Abstract
Previous investigations have shown that bisphenol A (BPA) induces a superfeminization syndrome in the freshwater snail Marisa cornuarietis at concentrations as low as 1 μg/L. Superfemales are characterized by the formation of additional female organs, enlarged accessory sex glands, gross malformations of the pallial oviduct, and a stimulation of egg and clutch production, resulting in increased female mortality. However, these studies were challenged on the basis of incomplete experimentation. Therefore, the objective of the current approach was to bridge several gaps in knowledge by conducting additional experiments. In an initial series of experiments, study results from the reproductive phase of the snails were evaluated in the sub-micrograms per liter range. Before and after the spawning season, superfemale responses were observed [NOEC (no observed effect concentration) 7.9 ng/L, EC10 (effective concentration at 10%) 13.9 ng/L], which were absent during the spawning season. A further experiment investigated the temperature dependence of BPA responses by exposing snails at two temperatures in parallel. The adverse effect of BPA was at least partially masked at 27°C (EC10 998 ng/L) when compared with 20°C (EC10 14.8 ng/L). In M. cornuarietis, BPA acts as an estrogen receptor (ER) agonist, because effects were completely antagonized by a co-exposure to tamoxifen and Faslodex. Antiandrogenic effects of BPA, such as a significant decrease in penis length at 20°C, were also observed. Competitive receptor displacement experiments indicate the presence of androgen- and estrogen-specific binding sites. The affinity for BPA of the estrogen binding sites in M. cornuarietis is higher than that of the ER in aquatic vertebrates. The results emphasize that prosobranchs are affected by BPA at lower concentrations than are other wildlife groups, and the findings also highlight the importance of exposure conditions.
Keywords: bisphenol A, endocrine disruptor, hazard assessment, low-dose effects, Marisa cornuarietis, prosobranchia, reproductive toxicity, superfemale, temperature, xenoestrogen
It has been shown that several xenobiotics in the environment are capable of inducing adverse effects in invertebrates and vertebrates, including humans, by interfering with the normal endocrine function of the organism (Colborn et al. 1993). Most studies have focused on endocrine disrupting chemicals (EDCs) that act as agonists at the estrogen receptor (ER) (Shelby et al. 1996). Among these xenoestrogens, bisphenol A (BPA, 4,4′-isopropylidine diphenol, CAS No. 80-05-7) has received particular attention because of its estimated worldwide production of 2.5 million metric tons in 2001 and its wide application in consumer household products (Staples et al. 2002). BPA is an intermediate used primarily in the production of polycarbonate and epoxy resins such as food and beverage containers, acrylic glass, and compact discs. Lesser amounts are used to produce fire retardants, thermo paper, tires, dental composites, and sealants, or as a stabilizer in polyvinyl chloride and other plastics (Staples et al. 2002).
With log KOW values of 2.20–3.82 (Heemken et al. 2001; Staples et al. 1998) and a water solubility of 300 mg/L (Staples et al. 1998), a moderate adsorption to sediments and accumulation in organisms can be expected. BPA is easily degraded, with a half-life of 2.5–4 days in water (Dorn et al. 1987) or 28 days in sewage treatment plants (Howard 1989). Nevertheless, BPA is detected continually in aquatic ecosystems, with concentrations up to 1.9 μg/L in Japanese rivers (Howard 1989). In the River Elbe (Germany), BPA was detected at concentrations from 9 to 776 ng/L in water and from 66 to 343 μg/kg in sediments (Heemken et al. 2001). Three years later aqueous concentrations in the River Elbe dropped to 4 to 92 ng/L, while sediment concentrations were almost identical (10–380 μg/kg) (Stachel et al. 2003). In five other German streams BPA was detected at maximum aqueous concentrations of 272 ng/L (Bolz et al. 2001).
BPA has been known to induce estrogenic activity at high doses in rodents since the 1930s (Dodds and Lawson 1936, 1938), although it exhibits only weak affinity to the vertebrate ER (Bolger et al. 1998). Acute toxicity of BPA to aquatic organisms appears to be low, with 96-hr LC50 (median lethal concentration) values in the 1–20 mg/L range for vertebrates and invertebrates (Hirano et al. 2004; Ike et al. 2002). In Japanese medaka (Oryzias latipes), BPA induced ovotestes at concentrations as low as 10 μg/L (Metcalfe et al. 2001). In a multi-generational study conducted with fathead minnows (Pimephales promelas) over a concentration range from 1 to 1,280 μg BPA/L, Sohoni et al. (2001) found adverse reproductive effects ≥ 640 μg/L. Kloas et al. (1999) found a significantly higher number of female phenotypes in Xenopus laevis after exposure of tadpoles to concentrations as low as 23 μg BPA/L. Although these studies indicate a weak estrogenic activity with effects beyond 10 μg/L, negative impacts on sexual differentiation in aquatic vertebrates were also reported at lower, environmentally relevant concentrations. In swordtails (Xiphophorus helleri) the development of the sword as a secondary sexual characteristic of male fish was inhibited in a concentration range from 0.2 to 20 μg/L (Kwak et al. 2001).
Only a few studies have investigated the effects of BPA as a xenoestrogen in aquatic invertebrates, although many researchers have examined such effects in vertebrates. It has been reported previously that exposure of a gonochoristic prosobranch mollusk, the ramshorn snail Marisa cornuarietis, to BPA induces a superfeminization syndrome at BPA concentrations as low as 1 μg/L (Oehlmann et al. 2000). Superfemales are characterized by the formation of additional female organs, enlarged accessory sex glands, gross malformations of the pallial oviduct, and a stimulation of egg and clutch production, resulting in increased female mortality. These results were challenged on the basis of incomplete experimentation (no analytical confirmation of test compounds, no replicates, no positive control). Staples et al. (2002) rated the study as “not valid,” and the results were not considered for the currently ongoing environmental risk assessment for BPA in Europe. However, the European risk assessor for BPA (U.K.) asked the plastics industry to replicate the original study. Subsequent discussions led to the decision that the design of the planned industry study will deviate from the original study in many aspects (e.g., exposure conditions, temperature, origin of test organisms). However, it is a cause for concern that experiments on BPA seem to be highly dependent on the experimental conditions employed, as shown in repetitions of the Kloas et al. (1999) study on BPA and X. laevis by Pickford et al. (2003) and Levy et al. (2004).
It was our objective to conduct additional BPA exposure experiments with M. cornuarietis to resolve the shortcomings of the original trial. We also sought to achieve the following additional goals: a) to determine NOEC and EC10 (effective concentration at 10%) for the super-female response, b) to analyze the influence of different reproductive phases of animals on the study outcome, c) to investigate the temperature-dependence of the effect, d) to explore whether the superfemale response is ER-mediated, and e) to find reasons for the high BPA sensitivity of M. cornuarietis compared with that of other aquatic species, particularly vertebrates.
Materials and Methods
Animals and exposures
Ramshorn snails, M. cornuarietis (Mesogastropoda: Ampullariidae), came from our laboratory breeding stock, which was maintained at a temperature of 22 ± 1°C. The stock was derived from Aquazoo Düsseldorf (Düsseldorf, Germany) in 1991 with regular crossbreeding of wild-caught animals from Florida to avoid inbreeding. Exposure experiments were performed as 24-hr (weekends, 48-hr) semistatic renewal systems in 60-L glass aquaria (water volume, 54 L), provided with an Eheim power filter and aeration (Eheim, Deizisau, Germany). Animals were fed regularly with TetraMin (Tetra, Melle, Germany) ad libitum, supplemented with lettuce in exposure series I. In exposure series II, TetraMin was used exclusively. Tests were performed at constant temperature with an equal light:dark cycle (12:12 hr). Water parameters (pH, conductivity, temperature, nitrite, O2 concentration, and saturation) were measured twice a week per tank. Initial values were pH 7.1–7.5, 809–924 μS/cm, < 1 mg NO2 (nitrogen dioxide)/L, and 80.8–102.2% O2 saturation.
Two experimental series were conducted with an exposure to different nominal concentration ranges of BPA (Merck Schuchardt, Hohenbrunn, Germany), including a solvent control (SC, ethanol concentration: 12.5 μL/L) and a positive control [PC; 17α-ethinylestradiol (EE2; Fluka, Buchs, Germany); nominal concentration: 0.01 μg/L, only exposure series I]. The applied ethanol concentrations do not affect reproduction in M. cornuarietis, as demonstrated in extensive lab studies with androgenic (tributyltin, triphenyltin, methyl testosterone, fenarimol, letrozole) and anti-androgenic compounds (p,p′-dichlorodi-phenyldichloroethylene, cyproterone acetate, linuron) in the European Union project COMPRENDO (Comparative Research on Endocrine Disrupters).
Exposure series I
Groups of 210 sexually mature snails (shell height > 20 mm, age > 18 months) were exposed to nominal BPA concentrations of 0, 0.05, 0.1, 0.25, 0.5, and 1 μg/L for 6 months (from September to March) in charcoal-filtered tap water at 22 ± 1°C. Thirty specimens were collected for analysis at the beginning of the experiment, and from each group at monthly intervals. Initial density in the tank was 3.89 snails/L, which dropped to a maximum of 0.56 snails/L during the last month.
Exposure series II
Two replicate groups of 30 sexually mature snails (shell height sssss> 20 mm, age > 18 months) were exposed to nominal BPA concentrations of 0, 0.25, 0.5, 1, and 5 μg/L alone, and to 5 μg BPA/L in combination with a potent antiestrogen [3 μg ICI 182,780/L (Faslodex; Tocris, Ellisville, MO, USA) or 10 μg tamoxifen/L (Sigma Chemical Co., Deisenhofen, Germany)] for 5 months (February to July) in fully reconstituted water at 20 ± 1°C and 27 ± 1°C in parallel. Animals were acclimatized to exposure temperatures 2 weeks before the start of the experiment. Thirty specimens were analyzed at the beginning, and all surviving animals were analyzed at the end. Initial density in the tank was 1.11 snails/L, which dropped to 0.72–1.00 snails/L at the end of the experiment, depending on the mortality in the different groups.
BPA analyses
Two-L water samples were taken in brown glass bottles during 24-hr cycles (at months 1, 3, and 5 for exposure series I, and at month 1 for exposure series II). Sampling began 15 min before the change of exposure media and ended 1 day later, before media were changed again (per cycle: n = 8). For extraction, solid-phase material (500 mg RP-C18 Bulk Sorbent; Separtis GmbH, Grenzahl-Wyhlen, Germany) was placed into glass cartridges, which were then conditioned by flushing with 1 × 2 mL hexane, followed by 1 × 2 mL acetone and 3 × 2 mL methanol. Cartridges were washed with 5 × 2 mL of water adjusted to pH 7. One-half liter of the water sample adjusted to pH 7 was glass-fiber filtered (< 1 μm) and spiked with bisphenol F as surrogate standard. Samples were sucked through packed glass cartridges at a flow rate of 20 mL/min. Subsequently, the solid phase was dried by a nitrogen stream for 1 hr, and analytes were eluted 4 times with 1 mL of acetone. Acetone extracts were evaporated to 200 μL by a gentle nitrogen stream.
For detection by gas chromatography–mass spectrometry (GC-MS), extracts were derivatized by adding 50 μL of the derivatization mixture [N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA)/trimethylsilylimidazole (TMSI)/dithioerytrol (DTE), 1,000 + 2 + 2, vol/vol/wt]. MSTFA and TMSI were purchased from Sigma (Deisenhofen, Germany), and DTE was obtained from Merck (Darmstadt, Germany). After 1 hr of reaction at 60°C, the solution was evaporated to dryness by a gentle nitrogen stream, and the residue was dissolved in 200 μL hexane. Finally, 100 μg mirex (Promochem, Wesel, Germany) was added to the final extract as an internal standard. For quantification, m/z = 357 and 372 were used for GC-MS detection in the SIM (selected ion monitoring) mode. A 10-point calibration was performed during the whole procedure after spiking deep groundwater with BPA in concentrations ranging from 0.01 to 5 μg/L. In each analysis series, a blank sample of deep groundwater was run in parallel.
The GC-MS system included an HP 5890 Series II coupled with an HP 5971 MS detector (Hewlett-Packard, Palo Alto, CA, USA). A Restek XTI-5 capillary column (Restek GmbH, Bad Homburg, Germany) was used at a head pressure of 85 kPa with a 3-μL splitless injector (250°C isotherm). The GC temperature program was set to a 50°C isotherm for 1.5 min, then ramped up at 20°C/min to 240°C/min, and at 1.5°C/min to 290°C/min, and finally set to a 290°C isotherm for 10 min.
Biological assessments
Mortality, numbers of eggs, clutches, and eggs per clutch in the tanks were recorded daily. Fecundity parameters were corrected for number of females per tank by taking into consideration the number of females analyzed in monthly intervals as well as mortality data with an assumed 1:1 sex ratio. Measures of variability for produced eggs and clutches could be calculated for exposure series II (two replicates) but not for series I (one replicate).
Snails were narcotized before analysis for a minimum of 1.5 hr (2.5% MgCl2 in distilled water), and their shells were cracked and removed. The extensions of all sex organs were measured to the nearest 0.1 mm under a dissection microscope, and malformations such as oviduct ruptures or excrescences on genital and other organs were recorded.
Competitive receptor displacement experiments
Cytosolic extracts were prepared from testes or ovaries of up to 10 individuals to achieve a fresh weight of 3 g, homogenized in 9 mL ice-cold incubation buffer [20 mM Tris–HCl (pH 7.4), 250 mM sucrose, 10 mM sodium molybdate, 5 mM dithiothreitol]. Homogenates were centrifuged at 12,000× g for 12 min, and the supernatant was centrifuged a second time at 100,000× g for 60 min at 4°C. The final supernatant represented the cytosolic extract, and it was used for displacement experiments. In time-course experiments for specific [3H]-E2 and testosterone ([3H]-T), binding conditions were optimized using incubation times of 18 and 16 hr for estrogen and androgen binding, respectively, when a steady state was obtained. Radiolabeled ligands ([3H]-E2, specific activity 41.8 μCi/mmol; PerkinElmer, Rodgau-Jugesheim, Germany; and [3H]-T, 95 μCi/mmol; Amersham Biotech, Freiburg, Germany) were dissolved in 5% ethanol. Incubations were performed in duplicate using 25μL [3H]-E2 (final concentration 24 nM) or [3H]-T (final concentration 22 nM), 10 μL unlabeled ligand or solvent, 150 μL incubation buffer, and 100 μL of cytosolic fractions from three preparations of pooled testes or ovaries. Unlabeled ligands E2, Tam (tamoxifen), BPA, T (testosterone), and MT (methyl testosterone) (Sigma, Deisenhofen, Germany) were diluted in 99.8% ethanol and added at final concentrations ranging from 10−9 to 10−3 M. Separation of receptor-bound and free ligands was accomplished by using dextran-coated activated charcoal solution [prepared by adding 3.75 g activated charcoal Norit A (4- to 7- μm particle size) (Serva, Heidelberg, Germany) and 0.375 g dextran T70 (Roth, Karlsruhe, Germany)] to 20 mM Tris–HCl buffer, pH 7.4. Three hundred microliters of charcoal solution were added to each sample, vortexed, incubated for 5 min, and centrifuged for 15 min at 3,500 × g and 4°C. Free ligands were adsorbed by activated charcoal and sedimented. Amounts of [3H]-E2 or [3H]-T bound to binding sites were determined by measuring the radioactivity of 400 μL supernatant in 3 mL scintillation cocktail (Ultima Gold TM;Packard BioScience, Groningen, the Netherlands), using a liquid scintillation counter (Tri Carb 1600; Canberra-Packard, Rodgau-Jugesheim, Germany). Samples were counted for 2 min, and measurements were corrected for background radiation.
Statistical analyses
The computer programs StatEasy (Wissenschaftliche Auswertungen, Hamburg, Germany) and Prism, version 4.02 (GraphPad Software, San Diego, CA, USA) were used for all statistical analyses. The chi-squared test was applied for mortality data, the t-test was used for the comparison of egg production between identical nominal treatment groups at different temperatures, and the Weir test was employed to detect imposex intensities. Analysis of covariance (ANCOVA) was used to ascertain cumulative egg and clutch numbers, and one-way analysis of variance (ANOVA) with the Student-Newman-Keuls post-hoc test was used to determine egg and clutch numbers per female and to make a comparison of genital organ lengths. The significance level for all tests was set at p < 0.05 when compared to solvent control. BPA half-lives in the exposure tanks were calculated using a one-phase exponential decay model, IC50 (median inhibitory concentration) values with a homologous competitive binding curve for one class of binding sites, and EC10 and EC50 values with a Weibull distribution (Prism 4.02).
Results
BPA analyses
Tables 1 and 2 are a summary of the BPA concentrations measured in exposure tanks during the two experiment series. Actual initial concentrations 15 min after renewal of exposure media were between 80.0 and 130% of nominals in exposure series I and between 96.0 and 110% of nominals in exposure series II, thereby indicating a spiking procedure of good quality. Probably because of adsorption to tank surfaces, snails, and food and/or degradation, a consequent loss of the test compound was apparent, so that median concentrations were between 39.0 and 48.3% of nominals except for the lowest concentration in exposure series I (15.8%). Calculated BPA half-life was between 2.53 and 6.35 hr, with the recurring exception of the lowest concentration in exposure series I (1.06 hr). Although ratios between analytically verified and nominal BPA concentrations in series II are in good accordance for the two exposures, measured concentrations were slightly lower, and calculated half-life was shorter for the higher temperature. This finding indicates a faster dissipation of BPA at 27°C compared with that at 20 or 22°C. Single positive determinations of BPA in the control tanks can probably be attributed to the leaching of the compound from the plastics of the Eheim power filters, including tubes. EC10, EC50, NOEC (no observed effect concentration), and LOEC (lowest observed effect concentration) for the different end points are calculated on the basis of measured mean concentrations in the tanks.
Table 1.
Comparison of nominal and measured BPA concentrations in M. cornuarietis exposure experiment I at 22°C (ng/L).
| Nominal concentrations | 0 (SC) | 50 | 100 | 250 | 500 | 1,000 |
|---|---|---|---|---|---|---|
| Measured concentrations | ||||||
| Range (min–max) | 0–40 | 0–40 | 0–180 | 0–360 | 0–590 | 0–1200 |
| Mean ± SD | 9.17 ± 16.4 | 7.92 ± 15.9 | 48.3 ± 51.1 | 104 ± 120 | 205 ± 223 | 404 ± 429 |
| BPA half-life (hr) | — | 1.06 | 3.03 | 3.76 | 6.35 | 5.89 |
| % of nominala | — | 80 | 130 | 121 | 111 | 110 |
Abbreviations: max, maximum; min, minimum.
Recovery of nominal concentrations is based on initial (i.e., directly after renewal) measured values to assess the quality of the spiking procedure.
Table 2.
Comparison of nominal and measured BPA concentrations in M. cornuarietis exposure experiment II at 20°C and 27°C (ng/L).
| Nominal concentrations | 0 (SC) | 250 | 500 | 1,000 | 5,000a |
|---|---|---|---|---|---|
| Measured concentrations (20°C) | |||||
| Range (min–max) | 0–30 | 0–270 | 0–510 | 0–1100 | 80–4900 |
| Mean ± SD | 3.75 ± 10.6 | 106 ± 113 | 224 ± 217 | 465 ± 460 | 2170 ± 1980 |
| BPA half-life (hr) | — | 3.04 | 3.90 | 3.67 | 3.75 |
| % of nominalb | — | 108 | 102 | 110 | 98 |
| Measured concentrations (27°C) | |||||
| Range (min–max) | 0–30 | 0–260 | 0–520 | 0–1100 | 40–4800 |
| Mean ± SD | 3.75 ± 10.6 | 97.5 ± 109 | 205 ± 204 | 436 ± 438 | 1990 ± 1930 |
| BPA half-life (hr) | — | 2.53 | 3.04 | 3.08 | 3.25 |
| % of nominalb | — | 104 | 104 | 110 | 96 |
Measured concentrations from the tank that received solely BPA. BPA concentrations in the tanks form combination experiments with ICI or Tam were not measured.
Recovery of nominal concentrations is based on initial (i.e., directly after renewal) measured values to assess the quality of the spiking procedure.
Exposure series I
During the experiment, which encompassed the main spawning season of snails from November to January, all water parameters were in an acceptable range (pH 6.5–8.0, conductivity 550–950 μS/cm, NO2− < 1 mg/L, O2 saturation > 80%). As in previous BPA experiments at higher concentrations (Oehlmann et al. 2000), the test compound did not induce imposex development in sexually mature M. cornuarietis (ANOVA and Weir test, p > 0.2). However, superfemales with oviduct malformations were observed in all groups except for the SC group and at 0.1 μg/L. The incidence of these malformations was 1.2, 0, 2.0, 3.1, and 3.2% in the groups receiving 0.05, 0.1, 0.25, 0.5, and 1 μg/L of BPA, respectively, and 2.4% in the PC group. We did not observe an increased mortality compared with the SC in any of the groups.
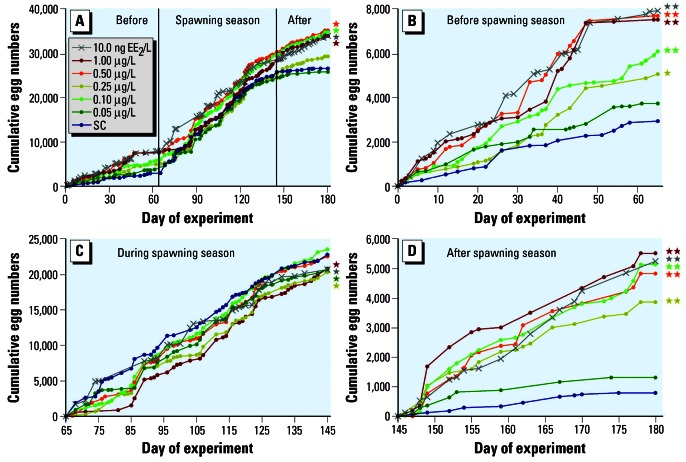
Females exposed to nominal BPA concentrations of 0.1, 0.5, and 1 μg/L, as well as those in the PC, produced significantly more clutches (data not shown) and eggs compared with the SC, provided that cumulative egg numbers were analyzed over the course of the entire experiment (Figure 1A; ANCOVA with Student-Newman-Keuls post-hoc test; p < 0.05). However, the sigmoid shape of cumulative egg production against time in the SC in Figure 1A indicates that snails passed through different phases of the reproductive cycle during the experiment, and therefore the effects of BPA should be assessed separately for the single phases (Figure 1B–D). Before the main spawning phase began (days 0–65), BPA exhibited a marked stimulation on clutch (data not shown) and egg production. Except for the 0.05-μg/L group, all BPA groups as well as the PC produced significantly more eggs than the SC (Figure 1B; ANCOVA with Student-Newman-Keuls post-hoc test, p < 0.05–0.01). Based on measured BPA concentrations, EC10 values were 13.9 and 14.6 ng/L for egg and clutch production, respectively (Table 3). The NOEC was 7.9 ng/L, and the LOEC was 48.3 ng/L for both end points. While control females spawned about 3,000 eggs from days 0–65, the number increased to 23,000 eggs during the following 80 days in the main spawning period of the animals (Figure 1C). Because of the high reproductive output, no further stimulation of egg production was observed. Moreover, snails in all treatments, except in the 0.1- and 0.5-μg BPA/L groups, produced significantly fewer eggs than control animals (ANCOVA with Student-Newman-Keuls post-hoc test, p < 0.05). During the last 35 days of the experiment (after the end of the spawning season), a significant increase in egg production was again observed for all groups exposed to BPA, as well as the PC, compared with the control, except for the group exposed to the lowest BPA concentration (Figure 1D; ANCOVA with Student-Newman-Keuls post-hoc test, p < 0.01). This result indicates that the effect of BPA in M. cornuarietis is totally masked during the main reproduction period of the animals.
Figure 1.
Marisa cornuarietis, exposure series I. Cumulative egg production in groups exposed to BPA and EE2 (positive control), normalized for number of females, for the entire experiment (A) and separated for the phases before (B), during (C), and after the main spawning season (D). All concentrations are provided on a nominal basis.
Significant differences from solvent control (SC): *p < 0.05; **p < 0.01, ANCOVA with Student-Newman-Keuls as post-hoc tests).
Table 3.
EC10 (95% confidence intervals) and NOEC values for the prespawning period of exposure experiment I and for the entire duration of experiment II with M. cornuarietis, based on measured BPA concentrations (ng/L).
| Exposure experiment (temperature) | Egg production | Clutch production |
|---|---|---|
| Exposure I (22°C) | 13.9 (12.8–15.0)
7.9 |
14.6 (13.8–15.4)
7.9 |
| Exposure II (20°C) | 14.8 (6.07–36.2)
< 106 |
18.0 (6.20–52.4)
< 106 |
| Exposure II (27°C) | 998 (161–6,200)
205 |
2090 (796–5,460)
≥ 1990 |
Exposure series II
During exposure series II all water parameters were again in an acceptable range (pH 6.4–8.2, conductivity 575–985 μS/cm, NO2− < 1 mg/L, O2 saturation > 80%). It was the main objective of the experiment to analyze the temperature dependence of the superfemale response in M. cornuarietis. To this end, tests were conducted with identical BPA concentration ranges at two temperatures in parallel, 20 and 27°C. Furthermore, snails were co-exposed to BPA and to potent antiestrogens to investigate the specificity of the superfemale syndrome (i.e., whether the response is ER-mediated).
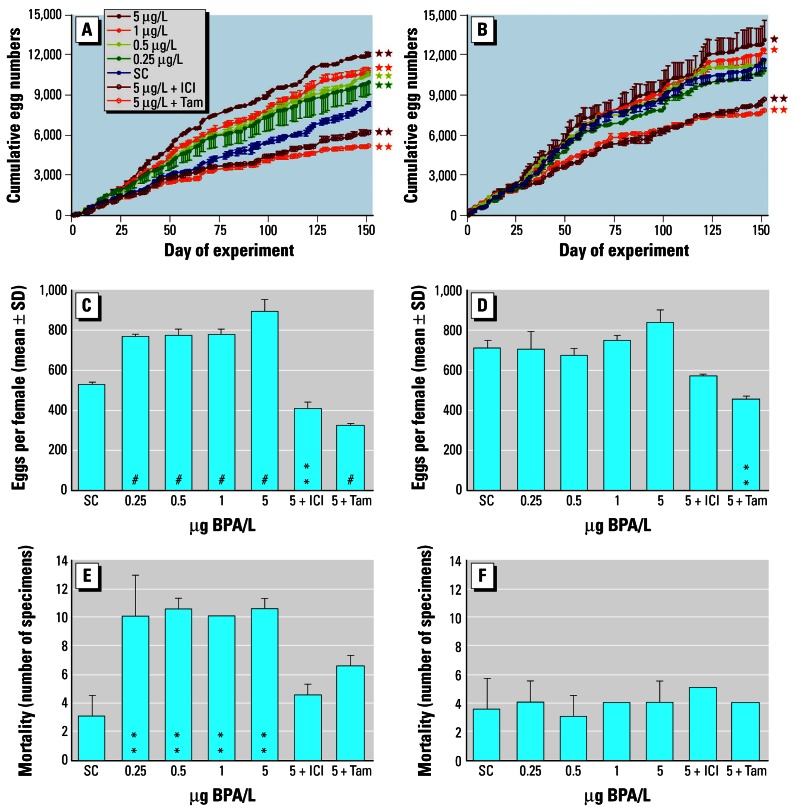
Ramshorn snails exposed to BPA produced significantly more clutches (data not shown) and eggs in the course of the 20°C experiment (Figure 2A; ANCOVA with Student-Newman-Keuls post-hoc test, p < 0.01) or, when expressed on a per-female basis, compared with the SC (Figure 2C; ANOVA with Student-Newman-Keuls post-hoc test, p < 0.001). Based on measured BPA concentrations, EC10 values were 14.8 and 18.0 ng/L for egg and clutch production, respectively (Table 3). These values are almost identical with the results for the pres-pawning phase of exposure series I at 22°C. The LOEC was 106 ng/L for both end points, and an NOEC could not be established because the lowest test concentration already exhibited significant effects. At the higher temperature of 27°C, none of the groups exposed solely to BPA produced significantly more clutches than control animals (data not shown). A significant increase in egg production was found only at 1 and 5 μg/L if cumulative egg numbers were analyzed over the course of the experiment (Figure 2B; ANCOVA with Student-Newman-Keuls post-hoc test; p < 0.05). None of the groups treated solely with BPA displayed a significant increase in egg production, expressed on a per-female basis (Figure 2D; ANOVA with Student-Newman-Keuls post-hoc test, p > 0.05). Based on measured BPA concentrations, EC10 values were 0.998 and 2.09 μg/L for egg and clutch production, respectively. These values are higher by a factor of 67 to 116 than those found at 20°C (Table 3). The NOEC was 205 ng/L and the LOEC 436 ng/L for egg production.
Figure 2.
Marisa cornuarietis exposure series II at 20°C (A,C,E) and 27°C (B,D,F ). (A,B) Cumulative egg production in groups exposed to BPA alone or in combination with either faslodex (ICI) or tamoxifen (Tam), normalized for number of females. Egg production per female (C,D) and mortality (E, F) during the entire experiment. Concentrations are provided on a nominal basis; biological parameters are given as mean values (n = 2) with SD.
Asterisks indicate significant differences from SC: *p < 0.05, **p < 0.01, #p < 0.001; ANCOVA with Student-Newman-Keuls as post-hoc tests in A and B; ANOVA with Student-Newman-Keuls as post-hoc tests in C and D; χ2 test in E and F).
These temperature-related differences of BPA effects in M. cornuarietis are a direct consequence of enhanced reproductive output at the higher temperature, with a significant increase from 529 ± 15.3 eggs per female at 20°C to 710 ± 39.1 at 27°C (t-test, p = 0.026). There were no significant differences in egg numbers per female for any of the groups exposed solely to BPA when comparing the output at both temperatures.
During the same experiment, additional groups of snails were simultaneously exposed to 5 μg BPA/L and a potent antiestrogen, either Faslodex (ICI) or tamoxifen (Tam), to investigate the specificity of the superfemale response. At both temperatures the stimulating effect of BPA on clutch (data not shown) and egg production was not only completely antagonized, but the reproductive output dropped even below control values in snails receiving both BPA and an antiestrogen (Figure 2A–D). These results indicate that BPA effects in M. cornuarietis can be blocked by compounds that act as antiestrogens in vertebrates.
Superfemales with oviduct malformations were exclusively observed at 20°C, with an incidence of 4.8, 8.0, 14.8, and 11.5 in the groups that received BPA at levels of 0.25, 0.5, 1, and 5 μg/L, respectively. No oviduct malformations were found in the SC, in the groups that received 5 μg BPA/L plus an antiestrogen (either ICI or Tam) at 20°C or in any of the groups at 27°C. Mortality increased significantly only in groups characterized by the occurrence of females with oviduct malformations (0.25, 0.5, and 1, 5 μg BPA/L at 20°C) when compared to the SC (Figure 2E). We found no increased mortality in any of the 27°C groups when compared to the SC (Figure 2F) and also no indication of significant differences in mortality between identical concentrations at the two temperatures, except for the groups exposed solely to BPA (χ2 test, p < 0.01).
BPA did not induce imposex development in M. cornuarietis at either temperature (ANOVA and Weir test, p > 0.5); however, in snails that received BPA and ICI simultaneously, imposex intensities increased when compared to the SC at both temperatures (Weir test, p < 0.05). The test compound did not affect the size of genital organs (penis pouch, penis sheath, and penis) in males at 20°C or 27°C, with the exception of penis length at 20°C. For this parameter we observed a concentration-dependent reduction in males exposed to BPA (data not shown; ANOVA with Student-Newman-Keuls post-hoc test, p < 0.01).
Competitive receptor displacement experiments
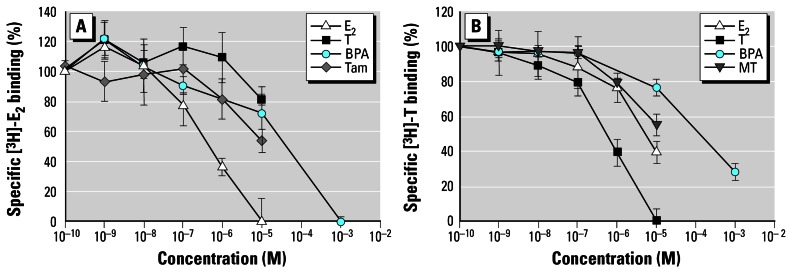
Specific binding for [3H]-E2 and [3H]-T was obtained in all cytosolic preparations. The specificities for [3H]-E2 and [3H]-T binding were almost identical in male and female gonads. Specific [3H]-E2 binding was displaced in the following ranking order by E2 >> Tam > BPA, while T possessed only weak displacement activity (Figure 3A). Calculated IC50 values are 753 nM, 1.47 μM, and 24.8 μM for E2, Tam, and BPA, respectively. Displacement of specific [3H]-T binding resulted in the order T > MT = E2 > BPA (Figure 3B). Calculated IC50 values are 849 nM, 1.15 μM, 1.58 μM, and 62.7 μM for T, MT, E2, and BPA, respectively.
Figure 3.
Marisa cornuarietis. Competitive displacement of specific [3H]-E2 (A) and [3H]-T binding (B). Mean curves (n = 3) with SE demonstrate displacment activities of T, E2, BPA, and Tam compared with [3H]-E2 and displacement activities of E2, T, BPA, and MT compared with [3H]-T.
Discussion
The present results confirm the findings of Oehlmann et al. (2000) and indicate that BPA affects M. cornuarietis at concentrations as low as 48.3 ng/L (LOEC in exposure series I). The concomitance of superfemales with oviduct malformations and increased mortality supports the earlier assumption of a higher female moribundity at BPA concentrations that are lower by a factor of 4,000 to 80,000 than reported LC50 values in invertebrates (Staples et al. 1998). It has been shown earlier that in female prosobranch snails exposed to tributyltin (TBT), comparable malformations with ruptures of the pallial oviduct do occur as a consequence of an oviduct blockade after the occlusion of the vaginal opening by proliferating vas deferens tissue. In such affected populations, an increased female mortality and a consequent shift of the sex ratio in favor of males was observed (Gibbs et al. 1987; Oehlmann et al. 1996). It can be assumed that not all M. cornuarietis superfemales with oviduct malformations were identified because it is likely that such females would not survive for long after the rupture occurred. However, although we found no indication of a statistically significant shift of sex ratios in favor of males in snails exposed solely to BPA, it must be considered that the sample size (with groups of 30 animals) was too small to be conclusive for this purpose.
Only a few studies to date have investigated hormone-mimetic effects of BPA in invertebrates. Hill et al. (2002) examined growth and development in freshwater sponges (Heteromyenia sp.) exposed to BPA, and they found significant reductions in growth rates over the concentration range 0.16–160 mg/L. Germination of Heteromyenia was completely inhibited at 80 mg/L BPA, and lower concentrations resulted in malformed water vascular systems in several replicates. Tominaga et al. (2003) performed a five-generation test with the nematode Caenorhabditis elegans. In the fourth generation, BPA reduced the fecundity rate at 1 and 10 nM, while the LC50 value was > 100 μM.
Some studies indicate that effects are dependent upon experimental conditions. For example, Zou and Fingerman (1997) found a reduced molting frequency in Daphnia magna exposed to BPA, while Caspers (1998) did not. Segner et al. (2003) and Watts et al. (2001) investigated BPA effects in a range of invertebrate species (Hydra vulgaris, Gammarus pulex, Chironomus riparius, Hyalella azteca, Lymnaea stagnalis). In long-term studies the impact of BPA on different developmental and reproductive parameters was assessed. The authors concluded that although a number of behavioral end points were affected at relatively high concentrations beyond environmental relevance, it would be unrealistic to attribute the effects to an endocrine-mediated process. Marcial et al. (2003) evaluated the impact of exposure of the copepod Tigriopus japonicus to environmentally relevant concentrations of BPA. BPA affected development in the parental and filial generation. Hahn et al. (2002) investigated the use of vitellogenesis as a marker for endocrine disruptors in the midge C. riparius. BPA exposure to concentrations between 1 and 3,000 μg/L reduced the yolk protein contents of freshly emerged midges by 20–25%.
A response with M. cornuarietis comparable to that observed in our study was reported by Andersen et al. (1999), with an increase of egg production in the copepod Acartia tonsa at 20 μg BPA/L. Superfemales are also reported for another prosobranch species by Duft et al. (2003). The authors investigated the effects of BPA exposure via sediments in the freshwater mudsnail Potamopyrgus antipodarum. The species-specific superfemale response in P. antipodarum is a time- and concentration-dependent increase of embryo numbers in the brood pouch, with an EC10 of 0.22 μg BPA/kg.
Although EDCs have great potential for interfering with reproductive health in wildlife and humans, there is little direct evidence that endocrine disruption has adversely affected fertility in any organism. Population declines, as a direct result of endocrine disruption, have been reported only for mollusks exposed to TBT (Gibbs et al. 1987; Oehlmann et al. 1996). Prosobranch snails are generally regarded as one of the invertebrate groups most sensitive to EDCs (DeFur et al. 1999). Their imposex response remains the best characterized example of endocrine disruption in wildlife, with adverse population-level effects in the low nanogram per liter range for a single chemical, TBT (Matthiessen and Gibbs 1998). More recently, Jobling et al. (2002) investigated the reproductive success in wild intersex roach (Rutilus rutilus) and demonstrated that gamete production, sperm motility, and the ability of sperm to fertilize eggs and successfully produce viable offspring were all reduced in intersex fish. The similarity of population-level effects in mollusks exposed to TBT and BPA, specifically oviduct ruptures and a consequent death of females, may indicate that comparable ecological consequences arise from both compounds. This possibility is also supported by a comparison of EC10 values for superfemale induction in M. cornuarietis via water (13.9 ng/L, this study) and in P. antipodarum via sediments (0.22 μg/kg, Duft et al. 2003). Reported concentrations of BPA in the environment (e.g., in the River Elbe, up to 776 ng/L in water and 343 μg/kg in sediments; Heemken et al. 2001) provide additional supporting evidence.
The present study demonstrates for the first time the existence of specific binding sites for [3H]-E2 and [3H]-T that probably represent functioning sexual steroid receptors in M. cornuarietis. The competitive displacement experiments indicate that the binding sites resemble two different types of receptors, specific for estrogens and androgens, respectively. The ER-like binding site has highest affinity for E2 (IC50, 753 nM) but only very weak affinity for T. In addition, BPA exhibits a clear binding (IC50, 24.8 μM). BPA/E2 affinity ratios, based on IC50 values, are 33 in Marisa, 831 in carp (Kloas et al. 2000), 719 in X. laevis (Lutz and Kloas 1999), and 12,400 for the human ERα (Matthews et al. 2001), thereby indicating considerable differences between the ramshorn snails and vertebrates. The anti-estrogen Tam also displays a moderate affinity to the ER-like binding sites in a range similar to that for BPA.
The AR-like binding site is characterized by the highest affinity for T (IC50, 849 nM), followed by displacement effects about one order of magnitude lower for the androgen MT as well as for E2. BPA, which is described as an antiandrogen in vertebrates (Lee et al. 2003), also displays a noticeable affinity (IC50, 62.7 μM) for T. The difference in the affinity ratio BPA/T between Marisa (74, this study) and humans (4,720; Fang et al. 2003) is slightly less than that for the ER. The different specificities of displacement effects for [3H]-E2 and [3H]-T by unlabeled ligands indicate the existence of two types of binding sites in M. cornuarietis, and it is likely that these sites resemble functioning estrogen and androgen receptors.
Conclusion
The results of the current study confirm previous findings that BPA induces a specific super-feminization syndrome in the ramshorn snail M. cornuarietis. The occurrence of superfemales is associated with adverse effects on reproduction and survival, even at sub-micrograms per liter concentrations of BPA (NOEC, 7.9 ng/L; EC10, 13.9 ng/L). However, the induction of superfemales in M. cornuarietis is at least partially masked if snails are exposed to BPA under conditions that maximize the reproductive output, particularly during the spawning season or at elevated temperatures. Super-female induction is mediated by a functional ER because the response can completely be antagonized by co-exposure to potent anti-estrogens. Furthermore, the high BPA sensitivity of M. cornuarietis and other prosobranch snails seems to be based on the higher affinity of the compound for the ER in this species compared with vertebrates. Overall, the results indicate that BPA poses a potential risk for prosobranch snail populations in the field at environmentally relevant concentrations.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
We are grateful to M. Duft, G. Elter, C. Stark, M. Tillmann, L. Weltje, and S. Ziebart for assistance, and also to two anonymous reviewers for their valuable comments.
Financial support was provided by the European Union (COMPRENDO project, contract EVK1-CT-2002-00129) and the Federal Environmental Agency Berlin (project code 297 65001/04).
References
- Andersen HR, Halling-Sørensen B, Kusk KO. A parameter for detecting estrogenic exposure in the copepod Acartia tonsa. Ecotoxicol Environ Saf. 1999;44:56–61. doi: 10.1006/eesa.1999.1800. [DOI] [PubMed] [Google Scholar]
- Bolger R, Wiese TE, Irvin K, Nestich S, Checovich W. Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect. 1998;106:551–557. doi: 10.1289/ehp.98106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz U, Hagenmaier H, Körner W. Phenolic xenoestrogens in surface water, sediments, and sewage sludge from Baden-Württemberg, south-west Germany. Environ Pollut. 2001;115:291–301. doi: 10.1016/s0269-7491(01)00100-2. [DOI] [PubMed] [Google Scholar]
- Caspers N. No estrogenic effects of bisphenol A in Daphnia magna Straus. Bull Environ Contam Toxicol. 1998;61:143–148. doi: 10.1007/s001289900741. [DOI] [PubMed] [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFur PL, Crane M, Ingersoll C, Tattersfield L, eds. 1999. Endocrine Disruption in Invertebrates: Endocrinology, Testing, and Assessment. Proceedings of the Workshops on Endocrine Disruption in Invertebrates, 12–15 December 1998, Noordwijkerhout, the Netherlands. Pensacola, FL:SETAC Press. Available: https://www.setac.net/setacssa/ecssashop.show_product_detail?p_product_serno=17&p_mode=detail&p_cust_id=&p_order_serno=&p_promo_cd=&p_price_cd= [accessed 3 April 2006].
- Dodds EC, Lawson W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. [Google Scholar]
- Dodds EC, Lawson W. Molecular structure in relation to oestrogenic activity. Compounds without a phenanthrene nucleus. Proc Royal Soc London B. 1938;125:222–232. [Google Scholar]
- Dorn PB, Chou CS, Gentempo JJ. Degradation of bisphenol A in natural waters. Chemosphere. 1987;16:1501–1507. [Google Scholar]
- Duft M, Schulte-Oehlmann U, Weltje L, Tillmann M, Oehlmann J. Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mud-snail Potamopyrgus antipodarum. Aquat Toxicol. 2003;64:437–449. doi: 10.1016/s0166-445x(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Fang H, Tong WD, Branham WS, Moland CL, Dial SL, Hong HX, et al. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol. 2003;16:1338–1358. doi: 10.1021/tx030011g. [DOI] [PubMed] [Google Scholar]
- Gibbs PE, Bryan GW, Pascoe PL, Burt GR. The use of the dog-whelk, Nucella lapillus, as an indicator of tributyltin (TBT) contamination. J Mar Biol Assoc UK. 1987;67:507–523. [Google Scholar]
- Hahn T, Schenk K, Schulz R. Environmental chemicals with known endocrine potential affect yolk protein content in the aquatic insect Chironomus riparius. Environ Pollut. 2002;120:525–528. doi: 10.1016/s0269-7491(02)00189-6. [DOI] [PubMed] [Google Scholar]
- Heemken OP, Reincke H, Stachel B, Theobald N. The occurrence of xenoestrogens in the Elbe river and the North Sea. Chemosphere. 2001;45:245–259. doi: 10.1016/s0045-6535(00)00570-1. [DOI] [PubMed] [Google Scholar]
- Hill M, Stabile C, Steffen LK, Hill A. Toxic effects of endocrine disrupters on freshwater sponges: common developmental abnormalities. Environ Pollut. 2002;117:295–300. doi: 10.1016/s0269-7491(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Hirano M, Ishibashi H, Matsumura N, Nagao Y, Watanabe N, Watanabe A, et al. Acute toxicity responses of two crustaceans, Americamysis bahia and Daphnia magna, to endocrine disrupters. J Health Sci. 2004;50:97–100. [Google Scholar]
- Howard PH, ed. 1989. Handbook of Environmental Fate and Exposure Data for Organic Chemicals. Vol I: Large Production and Priority Pollutants. Boca Raton, FL:Lewis Publishers.
- Ike M, Chen MY, Jin CS, Fujita M. Acute toxicity, mutagenicity, and estrogenicity of biodegradation products of bisphenol A. Environ Toxicol. 2002;17:457–461. doi: 10.1002/tox.10079. [DOI] [PubMed] [Google Scholar]
- Jobling S, Coey S, Whitmore JG, Kime DE, Van Look KJW, McAllister BG, et al. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol Reprod. 2002;67:515–524. doi: 10.1095/biolreprod67.2.515. [DOI] [PubMed] [Google Scholar]
- Kloas W, Lutz I, Einspanier R. Amphibians as a model to study endocrine disruptors: II. Estrogenic activity of environmental chemicals in vitro and in vivo. Sci Total Environ. 1999;225:59–68. doi: 10.1016/s0048-9697(99)80017-5. [DOI] [PubMed] [Google Scholar]
- Kloas W, Schrag B, Ehnes C, Segner H. Binding of xenobiotics to hepatic estrogen receptor and plasma sex steroid binding protein in the teleost fish, the carp (Cyprinus carpio) Gen Comp Endocrinol. 2000;119:287–299. doi: 10.1006/gcen.2000.7521. [DOI] [PubMed] [Google Scholar]
- Kwak HI, Bae MO, Lee MH, Lee YS, Lee BJ, Kang KS, et al. Effects of nonylphenol, bisphenol A, and their mixture on the viviparous swordtail fish (Xiphophorus helleri) Environ Toxicol Chem. 2001;20:787–795. [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Levy G, Lutz I, Krüger A, Kloas W. Bisphenol A induces feminization in Xenopus laevis tadpoles. Environ Res. 2004;94:102–111. doi: 10.1016/s0013-9351(03)00086-0. [DOI] [PubMed] [Google Scholar]
- Lutz I, Kloas W. Amphibians as a model to study endocrine disruptors: I. Environmental pollution and estrogen receptor binding. Sci Total Environ. 1999;225:49–57. doi: 10.1016/s0048-9697(99)80016-3. [DOI] [PubMed] [Google Scholar]
- Marcial HS, Hagiwara A, Snell TW. Estrogenic compounds affect development of harpacticoid copepod Tigriopus japonicus. Environ Toxicol Chem. 2003;22:3025–3030. doi: 10.1897/02-622. [DOI] [PubMed] [Google Scholar]
- Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem Res Toxicol. 2001;14:149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- Matthiessen P, Gibbs PE. Critical appraisal of the evidence for tributyltin–mediated endocrine disruption in mollusks. Environ Toxicol Chem. 1998;17:37–43. [Google Scholar]
- Metcalfe CD, Metcalfe TL, Kiparissis Y, Koenig BG, Khan C, Hughes RJ, et al. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes) Environ Toxicol Chem. 2001;20:297–308. [PubMed] [Google Scholar]
- Oehlmann J, Fioroni P, Stroben E, Markert B. Tributyltin (TBT) effects on Ocinebrina aciculata (Gastropoda: Muricidae): imposex development, sterilization, sex change and population decline. Sci Total Environ. 1996;188:205–223. [Google Scholar]
- Oehlmann J, Schulte-Oehlmann U, Tillmann M, Markert B. Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part I: Bisphenol A and octylphenol as xenoestrogens. Ecotoxicology. 2000;9:383–397. doi: 10.1023/a:1008972518019. [DOI] [PubMed] [Google Scholar]
- Pickford DB, Hetheridge MJ, Caunter JE, Hall AT, Hutchinson TH. Assessing chronic toxicity of bisphenol A to larvae of the African clawed frog (Xenopus laevis) in a flow-through exposure system. Chemosphere. 2003;53:223–235. doi: 10.1016/s0045-6535(03)00308-4. [DOI] [PubMed] [Google Scholar]
- Segner H, Caroll K, Fenske M, Janssen CR, Maack G, Pascoe D, et al. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project. Ecotoxicol Environ Safe. 2003;54:302–314. doi: 10.1016/s0147-6513(02)00039-8. [DOI] [PubMed] [Google Scholar]
- Shelby MD, Newbold RR, Tully DB, Chae K, Davis VL. Assessing environmental chemicals for estrogenicity using a combination of in vitro and in vivo assays. Environ Health Perspect. 1996;104:1296–1300. doi: 10.1289/ehp.961041296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoni P, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, et al. Reproductive effects of long-term exposure to bisphenol A in the fathead minnow (Pimephales promelas) Environ Sci Technol. 2001;35:2917–2925. doi: 10.1021/es000198n. [DOI] [PubMed] [Google Scholar]
- Stachel B, Ehrhorn U, Heemken OP, Lepom P, Reincke H, Sawal G, et al. Xenoestrogens in the River Elbe and its tributaries. Environ Pollut. 2003;124:497–507. doi: 10.1016/s0269-7491(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Staples CA, Weeks J, Hall JF, Naylor CG. Evaluation of aquatic toxicity and bioaccumulation of C8- and C9-alkylphenol ethoxylates. Environ Toxicol Chem. 1998;17:2470–2480. [Google Scholar]
- Staples CA, Woodburn K, Caspers N, Hall AT, Klec ka GM. A weight of evidence approach to the aquatic hazard assessment of bisphenol A. Hum Ecol Risk Assess. 2002;8:1083–1105. [Google Scholar]
- Tominaga N, Kohra S, Iguchi T, Arizono K. A multi-generation sublethal assay of phenols using the nematode Caenorhabditis elegans. J Health Sci. 2003;49:459–463. [Google Scholar]
- Watts MM, Pascoe D, Carroll K. Survival and precopulatory behaviour of Gammarus pulex (L.) exposed to two xenoestrogens. Water Res. 2001;35:2347–2352. doi: 10.1016/s0043-1354(00)00537-6. [DOI] [PubMed] [Google Scholar]
- Zou E, Fingerman M. Synthetic estrogenic agents do not interfere with sex differentiation but do inhibit molting of the Cladoceran Daphnia magna. Bull Environ Contam Toxicol. 1997;58:596–602. doi: 10.1007/s001289900376. [DOI] [PubMed] [Google Scholar]