986;28:17–20. 42–44. [Google Scholar]
- Hamada M, Inoue M. Seed production of the ivory shell, Babylonia japonica Reeve [in Japanese] Bull Tottori Prefect Fish Exp Station. 1993;11:193–195. [Google Scholar]
- Hamada M, Inoue M. Seed production of the ivory shell, Babylonia japonica Reeve [in Japanese] Bull Tottori Prefect Fish Exp Station. 1994;12:182–184. [Google Scholar]
- Hamada M, Inoue M. Seed production of the ivory shell, Babylonia japonica Reeve [in Japanese] Bull Tottori Prefect Fish Exp Station. 1995;13:204–206. [Google Scholar]
- Hamada F, Kanazawa T, Yamamoto E. Seed production of the ivory shell, Babylonia japonica Reeve [in Japanese] Bull Tottori Prefect Fish Exp Station. 1988;6:110–116. [Google Scholar]
- Hamada F, Kanazawa T, Yamamoto E. Seed production of the ivory shell, Babylonia japonica Reeve [in Japanese] Bull Tottori Prefect Fish Exp Station. 1989;7:103–109. [Google Scholar]
- Horiguchi T. 1993. Imposex Caused by Organotin Compounds in Marine Gastropods from Japan [PhD Thesis, in Japanese]. Bunkyo-ku, Tokyo:The University of Tokyo.
- Horiguchi T. Organotin compounds and reproductive abnormalities in marine snails [in Japanese] Kagaku. 1998;68:546–551. [Google Scholar]
- Horiguchi T. 2000. Molluscs. In: Problems of Endocrine Disruptors in Fisheries Environment [in Japanese] (Kawai S, Koyama J, eds) . Tokyo:Koseisha-Koseikaku, 54–72.
- Horiguchi T, Shimizu M. 1992. Effects on aquatic organisms, mainly on molluscs. In: Organotin Pollution and Its Effects on Aquatic Organisms [in Japanese] (Satomi Y, Shimizu M, eds). Tokyo:Koseisha-Koseikaku, 99–135.
- Horiguchi T, Shiraishi H, Shimizu M, Morita M. Imposex and organotin compounds in Thais clavigera and T. bonni in Japan. J Mar Biol Assoc UK. 1994;74:651–669. [Google Scholar]
- Horiguchi T, Shiraishi H, Shimizu M, Yamazaki S, Morita M. Imposex in Japanese gastropods (Neogastropoda and Mesogastropoda): effects of tributyltin and triphenyltin from antifouling paints. Mar Pollut Bull. 1995;31:402–405. [Google Scholar]
- Horiguchi T, Shiraishi H, Shimizu M, Morita M. Effects of triphenyltin chloride and five other organotin compounds on the development of imposex in the rock shell, Thais clavigera. Environ Pollut. 1997a;95:85–91. doi: 10.1016/s0269-7491(96)00093-0. [DOI] [PubMed] [Google Scholar]
- Horiguchi T, Shiraishi H, Shimizu M, Morita M. Imposex in sea snails, caused by organotin (tributyltin and triphenyltin) pollution in Japan: a survey. Appl Organomet Chem. 1997b;11:451–455. [Google Scholar]
- Horiguchi T, Cho HS, Shiraishi H, Shibata Y, Morita M, Shimizu M, et al. Temporal trends and current status of organotin contamination and imposex in gastropods from Japan [in Japanese] Bulletin on Coastal Oceanography. 2000a;37:89–95. [Google Scholar]
- Horiguchi T, Takiguchi N, Cho HS, Kojima M, Kaya M, Shiraishi H, et al. Ovo-testis and disturbed reproductive cycle in the giant abalone, Haliotis madaka: possible linkage with organotin contamination in a site of population decline. Mar Environ Res. 2000b;50:223–229. doi: 10.1016/s0141-1136(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Horiguchi T, Kojima M, Kaya M, Matsuo T, Shiraishi H, Morita M, et al. Tributyltin and triphenyltin induce spermatogenesis in ovary of female abalone, Haliotis gigantea. Mar Environ Res. 2002;54:679–684. doi: 10.1016/s0141-1136(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Horiguchi T, Shiraishi H, Morita M. 2003. Specific tissue distributions of organotin compounds in prosobranch gastropods [Abstract]. SETAC 24th Annual Meeting Abstract Book. Pensacola, FL:SETAC, 290.
- Horiguchi T, Kojima M, Takiguchi N, Kaya M, Shiraishi H, Morita M. Continuing observation of disturbed reproductive cycle and ovarian spermatogenesis in the giant abalone, Haliotis madaka, from an organotin-contaminated site of Japan. Mar Pollut Bull. 2005;51:817–822. doi: 10.1016/j.marpolbul.2005.06.045. [DOI] [PubMed] [Google Scholar]
- Inoue S, Oshima Y, Nagai K, Yamamoto T, Go J, Kai N, et al. Effect of maternal exposure to tributyltin on reproduction of the pearl oyster (Pinctada fucata martensii) Environ Toxicol Chem. 2004;23:1276–1281. doi: 10.1897/03-265. [DOI] [PubMed] [Google Scholar]
- International Maritime Organization (IMO). 2001. Adoption of the final act of the conference and any instruments, recommendations and resolutions resulting from the work of the conference. International Convention on the Control of Harmful Anti-fouling Systems on Ships, 2001 (AFS/CONF/26, 18 October 2001), London, UK. London:International Maritime Organization, 1–26.
- Kajikawa A. Sexual characteristics, spawning abilities, and their percentage occurrence in populations of Babylonia japonica Reeve [in Japanese] Bull Tottori Prefect Fish Exp Station. 1984;2:31–32. [Google Scholar]
- Kajikawa A, Yamamoto E, Masutani R. Sexual characteristics and spawning abilities in the ivory shell, Babylonia japonica Reeve [in Japanese] Bull Tottori Prefect Fish Exp Station. 1983;1:16–18. [Google Scholar]
- Lapota D, Rosenberger DE, Platter-Rieger MF, Seligman PF. Growth and survival of Mytilus edulis larvae exposed to low levels of dibutyltin and tributyltin. Mar Biol. 1993;115:413–419. [Google Scholar]
- LeBlanc GA, Campbell PM, den Besten P, Brown RP, Chang ES, Coats JR et al. 1999. The endocrinology of invertebrates. In: Endocrine Disruption in Invertebrates: Endocrinology, Testing, and Assessment (deFur PL, Crane M, Ingersoll C, Tattersfield L, eds). Pensacola, FL:SETAC Press, 23–106.
- Lee RF. Metabolism of tributyltin oxide by crab, oyster, and fish. Mar Environ Res. 1985;17:145–148. [Google Scholar]
- Le Guellec D, Thiard MC, Remy-Martin JP, Deray A, Gomot L, Adessi GL. In vitro metabolism of androstenedione and identification of endogenous steroids in Helix aspersa. Gen Comp Endocrinol. 1987;66:425–433. doi: 10.1016/0016-6480(87)90253-x. [DOI] [PubMed] [Google Scholar]
- Li Q, Osada M, Takahashi K, Matsutani T, Mori K. Accumulation and depuration of tributyltin oxide and its effect on the fertilization and embryonic development in the Pacific oyster, Crassostrea gigas. Bull Environ Contam Toxicol. 1997;58:489–496. doi: 10.1007/s001289900361. [DOI] [PubMed] [Google Scholar]
- Lu M, Horiguchi T, Shiraishi H, Shibata Y, Abo M, Okubo A, et al. Identification and quantitation of steroid hormones in marine gastropods by GC/MS [in Japanese] Bunseki Kagaku. 2001;50:247–255. [Google Scholar]
- Marshall DJ, Rajkumar A. Imposex in the indigenous Nassarius kraussianus (Mollusca: Neogastropoda) from South African harbours. Mar Pollut Bull. 2003;46:1150–1155. doi: 10.1016/S0025-326X(03)00191-7. [DOI] [PubMed] [Google Scholar]
- Matsuda R, Suzuki T, Saito Y. Metabolism of tri-n-butyltin chloride in male rats. J Agric Food Chem. 1993;41:489–495. doi: 10.1021/jf9903408. [DOI] [PubMed] [Google Scholar]
- Matthiessen P, Gibbs PE. Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ Toxicol Chem. 1998;17:37–43. [Google Scholar]
- Mensink B, Boon JP, ten Hallers-Tjabbes CC, van Hattum B, Koeman JH. Bioaccumulation of organotin compounds and imposex occurrence in a marine food chain (Eastern Scheldt, The Netherlands) Environ Technol. 1997;18:1235–1245. [Google Scholar]
- Morcillo Y, Porte C. Evidence of endocrine disruption in the imposex–affected gastropod Bolinus brandaris. Environ Res A. 1999;81:349–354. doi: 10.1006/enrs.1999.4002. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Oshima Y, Nagafuchi K, Hano T, Shimasaki Y, Honjo T. Early-life-stage toxicity in offspring from exposed parent medaka, Oryzias latipes, to mixtures of tributyltin and polychlorinated biphenyls. Environ Toxicol Chem. 2005;24:591–596. doi: 10.1897/04-157r.1. [DOI] [PubMed] [Google Scholar]
- Nishikawa J, Mamiya S, Kanayama T, Nishikawa T, Shiraishi F, Horiguchi T. Involvement of the retinoid X receptor in the development of imposex caused by organotins in gastropods. Environ Sci Technol. 2004;38:6271–6276. doi: 10.1021/es049593u. [DOI] [PubMed] [Google Scholar]
- Oberdörster E, McClellan-Green P. The neuropeptide APGWamide induces imposex in the mud snail (Ilyanassa obsoleta) Peptides. 2000;21:1323–1330. doi: 10.1016/s0196-9781(00)00274-6. [DOI] [PubMed] [Google Scholar]
- Oberdörster E, McClellan-Green P. Mechanism of imposex induction in mud snail, Ilyanassa obsoleta: TBT as a neuro-toxin and aromatase inhibitor. Mar Environ Res. 2002;54:715–718. doi: 10.1016/s0141-1136(02)00118-6. [DOI] [PubMed] [Google Scholar]
- Oehlmann J, Fioroni P, Stroben E, Markert B. Tributyltin (TBT) effects on Ocinebrina aciculata (Gastropoda: Muricidae): imposex development, sterilization, sex change, and population decline. Sci Total Environ. 1996;188:205–223. [Google Scholar]
- Ruiz JM, Bryan GW, Gibbs PE. Acute and chronic toxicity of tributyltin (TBT) to pediveliger larvae of the bivalve Scrobularia plana. Mar Biol. 1995;124:119–126. [Google Scholar]
- Schulte-Oehlmann U, Oehlmann J, Fioroni P, Bauer B. Imposex and reproductive failure in Hydrobia ulvae (Gastropoda: Prosobranchia) Mar Biol. 1997;128:257–266. [Google Scholar]
- Shim WJ, Kahng SH, Hong SH, Kim NS, Kim SK, Shim JH. Imposex in the rock shell, Thais clavigera, as evidence of organotin contamination in the marine environment of Korea. Mar Environ Res. 2000;49:435–451. doi: 10.1016/s0141-1136(99)00084-7. [DOI] [PubMed] [Google Scholar]
- Smith BS. Sexuality in the American mud snail, Nassarius obsoletus Say. Proc Malacol Soc Lond. 1971;39:377–378. [Google Scholar]
- Sole M, Morcillo Y, Porte C. Imposex in the commercial snail (Bolinus brandaris) in the northwestern Mediterranean. Environ Pollut. 1998;99:241–246. doi: 10.1016/s0269-7491(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Spooner N, Gibbs PE, Bryan GW, Goad LJ. The effect of tributyltin upon steroid titres in the female dogwhelk, Nucella lapillus, and the development of imposex. Mar Environ Res. 1991;32:37–49. [Google Scholar]
- Suzuki T, Kondo K, Uchiyama M, Murayama M. Some sulfur-containing metabolites of tri-n-butyltin chloride. J Agric Food Chem. 1999;47:4791–4798. doi: 10.1021/jf9903408. [DOI] [PubMed] [Google Scholar]
- Takamaru T, Fuji A. Reproductive cycles in populations of Neptunea arthritica from the southern coastal waters of Hokkaido, Japan [in Japanese] Suisan Zoshoku. 1981;29:78–87. [Google Scholar]
- Takeda N. Induction of egg-laying by steroid hormones in slugs. Comp Biochem Physiol. 1979;62A:273–278. [Google Scholar]
- Takeda N. 1983. Endocrine regulation of reproduction in the snail, Euhadra peliomphala In: Molluscan Neuro-Endocrinology (Lever J, Boer HH, eds). Amsterdam:North Holland Publishing, 106–111.
- Tanabe S, Prudente M, Mizuno T, Hasegawa J, Iwata H, Miyazaki N. Butyltin contamination in marine mammals from north Pacific and Asian coastal waters. Environ Sci Technol. 1998;32:193–198. [Google Scholar]
- ten Hallers-Tjabbes CC, Wegener JW, Van Hattum BA, Kemp JF, ten Hallers E, Reitsemae TJ, et al. Imposex and organotin concentrations in Buccinum undatum and Neptunea antiqua from the North Sea: relationship to shipping density and hydrographical conditions. Mar Environ Res. 2003;55:203–233. doi: 10.1016/s0141-1136(02)00217-9. [DOI] [PubMed] [Google Scholar]
- Terlizzi A, Delos AL, Garaventa F, Faimali M, Geraci S. Limited effectiveness of marine protected areas: imposex in Hexaplex trunculus (Gastropoda: Muricidae) populations from Italian marine reserves. Mar Pollut Bull. 2004;48:188–192. doi: 10.1016/j.marpolbul.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- Uki N. 1989. Sexual maturation, development, and growth in gastropods, and their control . In: Reproductive Biology of Fish and Shellfish [in Japanese] (Takashima F, Hanyu I, eds). Tokyo:Midori Shobo, 367–417.
- Yamabe Y, Hoshino A, Imura N, Suzuki T, Himeno S. Enhancement of androgen-dependent transcription and cell proliferation by tributyltin and triphenyltin in human prostate cancer cells. Toxicol Appl Pharmacol. 2000;169:177–184. doi: 10.1006/taap.2000.9067. [DOI] [PubMed] [Google Scholar]