Abstract
We have previously shown that exposure to exogenous androgens causes female sticklebacks (Gasterosteus aculeatus) to produce the glue protein, spiggin, in their kidneys. This protein can be quantified by an enzyme-linked immunosorbent assay developed and validated at the Centre for Environment, Fisheries and Aquaculture Science. Here we report the development of an in vivo test for the detection of environmental antiandrogens. The system involves the simultaneous exposure of female sticklebacks to 17α-methyltestosterone (a model androgen) at 500 ng/L and suspected environmental antiandrogens over a period of 21 days. The spiggin content of the kidneys is then measured, and any antiandrogenic activity is evaluated by comparing the spiggin levels of female fish exposed to antiandrogens to those of female fish exposed solely to the model androgen. The assay detects the antiandrogenic activity of flutamide, vinclozolin (both used at 250 μg/L), linuron (at 150 μg/L), and fenitrothion (at 15 and 150 μg/L). These results provide the first evidence of in vivo antiandrogenic activity of both linuron and fenitrothion in teleosts. Although there are other suggested fish species that could be used for this purpose, the stickleback is the only widely available species in which it is now possible to study both estrogenic and antiandrogenic end points in the same individual. Furthermore, the species is endemic and ubiquitous in Europe, and it possesses many ecological traits that make it better suited than other potential species for field research into endocrine disruption.
Keywords: antiandrogens, endocrine disruption, fenitrothion, flutamide, linuron, spiggin, stickleback, vinclozolin
Several field and laboratory studies have shown induction of adverse effects in wildlife species and populations upon exposure to endocrine-disrupting chemicals (EDCs). These effects vary from subtle changes in the physiology and sexual behavior of species to permanently altered sexual differentiation (Vos et al. 2000). The vast majority of endocrine disruption research in fish has focused on estrogenic xenobiotics. This work has used the egg yolk protein precursor, vitellogenin (VTG), as an unambiguous biomarker for estrogens in a variety of model species (Allen et al. 1999; Denslow et al. 1999; Folmar et al. 1996; Heppell et al. 1995; Purdom et al. 1994; Sumpter and Jobling 1995).
The role of environmental androgens and antiandrogens has until recently been overlooked, most likely because of the lack of a sensitive in vivo system for the detection of such activity.
Nevertheless, the only androgenic industrial effluent identified to date is pulp mill effluent. Several reports have focused on the masculinizing effect of pulp mill effluent on female mosquitofish (Cody and Bortone 1997; Howell and Denton 1989; Parks et al. 2001), male-biased eelpout embryos (Larsson et al. 2000), and induction of spiggin in female sticklebacks (Katsiadaki et al. 2002b). Although domestic effluent is suspected to have a high content of natural androgens, an advanced level of treatment, such as percolating filter beds and activated sludge systems, seems to be very efficient in removing the responsible agents (Thomas et al. 2002).
More recently, concerns were expressed that trenbolone acetate, a growth promoter used in livestock, is a strong androgen agonist (Ankley et al. 2003; Wilson et al. 2002).
Although androgens do not appear to be as widespread as estrogens in the environment, a nationwide survey of endocrine activity of final sewage effluents across the United Kingdom, using the yeast androgen screen assay, has revealed significant antiandrogenic activity (Environment Agency, in press).
Antiandrogens in general exert their effects by occupying the androgen receptor (AR) without activating it. Activation of the AR is induced by ligand binding through conformational changes that lead to specific gene expression. Antiandrogens compete with androgens for AR occupancy and subsequently block receptor action. Two classes of antiandrogens are currently recognized: steroidal derivatives, which possess mixed agonistic and antagonistic androgenic activity, and nonsteroidal derivatives or “pure” antiandrogens, exemplified by flutamide, (2-methyl-N-[4-nitro-3-(tri-flouromethyl) phenyl] propanamide) . Flutamide (FL) and its derivatives are the main representatives of the latter category, and they have been studied extensively because of their proven clinical efficacy in the treatment of prostate cancer (Singh et al. 2000). They are potent AR antagonists that compete with androgens for binding to the AR and prevent AR DNA binding and transcription of androgen-dependent genes.
Several environmental chemicals have been shown to compete with androgens at a receptor level. Kelce et al. (1994) and Gray et al. (1994) identified the first environmental antiandrogens that share the same mechanism of endocrine disruption with FL, as M1 and M2 metabolites of the fungicide vinclozolin. Vinclozolin (VZ) itself has poor affinity for the mammalian AR (Kelce et al. 1994). However, in vivo, VZ is hydrolyzed to two open-ringed metabolites, M1 (2-[[(3,5-dichlorophenyl)-carbamoyl]oxy]-2-methyl-3-butenoic acid) and M2 (3′, 5′-dichloro-2-hydroxy-2-methylbut-3-enanilide), which act as AR antagonists by preventing transcription of androgen-dependent genes (Wong et al. 1995).
The same mechanism of antiandrogenic action was identified in p,p′-DDE (dichloro-diphenyldichloroethylene), a persistent metabolite of the pesticide DDT (dichloro-diphenyltrichloroethane) that, as in the case of VZ, prevents gene transcription in mammals by binding to the AR (Kelce et al. 1995).
The fungicide procymidon alters sexual differentiation in the male rat by also acting as an AR antagonist both in vitro and in vivo (Ostby et al. 1999; Vinggaard et al. 1999).
Linuron (LN), an herbicide applied to suppress broadleaf and grassy weeds, is a weak competitive AR antagonist in vitro, induces a positive response in the immature and adult rat Hershberger assay, and suppresses androgen-dependent gene expression (Cook et al. 1993; Lambright et al. 2000; McIntyre et al. 2000).
Tamura et al. (2001) were first to demonstrate both in vitro and in vivo (rats, Hershberger test) the antiandrogenicity of fenitrothion (O,O-dimethyl-O-4-nitro-m-tolyl phosphorothioate), a widely used organophosphate pesticide. However, Sohoni et al. (2001) observed no conclusive antiandrogenic effects of fenitrothion (FN) in either intact or castrated male rats. In addition, Turner et al. (2002) failed to demonstrate significant alteration of androgen-dependent sexual differentiation in male rats exposed in utero, and they concluded that FN is only weakly antiandrogenic. Nevertheless, several other reports confirmed an androgen antagonism in vitro using a number of different systems (Freyberger and Ahr, 2004; Sohoni et al. 2001).
Antiandrogens may disrupt male sexual differentiation and performance by interfering with androgen signaling at levels other than the AR, such as androgen production, transport, and metabolism.
Inhibitors of the enzyme 5α-reductase (responsible for the conversion of testosterone in the more potent dihydrotestosterone in mammals), such as finasteride, provide another example of an antiandrogen that produces effects on androgen-regulated sexual differentiation by a mechanism that does not involve AR but, rather, causes interference with androgen production.
Administration of di-(n-butyl) phthalate during late gestation in rats also disrupts androgen-regulated male reproductive development (Mylchreest et al. 1999). Di-(n-butyl) phthalate and its metabolites do not show any affinity for the AR in vitro; thus, the mechanism of action is not by AR interaction.
Interestingly, fetal exposure to high doses of estrogens can produce cryptorchidism and hypospadias (Newbold 1995). In vitro studies have also confirmed the affinity of natural estrogens (Sohoni and Sumpter 1998) or environmental estrogens (Lee et al. 2003) for the AR. However, estrogens can also have antiandrogenic effects by acting on feedback mechanisms (inhibition of luteinizing hormone and follicle-stimulating hormone) and by preventing secretion of testosterone from the testes. Other researchers support the view that neonatal treatment of rats with xenoestrogens induces reproductive abnormalities by disturbing the androgen/estrogen balance (Rivas et al. 2002).
Regardless of the mechanism through which a xenobiotic interferes with the endocrine system, its significance depends on the timing of exposure. Indeed, when adult individuals are exposed to EDCs, the effects are reversible once the exposure ceases. In contrast, the consequences of exposure during organogenesis may result in irreversible deleterious developmental effects such as those observed after in ovo exposure to the pesticide DDT in birds (Fry 1995) and those observed after in utero exposure to diethylstilbestrol in rodents and humans (Herbst and Anderson 1990; Newbold et al. 1990).
The importance of the issue is highlighted by a large number of studies undertaken to link human adverse effects and exposure to EDCs in the last decade. Evidence for the effect of EDCs in human health includes the increased incidence of idiopathic hypospadias, alterations of the male genitalia, pseudo-hermaphroditism, decline in sperm counts, and the increasing incidence of breast cancer among females and testicular and prostate cancers among males (Kelce and Wilson 1997; Topari et al. 1996).
The only robust assay for antiandrogens thus far has been that described by Hershberger et al. (1953). The basis of the assay is that castrated sexually mature male rats undergo regression of androgen-sensitive tissues (testes, prostate, epididymis, seminal vesicle, and levator ani muscles). These tissues are restored to their original weight upon treatment with testosterone, and that growth can be blocked by the concomitant administration of an antiandrogen.
In view of the numerous clinical implications in human health and that the only reliable antiandrogen bioassay requires castrated rats, a simple in vivo test using intact fish to screen and identify environmental antiandrogens is very important and desirable. The purpose of this study was to test the possibility of adapting the stickleback androgen bioassay, based on spiggin production (Katsiadaki et al. 2002a), for the detection of environmental antiandrogens and to perform a preliminary screen for the antiandrogenic activity of selected environmental contaminants.
Materials and Methods
Chemicals, consumables, and equipment
Dihydrotestosterone (DHT), 17α-methyl-testosterone (17α-MT), FL, and ethinyl-estradiol (EE2) were obtained from Sigma-Aldrich Co. Ltd. (Dorset, UK). VZ, LN, and FN were purchased from QMX Laboratories Ltd (Thaxted, Essex, UK).
Fish
Wild three-spined sticklebacks were obtained with hand nets from U.K. rivers in the Kent and Reading areas when they were only a few weeks old, and they were kept in the laboratory for 6 months before the experiments.
Stock population
The fish were kept in brackish water at 12°C with a photoperiod of 8:16 hr light:dark, a regimen in which the fish will remain reproductively quiescent. Only fish that had been kept under these conditions, that weighed > 0.8 μg, and had no external signs of parasitism were used for the experiments.
Experimental populations
Fish (groups of 25 to 30) were transferred to 40-L glass aquaria containing fresh water. The photoperiod was 12:12 hr light:dark, and the temperature was 15°C. Both photoperiod and temperature for the experimental fish were chosen on the basis that these conditions were neither stimulatory nor inhibitory for the reproductive system. Tanks were aerated constantly. Daily readings from each tank were taken for temperature, dissolved oxygen, pH, and conductivity. The fish were fed every other day throughout the exposure period with a combination of frozen shrimp, artemia, and bloodworm.
Laboratory exposure to androgens/anti-androgens
The test system we used to assess antiandrogenic activity used female sticklebacks that were simultaneously treated with a model androgen. However, as stickleback sexing outside the breeding season is a difficult task, some males were present in the tanks.
Semistatic exposure
For this experiment, we used the synthetic model androgen 17α-MT at 5 and 0.5 μg/L to induce the glue protein spiggin. We chose the semistatic system for administration of the test compounds because of the large volumes of pesticides needed for a flow-through exposure. For this process, two-thirds of the aquarium water was removed every 48 hr and replaced with fresh water plus a fresh dose of compound. Previous exposures to 17α-MT have indicated that the androgen is stable in water over a period of 48 hr and appropriate to use for semistatic exposures during screening of xenobiotics (Katsiadaki et al. 2002a). The environmental antiandrogens were simultaneously administered via the water, using the same semistatic system. The highest concentrations tested were either 250 or 150 μg/L and were based on toxicity data in fish. FL, VZ, LN, and FN were screened for potential antiandrogenic activity.
Both suspected antiandrogens and 17α-MT were dissolved in methanol and applied via the water as described above. Appropriate volumes of vehicle solvents were added to the controls. All treatments were duplicated. The fish were exposed to the test compounds for 3 weeks. Water samples were taken each week before and immediately after the addition of fresh solutions for the analytical verification of the tested chemicals.
Flow-through
We used a continuous flow-through system for exposure to DHT (a nonaromatizable model androgen) at 5 μg/L and various concentrations of FL (to construct a dose–response curve). The flow rate was 100 mL/min. Previous unpublished data have indicated that DHT has a low stability in water, so it was not appropriate to use in semi-static exposure. Verification of the test compound concentration took place twice a week over the 3-week period.
Spiggin measurements
At the end of the exposure period, all fish were sacrificed by destruction of the brain and then snap frozen in liquid nitrogen. The fish were weighed to the nearest milligram, and their kidneys were dissected out, placed in individual vials, and labeled. The addition of 200 μL of a strong denaturing buffer [100 mM Tris–HCl, 10 mM EDTA, 8 M urea, 2% SDS (wt/vol), and 200 mM β-mercaptoethanol; pH 8.5] and heating of the kidneys at 70°C for 30 min followed. The enzyme-linked immunosorbent assay (ELISA) procedure for spiggin has been described in detail elsewhere (Katsiadaki et al. 2002a).
Histological analysis
In one experiment, the kidney was divided into two equal parts, one of which was used for the ELISA and the other for histological examination. We have previously described in detail the standard histological protocols used for light microscopy (Katsiadaki et al. 2002a).
Analytical verification of test concentrations
We used solvents that were all HPLC grade. Before extraction, a known amount of diuron was added to each tank water sample as an internal (surrogate or recovery) standard. Analytes were extracted from the samples using conditioned C 18 solid phase extraction (SPE) cartridges (Waters Ltd., Watford, UK). Water samples (1-L) were pumped through a prefilter (0.45-μm pore size; Pall Life Sciences, Portsmouth, Hampshire, UK) before passing through the SPE cartridge. After elution with methanol and hexane, the final extracts were reduced to near-dryness and prepared in methanol.
The separation and detection of LN, FL (including 2-hydroxyflutamide), and VZ (including metabolites M1 and M2) were performed by a HP1050 liquid chromatograph (Agilent Technologies Ltd., Wokingham, Berkshire, UK) coupled to a Platform II mass spectrometer (Micromass, Altrincham, Cheshire, UK). Analysis was conducted in the negative ionization mode, using an electro-spray interface. For FN, an atmospheric pressure chemical ionization interface was used, and detection was performed in the positive mode. An acetonitrile:water gradient was applied, and separation was achieved by using either phenyl-hexyl or C8 minibore analytical columns (Phenomenex, Torrance, CA, USA). Selective ion monitoring (SIM) was then performed in time-scheduled events.
Quantification of LN, FL, and VZ was achieved internally and relative to diuron; hydroxyflutamide was determined relative to the response of FL. Concentration data for FN were derived from external calibration curves.
A competitive ELISA and a radioimmunoassay were used to measure 17α-MT and DHT in water samples, respectively. We used commercially available antibodies as described previously (Katsiadaki et al. 2002a).
Statistical analysis
Differences between groups were analyzed by analysis of variance with a post-hoc Duncan’s test. Spiggin unit data were logarithmically transformed before analysis. Male and female fish were analyzed separately because of the large differences in spiggin contents between the sexes, particularly in control groups.
Results
In vivo tests.
During the early experiments, the amounts of FL added to the aquaria were 500 μg/L of ambient water (Figures 1, 2). Both the histological analysis of kidney epithelium height (Figure 3) and the immunoassay developed for spiggin demonstrated that FL is highly effective at inhibiting or reducing (depending on the androgen dose used) spiggin induction in androgen-treated sticklebacks or in photoperiodically stimulated male sticklebacks.
Figure 1.
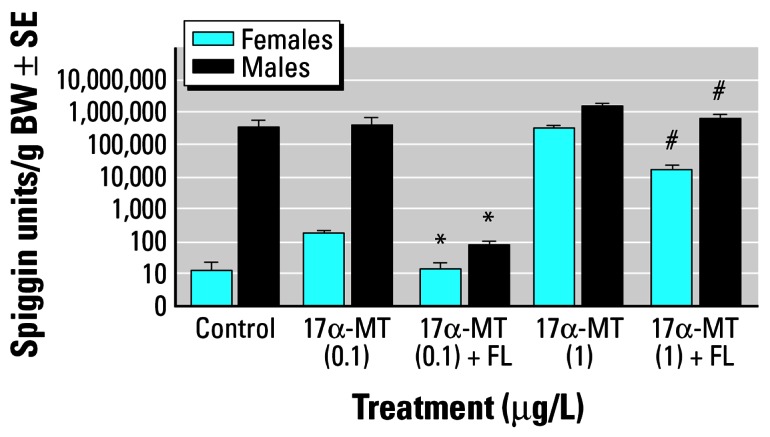
Amounts of spiggin per gram of body weight (BW; ± SE) in female and male sticklebacks exposed for 3 weeks to 17α-MT with or without flutamide.
*Significant (p < 0.05) inhibition of spiggin induction by flutamide. #Significant (p < 0.05) reduction in spiggin production by flutamide.
Figure 2.

Amounts of spiggin per gram of body weight (± SE) in male sticklebacks (controls and photoperiodically stimulated with or without flutamide).
*Significant (p < 0.05) inhibition of natural spiggin induction by flutamide in comparison to light-stimulated male fish.
Figure 3.
Kidney sections of female sticklebacks treated with 17α-MT (10 μg/L) alone (A), and with 17α-MT (10 μg/L) plus flutamide at 500 μg/L (B). Sections were stained with periodic acid–Schiff. Both microphotographs were taken under the same magnification. Scale bar = 50 μm. (A) Kidney epithelium height, 28.5 μm; (B) kidney epithelium height, 20.01 μm.
These early results suggested that the antiandrogenic effect of FL at a given dose depends on the ligand’s concentration, its binding affinity for the AR in the stickleback kidney, and on the presence of competing natural ligands (in the case of male fish). The antagonizing effect is not observed when large doses of androgens are used (i.e., 17α-MT at 500 μg/L, results not shown), is moderate at 1 μg/L 17α-MT, and is complete at 100 ng/L 17α-MT. The inhibitory effect of FL with the simultaneous administration of 17α-MT at 100 ng/L is evident in male fish only because 17α-MT at this concentration did not show much induction of spiggin in females (Figure 1).
Remarkably, inhibition of natural spiggin production in photoperiodically stimulated male sticklebacks was complete (Figure 2). It should be noted that in males, the magnitude of response to a temperature/photoperiod stimulus is dependent on the time of the year that the experiment is taking place. Generally, the response increases from January to April, where a maximum response is observed, remains high until August, and then decreases during November and December. The spiggin levels of control fish in November are statistically lower than those of the light-stimulated fish, whereas this difference was not observed in the control fish during February and April (Figure 2). This observation is in line with previous studies that have shown that extraretinal photoreception and strong circadian rhythms are operating in the male sticklebacks (Borg 1982; Bornestaf and Borg 2000). Social hierarchies also influence the magnitude of secondary sexual characters such as kidney hypertrophy, and therefore, a sound knowledge of stickleback reproductive biology is needed for the design of successful experiments using male fish. Although it is possible that the time of the year and the reproductive status of the female fish might influence the magnitude of response to androgen treatment, more data are needed to establish this effect.
The inhibitory effect of FL on the androgen-induced kidney hypertrophy in the stickleback was confirmed histologically (Figure 3).
We followed this interesting finding by selecting two environmental antiandrogens, namely, VZ at 250 and 25 μg/L and LN at 150 and 15 μg/L for screening. In addition, we tested FL at 250 and 25 μg/L as a positive control. Because of the large volumes of environmental antiandrogens used (i.e., 150–250 μg/L), it was decided that at least the preliminary screening test should be run in a semi-static manner. We have previously reported inhibition of DHT-induced (at 5 μg/L) kidney hypertrophy in female fish by FL at 500 μg/L (Katsiadaki et al. 2002b). We therefore chose the same concentration for 17α-MT for the screening of suspected environmental anti-androgens. The results are presented in Figure 4.
Figure 4.

Amounts of spiggin per gram of body weight (± SE) in female sticklebacks (control and 17α-MT–treated with simultaneous exposure to flutamide (FL), vinclozolin (VZ), and linuron (LN).
No inhibition of spiggin production was apparent even by FL at 250 μg/L when female fish were exposed to 5 μg/L 17α-MT (Figure 4). Although we failed to demonstrate antagonism by the test compounds, our results provided further evidence that 17α-MT strongly induces spiggin, as reported earlier (Katsiadaki et al. 2002a). However, in the followup experiment where 17α-MT was used at 0.5 μg/L, the conclusions are different. This study included the organophosphate pesticide FN at concentrations of 150 and 15 μg/L (Figure 5).
Figure 5.

Amounts of spiggin per gram of body weight (± SE) in female sticklebacks (control and 17α-MT–treated with simultaneous exposure to flutamide (FL), vinclozolin (VZ), fenitrothion (FN), and linuron (LN).
Treatments shown with the same letter do not differ significantly from each other (p < 0.05).
All tested antiandrogens inhibited or significantly reduced spiggin production by androgen-treated female fish. In particular, the inhibitory effects of FN are very impressive, implying an even higher antiandrogenic potency than the model FL.
When concerns were expressed that 17α-MT, which is aromatizable to an estrogen, was not an ideal androgen model, we decided to replace it with DHT, which cannot be converted to an estrogen. We therefore designed another semistatic system of exposure using DHT as a model androgen at 5 μg/L. We tested LN at 150 μg/L, procymidon at 150 μg/L, VZ at 250 μg/L, and p,p′-DDE at 250 μg/L. These results are not presented because only 20% of the female fish in the androgen control group were spiggin positive, thereby precluding any assessment on inhibition. Chemical analysis of DHT in the aquaria water confirmed the lack of DHT at the nominal concentration, thus suggesting that this model androgen is very unstable in water.
To confirm this observation we designed another flow-through test using DHT alone at 5 μg/L (positive control), DHT along with FL at 500 μg/L, and EE2 at 20 ng/L (negative control).
We were able to reproduce our previous data using the flow-through system (Figure 6). FL antagonized DHT more successfully than 17α-MT because the inhibition was complete at 5 μg/L DHT, thereby imposing a higher androgenic potency of 17α-MT over DHT, which was also indicated through the levels of spiggin units induced by the androgens alone. In addition, it appears that EE2 might have an antiandrogenic effect itself because there were no males in breeding condition in this group (Figure 6).
Figure 6.

Amounts of spiggin per gram of body weight (± SE) in male and female sticklebacks treated with DHT alone or in combination with flutamide. EE2 was used as a negative control.
*Significant (p < 0.05) inhibition of DHT-induced spiggin induction by flutamide.
The next step involved in adapting the spiggin bioassay for antiandrogens was to obtain a dose–response curve for FL-induced inhibition of spiggin production. Indeed, all flow-through experiments up to this point used FL 500 μg/L at a single concentration only. The results of this study indicated that FL successfully antagonized DHT at much lower concentrations than 500 μg/L (Figure 7).
Figure 7.

Inhibition of DHT-induced spiggin production by flutamide in a dose–response manner. All groups except controls received 5 μg/L of DHT.
Treatments shown with the same letter do not differ significantly from each other (p < 0.05).
The very smooth standard curve confirmed the suitability of the stickleback bioassay as a test for antiandrogens. Inhibition of DHT-induced spiggin production in females was complete at 125 μg/L FL and above and was significant at 10 μg/L. For the male fish, total inhibition was observed at 250 μg/L of FL, and significant reduction was noted at 50 μg/L.
Analytical verification of test concentrations
Table 1 presents the recoveries of test compounds in aquaria water. Analysis of methanol washings from the interior walls of the sample collection bottles as well as the pre-SPE filters indicated negligible (< 0.5 μg/L) concentrations per analyte. The recoveries of diuron from the extracts ranged between 80 and 93% in all preparations.
Table 1.
| Determined concentration (μg/L)
|
|||||||
|---|---|---|---|---|---|---|---|
| Compound | Nominal concentration (μg/L) | D3ba | D3ab | D10b | D10a | D17b | D17a |
| Linuron (R1c) | 150 | 91 | 116 | 63 | 167 | 107 | 111 |
| Linuron (R2d) | 150 | 91 | 94 | 61 | 177 | 94 | 101 |
| Linuron (R1) | 15 | 5.4 | 6.1 | 3.4 | 11 | 5.9 | 8.5 |
| Linuron (R2) | 15 | 5.9 | 6.3 | 3.9 | 12 | 6.6 | 8.2 |
| Control (R1) | 0 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| Control (R2) | 0 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| Flutamide (R1) | 250 | 231 | 243 | 176 | 251 | 195 | 212 |
| Flutamide (R2) | 250 | 232 | 248 | 178 | 256 | 191 | 210 |
| Flutamide (R1) | 25 | 17 | 18 | 13 | 18 | 16 | 18 |
| Flutamide (R2) | 25 | 18 | 17 | 11 | 18 | 13 | 15 |
| Control (R1) | 0 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| Control (R2) | 0 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| Vinclozolin (R1) | 250 | 3.5 | 177 | 3.8 | 127 | 1.9 | 178 |
| Vinclozolin (R2) | 250 | 3.5 | 210 | 4.5 | 92 | 2.7 | 103 |
| Vinclozolin (R1) | 25 | < 0.5 | 11 | < 0.5 | 8.8 | < 0.5 | 10 |
| Vinclozolin (R2) | 25 | < 0.5 | 7 | < 0.5 | 7.3 | < 0.5 | 7.2 |
| Control (R1) | 0 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| Control (R2) | 0 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| Fenitrothion (R1) | 150 | 119 | 141 | 127 | 133 | 129 | 135 |
| Fenitrothion (R2) | 150 | 137 | 140 | 134 | 137 | 124 | 131 |
| Fenitrothion (R1) | 15 | 12 | 13 | 11 | 12 | 9 | 10 |
| Fenitrothion (R2) | 15 | 8.5 | 10 | 12 | 13 | 11 | 12 |
| Control (R1) | 0 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| Control (R2) | 0 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 | < 0.5 |
| 17α-MT (R1) | 0.5 | 0.04 | 0.45 | 0.03 | 0.46 | 0.02 | 0.47 |
| 17α-MT (R2) | 0.5 | 0.03 | 0.46 | 0.02 | 0.49 | 0.03 | 0.48 |
| Control (R1) | 0 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| Control (R2) | 0 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| Determined concentration (μg/L)
|
|||||||
|---|---|---|---|---|---|---|---|
| Compound | Nominal concentration (μg/L) | W1ae | W1bf | W2a | W2b | W3a | W3b |
| FL (flow-through) | 500 | 396 | 394 | 361 | 335 | 380 | 392 |
| FL (flow-through) | 250 | 205 | 194 | 202 | 205 | 200 | 201 |
| FL (flow-through) | 125 | 99 | 114 | 104 | 96 | 101 | 103 |
| FL (flow-through) | 50 | 40 | 40 | 39 | 49 | 41 | 42 |
| FL (flow-through) | 10 | 6.7 | 8.1 | 8.5 | 8.2 | 7.5 | 7.9 |
| FL (flow-through) | 1 | < 0.1 | 3.3 | 3.2 | 3.3 | 2.1 | 2 |
| FL (flow-through) | 0 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 |
| Control (FL) | 0 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 | < 0.1 |
| DHT (flow-through)g | 5 | 4.99 | 4.92 | 4.95 | 4.93 | 4.96 | 4.95 |
| Control (DHT) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: D, day; R, replicate; W, week.
Day 3 before addition of fresh solution in the semistatic system.
Day 3 after addition in the semistatic system.
Tank replicate 1.
Tank replicate 2.
Week 1 first sample.
Week 1 second sample.
The recovered concentrations of DHT from the tanks were very similar and close to the nominal concentration; thus we report only average values from all tanks on each sampling occasion.
Discussion
The present study provides further evidence that the stickleback is a unique bioindicator species that can be invaluable in the evaluation of antiandrogenic chemicals, EDCs that appear to pose a significant threat of adverse effects to both wildlife and humans.
In the test we describe here, antiandrogenic activity is detected by comparing the kidney content of spiggin in female sticklebacks simultaneously exposed to a model androgen (17α-MT or DHT) and known antiandrogens, to female sticklebacks exposed solely to the model androgen. This bioassay represents the adaptation of the established stickleback androgen bioassay in which the androgen specific biomarker spiggin is measured using an ELISA (Katsiadaki et al. 2002a).
Male sticklebacks can also be used for the in vivo detection of antiandrogenic activity; female sticklebacks, however, provide more consistent results because of the absence of endogenous androgens. The bioassay can use male sticklebacks only under extremely controlled photoperiodic conditions before and during the experiment. The reproductive status must be fully synchronized to allow comparison between groups. Social hierarchies within tanks can preclude any conclusions, as dominant males can suppress the onset of breeding in repressed males in the control groups. In addition, the endogenous levels of androgens in the dominant males are extremely high (Mayer et al. 1990), thereby masking the antiandrogenic effect of the test compounds. The development of strict protocols to ensure that the fine balance between environment and reproductive status of male fish is achieved is certainly one area of further work that would be highly beneficial. In general, it appears to be easier to manipulate the reproductive status of male fish during the autumn and winter months when the levels of endogenous androgens and AR numbers are low.
At 5 μg/L, 17α-MT effectively overwhelmed the antiandrogenic activity of the test compounds at the concentration they were administered. Using this information we modified the experimental design for the second exposure experiment, during which 17α-MT was applied at a concentration of 0.5 μg/L. We believed that this concentration would be sufficient to induce spiggin production but would not be so high as to mask any antiandrogenic activity.
In the second semistatic exposure, the average spiggin content in the androgen-exposed females was approximately one-third of that for the first exposure experiment. FL, VZ, and LN significantly antagonized 17α-MT–induced spiggin production in the female stickleback (Figure 5). The additional antiandrogen FN proved to be exceptionally potent in vivo. At its lowest administered concentration, only 15 μg/L, its antiandrogenic activity was analogous to the activity of the pharmaceutical antiandrogen FL at 250 μg/L. Furthermore, at the highest administered concentration, 150 μg/L, FN completely inhibited spiggin production. The spiggin content at this concentration is statistically the same as that obtained for the water and solvent control groups. These results provide the first in vivo evidence of antiandrogenic activity for both LN and FN in teleosts.
When the administration of test compounds is waterborne, one should consider the differences in solubility, stability, and means of transport to target tissues that exist between the test compounds and that influence the magnitude of biological response. In this case the differences between the two androgens (17α-MT and DHT) could have more than one cause. Our own unpublished data suggest that 17α-MT induces higher kidney hypertrophy than DHT when used at the same concentration in both flow-through and semistatic exposure systems. Furthermore, measurement of the model androgens in the aquaria water under semistatic conditions suggests that 17 α-MT is far more stable than DHT.
Unfortunately, the number of studies on antiandrogens in fish is limited, and as far as the authors are concerned, this current study is only the third time that FL, a classic mammalian antiandrogen, has been shown to be antiandrogenic in teleosts.
Sower et al. (1983) showed that administration of FL along with 17α-MT to juvenile steelhead trout (incorporated in food pellets) prevented epidermal thickening and reduced growth rate, characteristics that were both caused by 17α-MT alone.
Wells and Van Der Kraak (2000) provided the first evidence that certain environmental chemicals bind to AR in fish but exhibit inter-species and tissue differences in their binding profile. The lack of AR binding of some classic antiandrogens such as FL and VZ (and its metabolites M1 and M2) in rainbow trout, goldfish, and fathead minnow must be considered, especially when these species are used as models for endocrine research purposes.
The induction of nuptial tubercles in female fathead minnows has been proposed as a biomarker of androgen exposure. The concentrations of 17α-MT used to induce the formation of these tubercles range from 200 to 2,000 μg/L (Ankley et al. 2001; Smith 1974), which is 400–4,000 times the amount required to induce spiggin production in female sticklebacks. Furthermore, administration of 17α-MT at high concentrations resulted in high mortalities in the study conducted by Ankley et al. (2001). The antiandrogenic activity of FL has been detected in male fathead minnows through a reduction in the number of nuptial tubercles (Panter et al. 2004). However, a FL concentration of 1,000 μg/L (4 times greater than that used in this study) was required to elicit this response. In addition, exposure of fat-head minnow embryos to VZ at concentrations ranging from 90 to 1,200 μg/L did not result in any adverse effects on sexual differentiation or reproductive health (Makynen et al. 2000). These findings draw into question the suitability of fathead minnow bioassays for the detection of EDCs with antiandrogenic activity.
More recently, Bayley et al. (2002) exposed juvenile guppies to FL, VZ, and p,p′-DDE via the food from birth to adulthood and concluded that all three chemicals had a clear demasculinizing effect (reduction of orange display coloration, gonopodium development, reduction in sperm count, and suppressed courtship behavior). However, the length of the study and the nonwater-borne exposure preclude any comparisons of the sensitivity of the bioassay with the stickleback assay.
In addition, Kinnberg and Toft (2003) exposed sexually mature male guppies to a number of estrogenic and antiandrogenic compounds. Although FL, p,p′ DDE, and estrogens blocked spermatogonial mitosis, VZ did not have any adverse effects.
The in vivo studies that failed to demonstrate androgen antagonism by mammalian and environmental antiandrogens in teleosts suggest profound differences in the AR binding specificities between species.
In the stickleback kidney, previous studies have shown that no specific binding of 11-ketotestosterone (11-KT) or testosterone was detected in either cytosolic or nuclear fractions, although displacement of tritiated 11-KT with unlabeled 11-KT was observed in the kidney membrane fraction (Jakobsson et al. 1996). More recently, molecular cloning of a nuclear AR in the stickleback kidney has revealed that it is the classic mammalian type, AR2 or AR beta (GenBank accession no. AAO83572/3; http://ncbi.nlm.nih.gov/Genbank), as we have previously speculated on the basis of androgen potency (Katsiadaki et al. 2002a). The presence of a nuclear AR homologous to the mammalian AR in the stickleback kidney has important implications regarding the use of the species as a model organism for EDC research.
The most promising mammalian assay for androgens and antiandrogens is the castrated male rat assay, widely known as the Hershberger assay (Ashby and Lefevre 2000; Stocker et al. 2000). However, the prerequisite for surgical castration of rats makes it a rather laborious test for chemical screening. The need for a more rapid test for antiandrogens is reflected by the increasing number of alternative tests such as the weanling male rat assay (Ashby and Lefevre 1997), the intact young male rat assay (O’Connor et al. 1999), the peripubertal intact male assay (Stocker et al. 2000), the use of androgen-stimulated immature intact male rats (Ashby et al. 2002), in utero exposure (Shultz et al. 2001), and the use of gonadotrophin release hormone–inhibited rats (Nellemann et al. 2003).
Although surgical castration is not required in these alternative tests, the majority of these assays rely on the same principle changes in the reproductive or accessory sex gland weights upon treatment. This end point has received criticism because chemical treatment may affect growth rate; thus the relationship between body weight and/or accessory gland weight is problematic (Marty et al. 2003).
It should be added that although in utero and in vivo exposure (You et al. 1998) of weaning rats to p,p′-DDE confirmed the antiandrogenic activity observed in vitro by Kelce et al. (1995), exposure of adult male rodents has failed to identify any antiandrogenic effects (Leavens et al. 2002). With the adaptation of the spiggin assay described here, a dramatic inhibition of photoperiodically or androgen-induced kidney hypertrophy is detectable in intact adult sticklebacks within 21 days of exposure, which highlights the advantages that the assay presents. The exposure period of 21 days was chosen because it is used as the standard period in the fish screening assay for the detection of endocrine-active substances (Organisation for Economic Cooperation and Development 2004). In our experience, a 3-week exposure period is sufficient for detecting changes in biomarkers (i.e., spiggin and VTG).
EE2 was used in one exposure as a negative control (Figure 6). As expected, EE2 at a concentration that is effective at inducing VTG production in a wide range of fish did not induce spiggin in females. Indeed, there is a suggestion that it inhibited/reduced spiggin production in males. Both findings are in line with previous observations by Oguro (1957), who first reported that estrogens do not stimulate kidney hypertrophy in the stickleback but do result in regression of kidney hypertrophy. Exposure to high concentrations of estradiol also results in lower spiggin levels in male fish (unpublished data). We are currently testing a number of estrogens in androgen-treated female fish to determine whether they display the same antiandrogenic effect as in the males.
It is as yet unclear whether the antiandrogenic effect of estrogens is exerted through their binding to the AR in a genomic way (Kelce and Wilson 1997; Sohoni and Sumpter 1998); in a nongenomic way, mediated by an estrogen membrane receptor (Loomis and Thomas 2000); or via other mechanism(s) involving feedback control of sex steroid levels to gonadotrophins (Shultz et al. 2001).
One of the great advantages of the stickleback as a model organism is that the simultaneous assessment of an androgen and estrogen end point (we have also developed a homologous ELISA for stickleback VTG) can provide vital clues regarding the mechanisms responsible for endocrine adverse effects. We applied the VTG ELISA to detect estrogenicity of the test compounds and found that none of the tested environmental antiandrogens induced VTG in male fish or increased the VTG content in female fish (results not shown). As the two protein markers are produced and stored in different organs (kidney for spiggin, plasma or liver for VTG), a single fish can be analyzed for androgenic and estrogenic activity. This versatility reduces the number of test organisms needed, which is of great importance both ethically and economically.
There are several other reasons why the stickleback is an ideal European bioindicator species, as discussed elsewhere (Katsiadaki et al. 2002b).
Conclusions
The stickleback androgen bioassay can be adapted to detect antiandrogens in two ways:
Simultaneous treatment of females with an androgen
Photoperiodic manipulation of male stickleback reproductive status
The degree of antagonism by a given compound depends on the following:
Type and concentration of model androgen used (17α-MT being more potent than DHT).
Stability/solubility of the androgen/ antiandrogen in water (particularly when semistatic exposure is employed).
Reproductive status of fish/experimental conditions (competition with natural ligands in male fish).
On the basis of the results of the present study, we propose the use of DHT at 5 μg/L for flow-through systems and 17α-MT at 0.5 μg/L for either flow-through or semistatic exposures in order to stimulate spiggin production in female fish.
This work represents the first study in teleosts to demonstrate that linuron is an androgen antagonist.
Fenitrothion is an exceptionally potent androgen antagonist in vivo (also first evidence in teleosts).
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
We gratefully acknowledge the financial assistance of the Department for Environment, Food and Rural Affairs (DEFRA) under contracts AE1149 and AE1150 and, in particular, P. Leonard from Science Directorate, Marine and Waterways Division, for his help and support.
References
- Allen Y, Matthiessen P, Scott AP, Haworth S, Feist S, Thain JE. The extent of oestrogenic contamination in the UK estuarine and marine environments—further surveys of flounder. Sci Tot Environ. 1999;233:5–20. doi: 10.1016/s0048-9697(99)00175-8. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2001;20:1276–1290. [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, et al. Effects of the androgenic growth promoter 17-beta-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ Toxicol Chem. 2003;22:1350–1360. [PubMed] [Google Scholar]
- Ashby J, Lefevre PA. The weanling male rat as an assay for endocrine disruption: preliminary observations. Regul Toxicol Pharmacol. 1997;26(3):330–337. doi: 10.1006/rtph.1997.1177. [DOI] [PubMed] [Google Scholar]
- Ashby J, Lefevre PA. The peripubertial male rat assay as an alternative to the Hershberger castrated male rat assay for the detection of anti-androgens, oestrogen and metabolic modulators. J Appl Toxicol. 2000;20:35–47. doi: 10.1002/(sici)1099-1263(200001/02)20:1<35::aid-jat633>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ashby J, Owens W, Lefevre PA. Concept evaluation: stimulated immature intact male rats as an assay for anti-androgens. Regul Toxicol Pharmacol. 2002;35:280–285. doi: 10.1006/rtph.2002.1543. [DOI] [PubMed] [Google Scholar]
- Bayley M, Junge M, Baatrup E. Exposure of juvenile guppies to three antiandrogens causes demasculinisation and a reduced sperm count in adult males. Aquat Toxicol. 2002;56:227–239. doi: 10.1016/s0166-445x(01)00210-7. [DOI] [PubMed] [Google Scholar]
- Borg B. Extraretinal photoreception involved in photoperiodic effects on reproduction in male three-spined sticklebacks, Gasterosteus aculeatus. Gen Comp Endocrinol. 1982;47:84–87. doi: 10.1016/0016-6480(82)90087-9. [DOI] [PubMed] [Google Scholar]
- Bornestaf C, Borg B. Endogenous breeding cycles in male three-spined sticklebacks, Gasterosteus aculeatus. Behaviour. 2000;137:921–932. [Google Scholar]
- Cody RP, Bortone SA. Masculinization of mosquitofish as an indicator of exposure to kraft mill effluent. Bull Environ Contam Toxicol. 1997;58:429–436. doi: 10.1007/s001289900352. [DOI] [PubMed] [Google Scholar]
- Cook JC, Mullin LS, Frame SR, Biegel LB. Investigation of a mechanism for Leydig cell tumorigenesis by linuron in rats. Toxicol Appl Pharmacol. 1993;119(2):195–204. doi: 10.1006/taap.1993.1060. [DOI] [PubMed] [Google Scholar]
- Denslow ND, Chow MC, Kroll KJ, Green L. Vitellogenin as a biomarker of exposure for estrogen and estrogen mimics. Ecotoxicology. 1999;8:385–398. [Google Scholar]
- Environment Agency. In press. Assessment of the (Anti-) oestrogenic and (Anti-)androgenic Activities of Final Effluents from Sewage Treatment Works. R&D Technical Report P6-021/TR. Available: http://publications.environmentagency.gov.uk/epages/eapublications.storefront
- Folmar LC, Denslow ND, Rao V, Chow M, Crain DA, Enblom J, et al. Vitellogenin induction and reduced serum testosterone concentrations in feral male carp (Cyprinous carpio) captured near major metropolitan sewage treatment plant. Environ Health Perspect. 1996;104:1096–1101. doi: 10.1289/ehp.961041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyberger A, Ahr HJ. Development and standardization of a simple binding assay for the detection of compounds with affinity for the androgen receptor. Toxicology. 2004;195:113–126. doi: 10.1016/j.tox.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Fry DM. Reproductive effects in birds exposed to pesticides and industrial chemicals. Environ Health Perspect. 1995;103(suppl 7):165–171. doi: 10.1289/ehp.95103s7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Jr, Ostby JS, Kelce WR. Developmental effects of an environmental antiandrogen: the fungicide vinclozolin alters sex differentiation in the male rat. Toxicol Appl Pharmacol. 1994;129:46–52. doi: 10.1006/taap.1994.1227. [DOI] [PubMed] [Google Scholar]
- Heppell SA, Denslow ND, Folmar LC, Sullivan CV. Universal assay of vitellogenin as a biomarker for environmental estrogens. Environ Health Perspect. 1995;103:9–15. doi: 10.1289/ehp.95103s79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst AL, Anderson D. Clear cell adenocarcinoma of the vagina and cervix secondary to intrauterine exposure to diethylstilbestrol. Semin Surg Oncol. 1990;6:343–346. doi: 10.1002/ssu.2980060609. [DOI] [PubMed] [Google Scholar]
- Hershberger LG, Shipley EG, Meyer RK. Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method. Proc Soc Exp Biol Med. 1953;83:175–180. doi: 10.3181/00379727-83-20301. [DOI] [PubMed] [Google Scholar]
- Howell WM, Denton TE. Gonopodial morphogenesis in female mosquitofish, Gambusia affinis affinis, masculinized by exposure to degradation products from plant sterols. Environ Biol Fish. 1989;24:43–51. [Google Scholar]
- Jakobsson S, Mayer I, Schulz RW, Blankenstein MA, Borg B. Specific binding of 11-ketotestosterone in an androgen target organ, the kidney of the male three-spined stickleback, Gasterosteus aculeatus. Fish Physiol Biochem. 1996;15:459–467. doi: 10.1007/BF01874920. [DOI] [PubMed] [Google Scholar]
- Katsiadaki I, Scott AP, Hurst MR, Matthiessen P, Mayer I. Detection of environmental androgens: a novel method based on enzyme-linked immunosorbent assay of spiggin, the stickleback (Gasterosteus aculeatus) glue protein. Environ Toxicol Chem. 2002a;21(9):1946–1954. [PubMed] [Google Scholar]
- Katsiadaki I, Scott AP, Mayer I. Potential of the stickleback as a combined biomarker for oestrogens and androgens in European waters. Mar Environ Res. 2002b;54:725–728. doi: 10.1016/s0141-1136(02)00110-1. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Monnosson E, Gamcsik MP, Laws SC, Gray LE., Jr Environmental hormone disruptors: evidence that vinclozolin developmental toxicity is mediated by antiandrogenic metabolites. Toxicol Appl Pharmacol. 1994;126:276–285. doi: 10.1006/taap.1994.1117. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CS, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite, p,p′-DDE, is a potent androgen receptor antagonist. Nature. 1995;375:581–585. doi: 10.1038/375581a0. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Wilson EM. Environmental antiandrogens: developmental effects, molecular mechanisms, and clinical implications. J Mol Med. 1997;75:198–207. doi: 10.1007/s001090050104. [DOI] [PubMed] [Google Scholar]
- Kinnberg K, Toft G. Effects of estrogenic and anti-androgenic compounds on the testis structure of the adult guppy (Poecilia reticulata) Ecotoxicol Environ Saf. 2003;54:16–24. doi: 10.1016/s0147-6513(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Lambright C, Ostby J, Bobseine K, Wilson V, Hotchkiss AK, Mann PC. Cellular and molecular mechanisms of action of linuron: an antiandrogenic herbicide that produces reproductive malformations in male rats. Toxicol Sci. 2000;56(2):389–399. doi: 10.1093/toxsci/56.2.389. [DOI] [PubMed] [Google Scholar]
- Larsson DGJ, Hällman H, Förlin L. More male fish embryos near a pulp mill. Environ Toxicol Chem. 2000;19:2911–2917. [Google Scholar]
- Leavens TL, Sparrow BR, Devito MJ. Lack of antiandrogenic effects in adult male rats following acute exposure to 2,2-bis(4-chlorophenyl)-1,1-dichloro-ethylene (p,p′-DDE) Toxicology. 2002;174:69–78. doi: 10.1016/s0300-483x(02)00072-0. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Loomis AK, Thomas P. Effects of estrogens and xenoestrogens on androgen production by Atlantic croaker testes in vitro: evidence of a non-genomic action mediated by an estrogen membrane receptor. Biol Reprod. 2000;65:995–1004. doi: 10.1095/biolreprod62.4.995. [DOI] [PubMed] [Google Scholar]
- Makynen EA, Kahl MD, Jensen KM, Tietge JE, Wells KL, Van Der Kraak G, et al. Effects of the mammalian antiandrogen vinclozolin on development and reproduction of the fathead minnow (Pimephales promelas) Aquat Toxicol. 2000;48:461–475. doi: 10.1016/s0166-445x(99)00059-4. [DOI] [PubMed] [Google Scholar]
- Marty MS, Johnson KA, Carney EW. Effect of feed restriction on Hershberger and pubertal male assay points. Birth Defects Res (B) 2003;68:363–374. doi: 10.1002/bdrb.10028. [DOI] [PubMed] [Google Scholar]
- Mayer I, Borg B, Schulz R. Seasonal changes in and effect of castration/androgen replacement on the plasma levels of five androgens in the male three-spined stickleback, Gasterosteus aculeatus L. Gen Comp Endocrinol. 1990;79:23–30. doi: 10.1016/0016-6480(90)90084-y. [DOI] [PubMed] [Google Scholar]
- McIntyre BS, Barlow NJ, Wallace DG, Maness SC, Gaido KW, Foster PMD. Effects of in utero exposure to linuron on androgen-dependent reproductive development in the male Crl:CD(SD)BR rat. Toxicol Appl Pharmacol. 2000;167:87–99. doi: 10.1006/taap.2000.8998. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley R, Foster P. Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol Appl Pharmacol. 1999;156:81–95. doi: 10.1006/taap.1999.8643. [DOI] [PubMed] [Google Scholar]
- Nellemann C, Lefevre PA, Ashby J. Comparison of prostate gene expression and tissue weight changes as monitors of antiandrogen activity in GNRH-inhibited rats. Birth Defects Res (B) 2003;68:344–354. doi: 10.1002/bdrb.10030. [DOI] [PubMed] [Google Scholar]
- Newbold R. Cellular and molecular effects of developmental exposure to diethylstilbestrol: implications for other environmental estrogens. Environ Health Perspect. 1995;103(suppl 7):83–87. doi: 10.1289/ehp.95103s783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with oestrogens: a model for hormonal carcinogenesis. Cancer Res. 1990;50:7677–7681. [PubMed] [Google Scholar]
- O’Connor JC, Frame SR, Davis LG, Cook JC. Detection of the environmental antiandrogen p,p′-DDE in CD and Long-Evans rats using a Tier 1 screening battery and Hershberger assay. Toxicol Sci. 1999;51:44–53. doi: 10.1093/toxsci/51.1.44. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development. 2004. OECD Draft Report of the Initial Work Towards the Validation of the Fish Screening Assay for the Detection of Endocrine-Active Substances. Phase 1A, Paris:Organisation for Economic Co-operation and Development.
- Oguro C. Notes on the change in the kidneys of the three-spined stickleback, Gasterosteus aculeatus aculeatus L. caused by oestrogen administration. J Fac Sci Hokkaido Univ Ser VI Zool. 1957;13:404–407. [Google Scholar]
- Ostby J, Kelce WR, Lambright C, Wolf CJ, Mann P, Gray LE., Jr The fungicide procymidon alters the sexual differentiation in the male rat by acting as an androgen-receptor antagonist both in vitro and in vivo. Toxicol Ind Health. 1999;15:80–93. doi: 10.1177/074823379901500108. [DOI] [PubMed] [Google Scholar]
- Panter GH, Hutchinson TH, Hurd KS, Sherren A, Stanley RD, Tyler CR. Successful detection of (anti-)androgenic and aromatase inhibitors in pre-spawning adult fathead minnows (Pimephales promelas) using easily measured endpoints of sexual development. Aquat Toxicol. 2004;70:11–21. doi: 10.1016/j.aquatox.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Parks LG, Lambright CS, Orlando EF, Guillette LJ, Jr, Ankley GT, Gray LE., Jr Masculinization of female mosquitofish in kraft mill effluent-contaminated river water is associated with androgen receptor agonist activity. Toxicol Sci. 2001;62:257–267. doi: 10.1093/toxsci/62.2.257. [DOI] [PubMed] [Google Scholar]
- Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP. Estrogenic effects of effluents from sewage treatment works. Chem Ecol. 1994;8:275–285. [Google Scholar]
- Rivas A, Fisher JS, McKinnell C, Atanassova N, Sharpe RM. Induction of reproductive tract developmental abnormalities in the male rat by lowering androgen production or action in combination with a low dose of diethylstilbestrol: evidence for importance of the androgen-estrogen balance. Endocrinology. 2002;143:4797–4808. doi: 10.1210/en.2002-220531. [DOI] [PubMed] [Google Scholar]
- Shulz VD, Phillips S, Sar M, Foster PMD, Gaido KW. Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl)phthalate. Toxicol Sci. 2001;64:233–242. doi: 10.1093/toxsci/64.2.233. [DOI] [PubMed] [Google Scholar]
- Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (anti-androgens): structure-activity relationships. Curr Med Chem. 2000;7:211–247. doi: 10.2174/0929867003375371. [DOI] [PubMed] [Google Scholar]
- Smith RJF. Effects of 17-methyltestosterone on the dorsal pad and tubercles of fathead minnows (Pimephales promelas) Can J Zool. 1974;52:1031–1038. doi: 10.1139/z74-137. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Lefevre PA, Ashby J, Sumpter JP. Possible androgenic/anti-androgenic activity of the fungicide fenitrothion. J Appl Toxicol. 2001;21:173–178. doi: 10.1002/jat.747. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Sumpter JP. Several environmental estrogens are also anti-androgens. J Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Sower SA, Schreck CB, Evenson M. Effects of steroids and steroid antagonists on growth, gonadal development, and RNA/DNA ratios in juvenile steelhead trout. Aquaculture. 1983;32:243–254. [Google Scholar]
- Stocker TE, Parks LG, Gray E, Cooper RL. Endocrine-disrupting chemicals: prepubertal exposures and effects on sexual maturation and thyroid function in the male rat. A focus on the EDSTAC recommendations. Endocrine Disrupter Screening and Testing Advisory Committee. Crit Rev Toxicol. 2000;30:197–252. doi: 10.1080/10408440091159194. [DOI] [PubMed] [Google Scholar]
- Sumpter JP, Jobling S. Vitellogenin as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect. 1995;103:173–178. doi: 10.1289/ehp.95103s7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Maness SC, Reischmann K, Dorman DC, Gray LE, Gaido KW. Androgen receptor antagonism by the organophosphate insecticide fenitrothion. Toxicol Sci. 2001;60:56–62. doi: 10.1093/toxsci/60.1.56. [DOI] [PubMed] [Google Scholar]
- Thomas KV, Hurst MR, Matthiessen P, McHugh M, Smith A; Waldock MJ. An assessment of in vitro androgenic activity and the identification of environmental androgens in United Kingdom estuaries. Environ Toxicol Chem. 2002;20:1456–1461. [PubMed] [Google Scholar]
- Toppari J, Larsen J, Christiansen P, Giwercman J, Grandjean P, Guillette L, et al. Male reproductive health and environmental xenoestrogens. Environ Health Prespect. 1996;104:741–776. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KJ, Barlow NJ, Struve MF, Wallace DG, Gaido KW, Dorman DC, et al. Effects of in utero exposure to the organophosphate insecticide fenitrothion on androgen-dependent reproductive development in the Crl:CD(SD)BR rat. Toxicol Sci. 2002;68:174–183. doi: 10.1093/toxsci/68.1.174. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Jørgensen ECB, Larsen JC. Rapid and sensitive reporter gene assays for detection of antiandrogenic and estrogenic environmental chemicals. Toxicol Appl Pharmacol. 1999;155:150–160. doi: 10.1006/taap.1998.8598. [DOI] [PubMed] [Google Scholar]
- Vos JG, Dybing E, Greim HA, Ladefoged O, Lambrac C, Tarazona JV, et al. Health effects of endocrine-disrupting chemicals on wildlife with special reference to the European situation. Crit Rev Toxicol. 2000;30:71–133. doi: 10.1080/10408440091159176. [DOI] [PubMed] [Google Scholar]
- Wilson VS, Lambright C, Ostby J, Gray LE., Jr In vitro and in vivo effects of 17α-trenbolone: a feedlot effluent contaminant. Toxicol Sci. 2002;70:202–211. doi: 10.1093/toxsci/70.2.202. [DOI] [PubMed] [Google Scholar]
- Wells K, Van Der Kraak G. Differential binding of endogenous steroids and chemicals to androgen receptors in rainbow trout and goldfish. Environ Toxicol Chem. 2000;19:2059–2065. [Google Scholar]
- Wong CI, Kelce WR, Sar M, Wilson EM. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J Biol Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- You L, Casanova M, Chan, Archibeque-Engle S, Madhabananda S, Fan L, et al. Impaired male sexual development in peri-natal Spraque-Dawley and Long-Evans hooded rats exposed in utero and lactationally to p,p′-DDE. Toxicol Sci. 1998;45:162–173. doi: 10.1093/toxsci/45.2.162. [DOI] [PubMed] [Google Scholar]



