Abstract
The effects of simple mixtures of chemicals, with similar mechanisms of action, can be predicted using the concentration addition model (CA). The ability of this model to predict the estrogenic effects of more complex mixtures such as effluent discharges, however, has yet to be established. Effluents from 43 U.K. wastewater treatment works were analyzed for the presence of the principal estrogenic chemical contaminants, estradiol, estrone, ethinylestradiol, and nonylphenol. The measured concentrations were used to predict the estrogenic activity of each effluent, employing the model of CA, based on the relative potencies of the individual chemicals in an in vitro recombinant yeast estrogen screen (rYES) and a short-term (14-day) in vivo rainbow trout vitellogenin induction assay. Based on the measured concentrations of the four chemicals in the effluents and their relative potencies in each assay, the calculated in vitro and in vivo responses compared well and ranged between 3.5 and 87 ng/L of estradiol equivalents (E2 EQ) for the different effluents. In the rYES, however, the measured E2 EQ concentrations in the effluents ranged between 0.65 and 43 ng E2 EQ/L, and they varied against those predicted by the CA model. Deviations in the estimation of the estrogenic potency of the effluents by the CA model, compared with the measured responses in the rYES, are likely to have resulted from inaccuracies associated with the measurement of the chemicals in the extracts derived from the complex effluents. Such deviations could also result as a consequence of interactions between chemicals present in the extracts that disrupted the activation of the estrogen response elements in the rYES. E2 EQ concentrations derived from the vitellogenic response in fathead minnows exposed to a series of effluent dilutions were highly comparable with the E2 EQ concentrations derived from assessments of the estrogenic potency of these dilutions in the rYES. Together these data support the use of bioassays for determining the estrogenic potency of WwTW effluents, and they highlight the associated problems for modeling approaches that are reliant on measured concentrations of estrogenic chemicals.
Keywords: concentration addition, effluents, estradiol, estrogen, estrone, ethinylestradiol, mixtures, nonylphenol
Environmental risk assessment relies principally on effect assessments derived for single chemicals tested under laboratory conditions, yet many waterways receive discharges from wastewater treatment works (WwTW) that are contaminated with tens of thousands of chemicals. In reality, aquatic wildlife is rarely exposed to single chemicals in isolation but rather to complex mixtures of chemicals. Because mixtures of chemicals are generally expected to induce greater biological effects than single compounds (European Inland Fisheries Advisory Commission 1987; Scientific Committee on Problems of the Environment 1987), there is a need to consider the combined activity of chemicals in environmental risk assessment.
Two general methods for assessing the environmental risk of chemical mixtures exist, one empirical and the other model-based (Ankley and Mount 1996). The empirical method evaluates the biological effects of complex mixtures as a single entity and, consequently, does not require a priori knowledge of the contaminants of concern (Ankley and Mount 1996). This method offers the advantage that any uncertainties regarding bio-availability and chemical interactions are addressed, but it provides little information on the chemical cause(s) of the effect seen. The causative agents, however, can then be identified through the application of toxicity-based fractionation procedures, which allow the specific chemicals, or classes of chemicals, responsible for the observed effect to be isolated. This approach has been applied recently with success in identifying four chemicals that are principally responsible for the estrogenic activity of treated WwTW effluents in the United Kingdom. These chemicals include the natural steroids, 17β-estradiol (E2) and estrone (E1), the synthetic steroid, 17α-ethinylestradiol (EE2),and the alkylphenol nonylphenol (NP) (Desbrow et al. 1998; Rodgers-Gray et al. 2000; Sheahan et al. 2002).
Model-based methods for assessing the environmental risk of complex mixtures use toxicological models to relate measured environmental concentrations of chemicals to a predicted biological effect (Ankley and Mount 1996). The modeling approach can be used to predict the expected biological activity of a mixture. The successful application of the models, however, depends on a measure of the contaminant concentrations to which organisms are exposed, a measure of the biological potency of individual contaminants for the end point of interest, and an expression of how the potency of individual contaminants is modified or integrated when they are in a mixture. Where these criteria are met, it has been demonstrated that the model of concentration addition (CA; Loewe 1953) can be used to predict the toxicity of mixtures of chemicals with similar modes of action in those of fish and other aquatic organisms (Alabaster et al. 1994; Bailey et al. 1997; Matthiessen et al. 1988; Walker et al. 1996).
More recently, the model of CA has been demonstrated to predict accurately the in vivo estrogenic activity of binary mixtures of E2 with EE2 or NP in fish (using the estrogen-dependent endpoint of vitellogenin induction; Brian et al. 2005; Thorpe et al. 2001, 2003). This modeling approach, however, has yet to be proved for the assessment of the estrogenic activity of complex chemical mixtures contained in effluent discharges. The model of CA has been shown to be capable of predicting the toxicity of effluents to freshwater fish (Alabaster et al. 1994) on the basis of the predicted contribution of toxicants known to be present. However, this approach was less effective for predicting the sublethal effects of the effluents (Alabaster et al. 1994).
In the present study, our primary aim was to investigate the ability of the model of CA to predict the estrogenic activity of effluent discharges on the basis of the predicted contribution of the major estrogens known to be present. We focused on the four more potent estrogens, E2, E1, EE2, and NP, that are present in most WwTW effluents in both the United Kingdom (Desbrow et al. 1998; Rodgers-Gray et al. 2000; Sheahan et al. 2002), and more widely in Europe (Belfroid et al. 1999; Cargouët et al. 2004; Pawlowski et al. 2003; Rutishauser et al. 2004). Initial investigations were conducted to establish the potencies of the individual estrogenic chemicals in a recombinant yeast estrogen screen (rYES) and to demonstrate that the potency of binary combinations of these chemicals could be calculated on the basis of CA. The rYES employed has been used widely to assess the estrogenicity of individual chemicals (Routledge and Sumpter 1996; Segner et al. 2003; Van den Belt et al. 2004). More recently, this rYES has been used to investigate the estrogenic potency of predefined chemical mixtures (Payne et al. 2000; Silva et al. 2002) and complex chemical mixtures such as effluents extracts collected from WwTW (Aerni et al. 2004; Murk et al. 2002; Rutishauser et al. 2004). After demonstrating that the model of CA could be used to predict the activity of mixtures of the target estrogenic chemicals, we estimated the relative estrogenic potencies of 43 WwTW effluents throughout England and Wales on the basis of the measured concentrations of the target estrogens in spot samples collected for each effluent and on their estrogenic potencies in vitro (rYES) and in vivo [via induction of vitellogenin (VTG) in juvenile rainbow trout]. The prediction for the in vitro estrogenic activity of each effluent sample was then validated against the measured activity in the rYES and E2 EQ concentrations determined. We investigated further the estrogenic activity of a WwTW effluent in vitro versus in vivo by comparing the measured responses of extracted effluent samples in the rYES with the vitellogenic response in effluent-exposed adult fathead minnows (Pimephales promelas). In combination, the findings from these studies provide a critical insight into the practical difficulties in applying the model of CA to “real-world” complex mixtures of estrogenic chemicals.
Materials and Methods
Reference chemicals
The natural estrogens, E2 (98% purity) and E1 (99% purity), and the synthetic steroid estrogen, EE2 (98% purity), were purchased from Sigma Chemical Co. Ltd. (Dorset, UK). The alkylphenol NP (99% purity) was purchased from ACROS, Fisher Scientific (Leicester, UK).
The rYES was supplied by J. Sumpter, Brunel University, and the assay was run as described by Routledge and Sumpter (1996). Briefly, individual chemicals were diluted serially12 times in ethanol, and 10-μL aliquots of each concentration transferred in duplicate to an optically flat 96-well microtiter plate (Linbro/Titertek; ICN FLOW, Bucks, UK). The plates were then left at room temperature to allow the ethanol to evaporate. To prepare the fixed-ratio binary mixtures, the first chemical was diluted serially, transferred as described above for single chemicals, and the ethanol was evaporated. The second chemical was then diluted serially in ethanol on a separate plate, and 10-μL aliquots were transferred to the duplicate rows containing the first chemical. Aliquots (200 μL each) of assay medium (containing the recombinant yeast and the chromogenic substrate, chlorophenol red-β-d-galactopyranoside) were dispensed into each sample well containing the chemical(s). The plates were sealed with autoclave tape, shaken for 2 min, and then incubated at 32°C. After an incubation period of 3 days, color development in the medium was measured at an absorbance of 540 nm, and turbidity of the yeast was measured at 620 nm using a Spectramax Plus, microtiter plate reader (Molecular Devices, Berkshire, UK). All the individual chemicals and the binary mixtures were tested simultaneously to avoid interassay variations, and each assay was repeated 5 times.
The in vivo concentration–response curves for VTG induction in rainbow trout exposed to estrogenic chemicals and their mixtures described in this article are based on an analysis of an earlier series of experiments. Full details of the individual experiments can be found in Thorpe et al. (2000, 2001, 2003). Briefly, juvenile female rainbow trout were exposed under flow-through conditions for 14 days to a dilution water control, a methanol control, and a series of concentrations of the individual reference chemicals. In total, E2 was tested in five exposures at mean measured concentrations ranging from 1.0 to 723 ng/L, EE2 was tested in two exposures at concentrations ranging from 0.04 to 34 ng/L, and NP was tested in three exposures at concentrations ranging from 0.25 to 53 μg/L. Estrone was tested in a single exposure at a concentration range of 0.74 to 319 ng/L. At the end of each exposure plasma samples were collected from the fish and tested in homologous immunological assays to quantify plasma VTG concentrations. The results from all the exposures were collated to construct a single VTG concentration response curve for each chemical.
WwTW effluents
The effluent samples were collected and analyzed as part of a national survey to assess the estrogenic activity of WwTW effluents discharged into U.K. rivers, in collaboration with the U.K. Environment Agency. Briefly, spot samples were taken on two different occasions (collected at similar times of the day) from the final effluent from 43 WwTW operated by 10 water companies (Anglian Water, Northumbrian Water, Severn-Trent Water, Southern Water, South West Water, Thames Water, United Utilities, Welsh Water, Wessex Water, and Yorkshire Water). The first 25 WwTW were sampled during 1 April 2003 to 21 May 2003, while the final 18 WwTW were sampled during 16 July 2003 to 19 August 2003. Each sample collected was split into three separate solvent-rinsed containers for subsequent analysis of estrogenic activity in the rYES (carried out at the University of Exeter, Exeter, Devon, UK), for measurement of concentrations of E2, E1, and EE2 [carried out by Brixham Environmental Laboratory (Brixham, Devon, UK) for the first 25 WwTW and by WRc plc (Swindon, Wilts, UK) for the final 18 WwTW], and for the measurement of NP (carried out by WRc plc).
To determine the estrogenic activity of the WwTW effluents, effluent samples (nominally 750 mL) were concentrated onto primed solid-phase extraction columns and stored at 4°C. An additional four effluent samples and five HPLC-grade water samples were collected and spiked with a mixture of E2, E1, EE2, and NP to determine the recovery of the estrogenic activity during the extraction procedure. The final concentrations of the spiking chemicals in each sample, before extraction, were 20, 40, 8, and 4,000 ng/L for E2, E1, EE2, and NP, respectively. Before analysis in the rYES, columns containing the extracted samples were eluted with 5 mL methanol. The methanol was removed under a stream of nitrogen, and the extracts were resuspended in 1 mL of ethanol and stored at 4°C overnight. The following morning, five serial dilutions of each extract were performed in ethanol, and 10-μL aliquots of each dilution were transferred in duplicate to the 96-well microtiter plates. Twelve serial dilutions of E2 were run as an internal standard. After evaporation of the ethanol, aliquots (200 μL each) of the rYES assay medium were dispensed into each well, and the plates were sealed, shaken, and incubated in the same manner as the reference chemicals. Two rYES assays were performed on the WwTW effluents, one for each of the sample collections.
For chemical analysis of the natural and synthetic steroids, 5 L of each effluent sample was spiked with an initial concentration of 5 ng/L of each of the deuterated steroids (E2, E1, and EE2). The samples were then filtered using a 0.2-μm filter before extraction onto a preconditioned (with HPLC-grade methanol and double-distilled water) 5-g C18 solid-phase extraction column. The columns were eluted with 20 mL 85:15 methanol:water, and the eluent was evaporated to dryness under a stream of nitrogen. The residues were derivatized by adding 0.2 mL pyridine and 0.3 mL N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide and heating the vial to 60°C for 30 min. After cooling, 0.3 mL bis(trimethylsilyl)trifluoroacetamide was added, and the vial was heated for a further 120 min at 120°C. The vial was then allowed to cool, and the reagents removed under a gentle stream of nitrogen. The residue was resuspended in 2 mL dichloromethane (DCM) and passed through a 500-mg Waters Sepak Plus (Millipore UK Ltd, Watford, Hertfordshire, UK) silica solid-phase extraction column as a postderivatization cleanup. Further DCM was applied to the cartridge until approximately 3 mL had been collected. The eluent was evaporated to dryness and resuspended in 250 μL DCM. The derivatized samples were analyzed on a Polaris ion trap gas chromatography–mass spectrometry (GC-MS) (Thermoquest; Thermofinnigan, San Jose, CA). The analysis conditions were as follows: sample volume, 5 μL; GC column, 30 m × 0.25 mm (i.d.), DB5-MS (Agilent J&W, Cheshire, UK); carrier gas, helium, 1.0 mL/min; injector temperature, 300°C; column program: a) 65°C for 10 min, b) increase to 250°C at 15°C/min, c) increase to 285°C at 2°C/min, d) isothermal at 284°C for 1 min. The MS was operated in the electron ionization mode and set up to carry out MS-MS experiments. Precursor and product ion values for the steroids and their deuterated analogs are given in Table 1. Quantification was based on the use of the deuterated analogs as internal standards. The limits of detection were 0.25, 0.25, and 0.15 ng/L for E2, E1, and EE2, respectively.
Table 1.
Ion trap parameters.
| Compound | Time (min) | Precursor ion (amu) | Product ions (amu) |
|---|---|---|---|
| Estrone | 32.50 | 384 | 200–390 |
| d4-Estrone | 32.50 | 388 | 200–390 |
| Estradiol | 33.45 | 458 | 300–470 |
| d4-Estradiol | 33.45 | 462 | 300–470 |
| Ethinylestradiol | 35.80 | 482 | 300–490 |
| d4-Ethinylestradiol | 35.80 | 486 | 300–490 |
For chemical analysis of nonylphenol, 500-mL samples were spiked with 100 μL of labeled 4-n-NP, filtered using a 0.45-μm filter, and the pH of the samples was adjusted to pH 2 using 10% sulfuric acid. The samples were extracted onto a 500-mg C18 solid-phase extraction column and eluted using 2 × 50 mL DCM. The eluent was evaporated to dryness under a stream of nitrogen and resuspended in 1 mL DCM before analysis by GC-MS. The GC-MS system consisted of a HP 5980GC (Agilent Technologies UK Ltd, Wokingham, Berkshire, UK) directly coupled to a VG Trio-1 mass spectrometer (Micromass UK Ltd, Wythenshawe, Greater Manchester, UK) that was operated in the selected ion recording mode with electron impact ionization. The analysis conditions were as follows: sample volume, 1 μL (using a cool on-column injector); GC column , DB-1 30 m × 0.25 mm (i.d.) (Agilent Technologies); carrier gas, helium, 1.0 mL/min; column program: a) 30°C for 4 min, b) increase to 300°C at 8°C/min. Ions monitored were those at m/z 107, 121, 135, and 220. Quantification was based on the labeled internal standard. The limit of detection for NP was 1.0 μg/L.
In vitro versus in vivo comparison of the estrogenic potency of a WwTW final effluent.
To determine the utility of the rYES to predict the in vivo estrogenic potency of an effluent, we compared the vitellogenic response in adult fathead minnows exposed for 14 days to graded concentrations (0, 25, 50, and 100%) of a treated WwTW effluent with the potency of extracts from the effluent in the rYES. The effluent was collected from a single WwTW final effluent stream between 0800 and 1100 hr on five occasions over a 14-day period during March 2005 and transported to the testing laboratory at ambient air temperature (between 4 and 10°C) in leached plastic carboys. The effluent was stored outside at ambient temperatures (between 4 and 10°C), and 50-L subsamples were transferred, twice daily (am and pm) to an effluent tank held in the testing laboratory at 25°C. Effluent and/or dilution water was pumped via peristalsis from the effluent tank and/or from a dilution water header tank to each exposure tank (20-L glass aquaria) at a total flow rate of 20 mL/min. Each treatment comprised two replicate tanks, each containing eight adult male and eight adult female fathead minnows. All tanks were gently aerated to ensure that dissolved oxygen concentrations were maintained above 70%. At 0800 hr each day, before the introduction of fresh subsamples of the effluent, water/effluent samples (700 mL each) were removed from each exposure tank (and from the effluent holding tank) and extracted onto primed C18 solid-phase extraction columns for assessment of estrogenic activity in the rYES, following the procedures described above. At the end of the 14-day exposure period, the fish were sacrificed using a lethal dose of anesthetic, blood was sampled via cardiac puncture, and plasma samples were analyzed for VTG concentrations, using a carp enzyme-linked immunosorbent assay (ELISA) (Tyler et al. 1999).
Mathematical modeling
For the description of the in vitro and in vivo concentration–response relationships for the individual test compounds and for the binary mixtures, a four-parameter logit regression model was used, as shown in Equation 1:
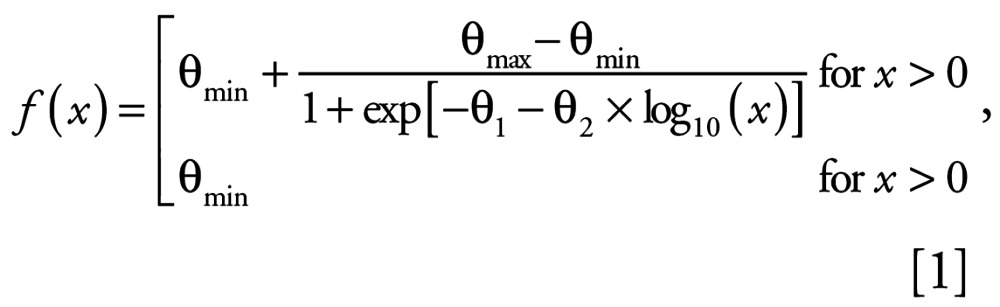 |
where x = concentration and f(x) = mean effect. The model parameter θmin describes the minimal mean effect (control response), θmax describes the asymptotical maximal effect, θ1 is termed the “location” parameter, and θ2 characterizes the “steepness” of the concentration–response relationship. Because of heterogeneous nonrandom variabilities in the replicated data (heteroscedasticity), each model was fitted using the estimation method of generalized least squares (Scholze et al. 2001). For the in vivo studies, to fulfill the statistical prerequisite of symmetrically distributed effect data for this estimation method, the plasma VTG concentrations were log10-transformed. The mean effect f (x) in Equation 1, for the in vivo data, therefore, always corresponds to the log10-transformed VTG concentration. Effect concentrations were determined on the basis of the estimated regression Equation 1 by its functional inverse as Equation 2:
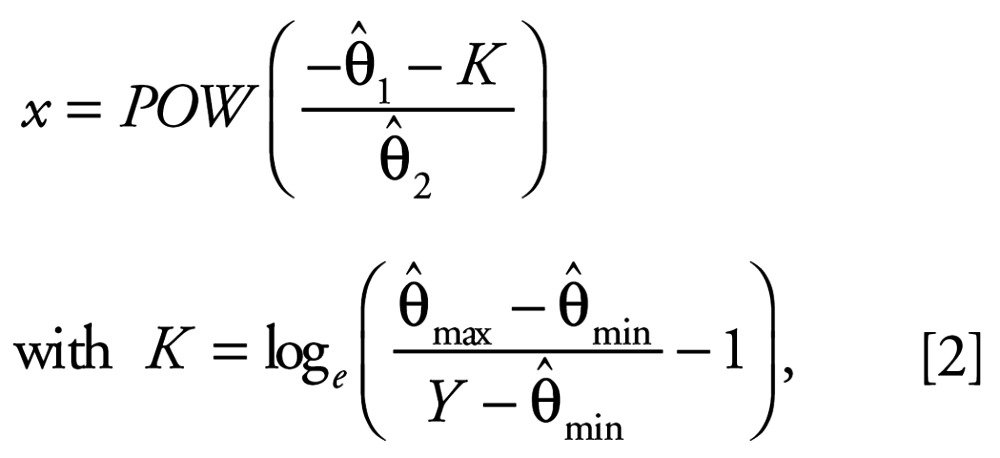 |
where Y is a given effect, POW(t) is 10 raised to the power t, and
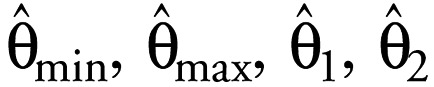 are the estimates of the unknown model parameters θmin, θmax, θ1, and θ2. The median effect is defined as the average between the mean control and the mean effect produced by the highest tested concentration of E2. Relative estrogenic potencies were calculated for the individual reference chemicals by dividing the median effect concentration for E2 by the concentration of each chemical (determined using Equation 2) required to cause the same level of effect.
are the estimates of the unknown model parameters θmin, θmax, θ1, and θ2. The median effect is defined as the average between the mean control and the mean effect produced by the highest tested concentration of E2. Relative estrogenic potencies were calculated for the individual reference chemicals by dividing the median effect concentration for E2 by the concentration of each chemical (determined using Equation 2) required to cause the same level of effect.
The model of CA was used to model the theoretical concentration response relationship for the fixed-ratio binary mixtures (Loewe 1953). The model of CA is based on the assumption that chemicals act via a similar mechanism to elicit an effect, such that one chemical acts as a dilution of the other and can be substituted at a constant proportion for the other. This model is usually defined for a binary mixture of substances 1 and 2 by Equation 3:
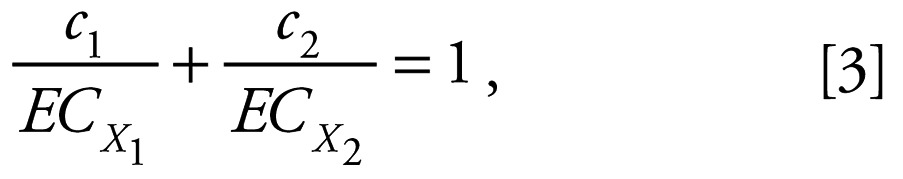 |
where c1 and c2 are the individual concentrations of the substances 1 and 2 constituting the mixture that produces an effect x, and ECx1 and ECx2 denote the equivalent effect concentrations of the single substances 1 and 2 that alone would produce the same effect x as the mixture. The sum of c1 and c2 equals the total concentration that produces the combined effect x, i.e., ECxmixture; therefore, the individual concentrations c1 and c2 can be expressed as proportions p1 and p2 of the total concentration, i.e., p1 = c1/ECxmixture and p2 =c2/ECxmixture. Equation 3 can, therefore, be rearranged as:
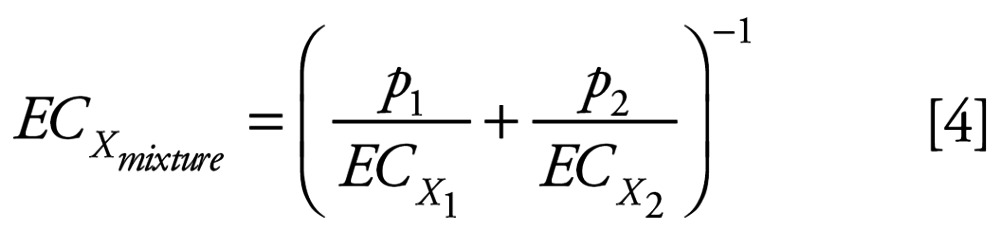 |
The individual effect concentrations ECx1 and ECx2 can be derived from Equation 2 on the basis of the estimated regression functions (Equation 1). Holding the ratio p1:p2 fixed, the calculations can be performed for different given combined effects x (assuming that the corresponding effect concentrations of the individual components exist), thereby leading to a graph of the concentration effect curve for the mixture. This estimated concentration response relationship is then compared with the observed concentration response for the experimental mixture.
For the effluent samples, E2 equivalent concentrations (EQ) were calculated by determining the dilution of effluent concentrate required to produce the median effect concentration of E2. For less potent effluents, the concentration of E2 required to produce the level of effect observed for the highest concentration of effluent (100% of a 750-fold concentrate) tested was determined.
Results and Discussion
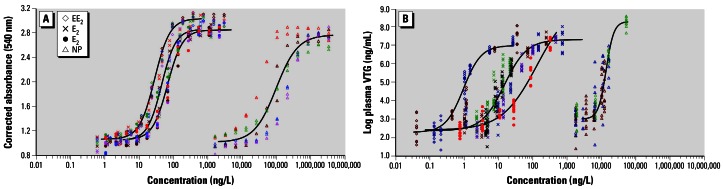
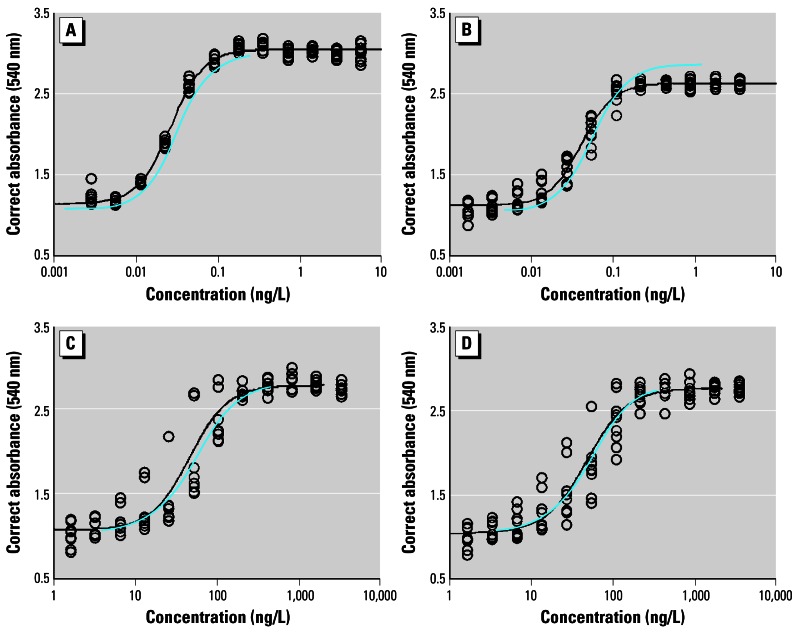
The primary aim of these investigations was to evaluate whether the estrogenic effects of treated WwTW effluents in the United Kingdom could be predicted using a simple mathematical model, the model of CA. To predict the estrogenic activity of a chemical mixture, it is necessary to have an understanding of the biological potency of the individual chemicals present in the effluents that are contributing to the estrogenic activity, as well as an expression of how the potency of the individual estrogenic chemicals is modified or integrated when in a mixture. In an initial series of investigations, potency estimates were derived for the four principal estrogenic chemicals of interest, E2, E1, EE2, and NP in the in vitro rYES. Full concentration–response curves were obtained for all of the chemicals (Figure 1A), and the median effect concentrations (EC50 values) were determined. Ethinylestradiol was the most potent chemical tested, followed by E2, then E1, and last, NP, with respective EC50 values of 21.2, 37.7, 55.3, and 81045 ng/L. The relative estrogenic potencies of 1.8, 0.68, and 0.00047 for EE2, E1, and NP, respectively, compared with those of E2 are consistent with those reported by others using the rYES (Céspedes et al. 2004; Van den Belt et al. 2004). These potency estimates were used to calculate concentration-response curves for binary combinations of E2, E1, EE2, and NP, using the model of CA. The calculated curves were compared with measured responses for each binary mixture in the rYES (examples are shown in Figure 2) to determine the accuracy of the calculations. For all of the binary combinations, the calculated curves were within the range of estrogenic responses observed for each mixture concentration tested, thereby demonstrating that the model of CA can be used to accurately predict the effects of binary mixtures of these estrogenic chemicals in the rYES. This finding is consistent with the results of earlier investigations, where it was shown that the model of CA could be applied to assess the effects of binary mixtures of these estrogens on the in vivo induction of VTG in rainbow trout (Thorpe et al. 2001, 2003). Other investigators have demonstrated that the model of CA can similarly be applied to other estrogenic chemicals to calculate responses for mixtures of up to eight estrogenic chemicals in the rYES (Payne et al. 2000; Silva et al. 2002) and five estrogenic chemicals in vivo (Brian et al. 2005). These findings further support the use of this model for predicting the mixture effects of estrogens.
Figure 1.
Concentration–response curves for EE2, E2, E1, and NP obtained in a (A) recombinant yeast estrogen screen, and (B) 14-day juvenile rainbow trout screening assay (VTG induction). The different colored symbols represent the individual responses at each concentration in a series of independent experiments for each chemical. Black regression lines for each chemical were calculated using a four-parameter logit regression model and are based on the results of five in vitro assays (each chemical tested in duplicate) and two, five, one, and three in vivo exposures conducted for EE2, E2, E1, and NP, respectively.
Figure 2.
Concentration–response curves for binary mixtures of E2 and EE2 (A), E2 and E1 (B), E2 and NP (C), and E1 and NP (D) obtained in a recombinant yeast estrogen screen. (○) Represents individual responses at each concentration. (—) Regression line showing the observed mixture response and is based on the results of five in vitro assays in which each chemical mixture was tested in duplicate. (
 ) Regression line showing the predicted response for each mixture based on the concentration–response curves in Figure 1 for each individual chemical. All lines were calculated using a four-parameter logit regression model.
) Regression line showing the predicted response for each mixture based on the concentration–response curves in Figure 1 for each individual chemical. All lines were calculated using a four-parameter logit regression model.
Applying the model of CA to predict the estrogenic potency of a real-world chemical mixture such as WwTW effluent is more challenging, given the complex chemical matrices involved. To investigate this area, concentrated effluent extracts from 43 WwTW in the United Kingdom were chemically analyzed for the presence of the four estrogens, E2, EE2, E1, and NP, and predicted E2 EQ concentrations for each effluent were calculated using the model of CA. To assess the applicability of the model of CA for calculating the estrogenic effects of the effluents, we compared the calculated E2 EQ concentrations for each effluent with the estrogenic potencies of effluent extracts measured in the rYES.
The estrogens were present in the effluents studied at the following concentrations; E1, between 0.25 and 87 ng/L; NP, between 1,000 and 6,750 ng/L; E2, between 0.25 and 20 ng/L; and EE 2 , between 0.15 and 2.85 ng/L. These concentrations of environmental estrogens are similar to those reported previously for effluents from WwTW in the United Kingdom (Desbrow et al. 1998; Rodgers-Gray et al. 2000). The ratios of the measured concentrations of the chemicals present in each effluent, and the estrogenic potency estimates derived for the individual chemicals in the rYES, were used in the model of CA to calculate a concentration-response curve for each effluent extract. Through comparison of the calculated curve with a reference E2 standard curve, theoretical E2 EQ concentrations could be estimated for each effluent. As an example, in one effluent sample, the measured concentrations of E1, E2, EE2, and NP were 70, 14, 0.83, and 2,850 ng/L, respectively. Therefore, the total estrogen concentration was 2,922 ng/L, and the proportions of E1, E2, EE2, and NP in the mixture were 0.024, 0.00050, 0.00028, and 0.98, respectively. Through comparison of the calculated concentration–response curve for this specific effluent with the reference E2 standard curve, it was predicted that the total concentration of estrogens present in this effluent would produce an absorbance of 2.06 nm in the rYES. This absorbance is equivalent to the absorbance produced by an E2 concentration of 50 ng/L.
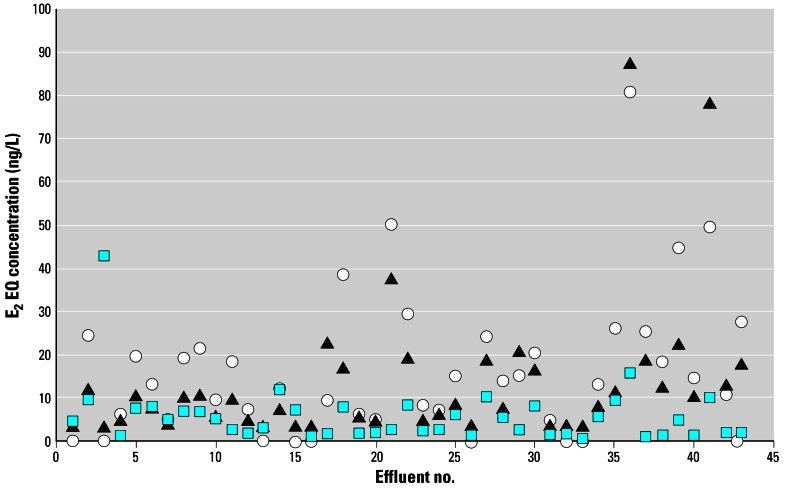
This approach was also repeated to obtain predicted E2 EQ concentrations based on the in vivo relative potencies of the individual chemicals for the induction of VTG in juvenile rainbow trout (Figure 1B). Using this approach for each effluent sample, E2 EQ concentrations were predicted to range from < 5 to 81 ng/L in the rYES and from < 5 to 87 ng/L for the induction of VTG (Figure 3). A very good correlation was observed between the in vitro and in vivo predictions (R2 = 0.75, p < 0.05). However, for 70% of the effluents tested, the in vitro predictions tended to be higher than the in vivo predictions (range 1 to 2.3-fold higher, average 1.5-fold). These variations in the in vitro and in vivo predictions likely result from differences in the estrogenic potencies of the individual chemicals in the two assays, particularly the higher relative in vitro potency of E1, the predominant steroid measured in most of the WwTW effluents, compared with its potency in vivo (Figure 1). The predicted E2 EQ concentrations for the 43 effluents are consistent with levels of estrogenic activity that have been reported for WwTW effluents across Europe tested in the rYES (Aerni et al. 2004; Desbrow et al. 1998; Murk et al. 2002; Pawlowski et al. 2003; Rutishauser et al. 2004; Witters et al. 2001). The predicted E2 EQ concentrations are also representative of levels of VTG induction that have been previously observed in U.K. effluents (Harries et al. 1996, 1999; Purdom et al. 1994), and they equate to plasma VTG concentrations that range from 1 to 8,000 μg VTG/mL of plasma in rainbow trout. This result indicates that the modeling approach does provide a realistic representation of estrogenic activity in WwTW effluents and that it predicts E2 EQ concentrations that are within the same order of magnitude as those that are reported in the environment.
Figure 3.
Comparison of calculated and measured E2 EQs determined for final WwTW effluents sampled from 43 locations across England and Wales. (
 ) Represents the measured E2 EQs in the recombinant yeast screen. (○, ▵) Represent the predicted E2 EQs calculated based on the measured concentrations of the four target estrogenic chemicals in the individual effluents and their relative estrogenic potencies in the recombinant yeast screen (○) and for the induction of VTG in juvenile rainbow trout (▴). Both the measured and predicted responses are based on results from two sampling occasions for each effluent.
) Represents the measured E2 EQs in the recombinant yeast screen. (○, ▵) Represent the predicted E2 EQs calculated based on the measured concentrations of the four target estrogenic chemicals in the individual effluents and their relative estrogenic potencies in the recombinant yeast screen (○) and for the induction of VTG in juvenile rainbow trout (▴). Both the measured and predicted responses are based on results from two sampling occasions for each effluent.
To examine further the accuracy of the predicted E2 EQ concentrations, we tested the effluent extracts in the rYES to obtain measured E2 EQ concentrations. Ideally, these comparisons would also have been conducted in vivo, but because of the time and expense involved in conducting such in vivo exposures, it was not practicable to do so. The measured E2 EQ concentrations in the rYES for all the 43 effluents tested were generally lower than those calculated using the model of CA and ranged between 0.65 and 43 ng E2 EQ/L (Figure 3). A positive correlation (R2 = 0.349, p < 0.05) was observed between the calculated versus measured E2 EQ concentrations in the rYES, but the two values aligned closely only in two cases. In general, the calculated E2 EQ concentrations were higher than those measured in the rYES, by between 2- and 3-fold for 16 of the 43 effluents tested, by 3- to 6-fold for 11 effluents, and by between 7- and 24-fold for 6 effluents.
Aerni et al. (2004) also reported a positive correlation between calculated and measured E2 EQ concentrations in a survey of the estrogenic potency of five WwTW in mainland Europe, but as observed here, they also found that the calculated E2 EQ concentrations tended to overestimate the E2 EQ concentrations in the rYES. Similarly, Rutishauser et al. (2004) found that although calculated and measured E2 EQ concentrations were highly consistent for effluent samples from one WwTW, calculated E2 EQs were approximately 5-fold higher than measured E2 EQs on three of four sampling occasions for a second WwTW. Differences in the calculated versus measured E2 EQ concentrations were also reported in a survey of estrogenic activity of WwTW effluent discharges in Japan (Tanaka et al. 2001), with the calculated E2 EQ concentrations overestimating the measured estrogenic activity in 50% of the samples studied. In their investigation Tanaka et al. (2001) also found that in a number of the WwTW studied, the estrogenic activity was higher in the rYES than predicted based on the measured chemical concentrations. Pawlowski et al. (2003) also reported a lower calculated estrogenic activity, based on chemical concentrations, compared with measured estrogenicity in the rYES for two WwTW effluents in Germany. In our investigation, although calculated E2 EQ concentrations were generally higher than those measured in the rYES, for eight of the effluents studied the opposite was observed. No estrogenic activity was expected for these eight effluents, based on the measured concentrations of the four estrogens, yet E2 EQ concentrations measured in the rYES ranged between 0.65 and 7.2 ng E2 EQ/L for seven of the effluents and measured 43 ng/L for the remaining effluent. Although this latter response deviates considerably from all the other comparisons for the analytical chemistry versus the rYES data, both the analytical chemistry data and rYES responses were highly consistent for the two spot samples taken at the different sampling times, thus indicating that they were true representations of the estrogenic profile for this WwTW effluent, using the different techniques.
The differences observed between the calculated and measured estrogenic potencies of the WwTW effluents studied demonstrate a weakness of the modeling approach for predicting the estrogenic potency of complex mixtures. This deficiency is not caused by a failure of the CA model itself, and indeed, the results of the initial investigations demonstrated that the model could accurately predict the estrogenic effects of defined chemical mixtures. The weakness of the modeling approach when applied to environmental samples arises more as a consequence (at least in part) of an incomplete knowledge of the chemical composition of the WwTW effluent and constraints caused by uncertainties in the accuracy of the data that are input into the model.
The analyses in this investigation focused on measurements of only four estrogenic chemicals; however, other estrogenic compounds that are capable of invoking an estrogenic response (e.g., estriol and other steroidal estrogens and conjugates of steroidal estrogens, phytoestrogens, and industrial chemicals such as bisphenol A, phathalates) have been shown to occur in some WwTW effluents (Aerni et al. 2004; Liney et al. 2005; Pawlowski et al. 2003; Ruithauser et al. 2004; Tanaka et al. 2001). Similarly, the presence of estrogen receptor antagonists in effluents and/or other compounds that inhibit a part of the estrogen responsive pathway (e.g., the activation of the estrogen response elements) may alter the estrogenic potency of the mixture and could lead to a reduction in the estrogenic activity. This possibility is supported by recent measurements of antiestrogenic activity in the rYES in some U.K. WwTW effluents (E. Hill, personal communication). Additionally, any uncertainties in the analytical procedures used for the measurements of the estrogenic chemicals would result in uncertainties in the accuracy of the predictions. In this investigation, mean recoveries of the steroidal estrogens in the analytical procedures were 77.8% for E2, 88.2% for E1, and 92.5% for EE2. However, in some cases, quantifiable peaks for either the inherent steroids or the internal standards present in the samples were not identified. This lack of identification was particularly true for the quantification of EE2, thus leading to some uncertainty for some of the measurements of EE2 and the other steroidal estrogens.
Other investigators have also reported a high degree of uncertainty in the measurement of steroidal estrogens in WwTW effluents (Aerni et al. 2004; Ruithauser et al. 2004). Uncertainties in the quantification of some of the steroidal estrogens are likely to have contributed, at least in part, to some of the differences seen here between the calculated and measured E2 EQ concentrations. There was also some degree of uncertainty in the quantification of NP in the WwTW effluents. The mean recovery of NP was good at 109%, but the high limit of quantification (1.0 μg/L) for NP may have resulted in an overestimation of NP concentrations in the effluent samples where concentrations were at or below this limit. In contrast with the steroidal estrogens, however, the contribution of NP to the estrogenic activity of the effluent samples was relatively minor. Such uncertainties regarding the analysis of NP would not affect the mixture analyses and did not contribute to the differences between the calculated and measured E2 EQ concentrations in this investigation.
In the analysis of the data sets thus far, the focus has been on the limitations surrounding the modeling approach, with the assumption that the measured E2 EQ concentrations in the rYES are accurate. There was no evidence to suggest otherwise in this investigation. The rYES was highly reproducible, and the results of the standard reference estrogen were comparable with historical data sets both within our laboratory (unpublished data) and at others (Aerni et al. 2004; Routledge and Sumpter 1996). No loss of total estrogenic activity as a consequence of interaction between the chemicals within the rYES, or during the extraction procedures was observed. Also, the estrogenic activity of the HPLC-grade water samples spiked with a mixture of the four estrogens compared favorably with the predicted activity for the mixture of these estrogenic chemicals. Similarly, there was no evidence of a loss of estrogenic activity during the extraction procedure for effluent samples spiked with a mixture of the four estrogens, and recovery of total estrogenic activity was between 113 and 124%. This result suggests that the measured rYES responses are reliable. It also indicates that the differences between the calculated and measured E2 EQ concentrations are most likely a consequence of the limitations in the modeling procedure caused by an incomplete knowledge of the chemical composition of the mixture and/or uncertainties in the concentrations of the measured estrogens. This finding highlights the need for further work to improve the detection limits for estrogenic chemicals in complex chemical matrices and to identify other chemicals that may be present in WwTW effluents that are able to alter the estrogenic response. Until these issues have been more fully addressed, predictions of the estrogenic activity of WwTW and other complex mixtures, based on a modeling approach, should be treated with caution. This conclusion is especially true for effluents where no estrogenic activity is predicted, based on concentrations of the individual estrogenic chemicals measured, yet measurable estrogenic activity is detected in the rYES.
Given the limitations of a modeling approach for assessing the estrogenic activity of complex mixtures of chemicals, it is valuable to consider other methods such as the empirical method in which the biological effects of the mixture are evaluated as a single entity. Ideally, any assessments of the estrogenic activity of a complex mixture such as WwTW effluents would be conducted using long-term in vivo exposures that assess the potential of chemicals to bioaccumulate within the organism and that incorporate population-relevant end points such as reproduction. Conducting such exposures, however, is time consuming and expensive and is not practical or ethical given the desire to test very large numbers of WwTW effluents. Rather, priority is given to rapid and cost-effective testing methods for the assessment of the estrogenic activity of WwTW effluents. The rYES offers considerable value in this respect; however, from the perspective of environmental risk assessment, it is important to understand how in vitro effect measurements relate to effects in vivo.
To evaluate the rYES as a method for assessing the estrogenic potency of a WwTW effluent, estrogenic responses in the rYES were compared with the vitellogenic response in adult male fathead minnows exposed to effluent from a single WwTW (Figure 4). Effluent dilutions of 25, 50, and 100% induced a concentration-dependent (p < 0.05) increase in plasma concentrations of VTG in male fathead minnows exposed for 14 days under flow-through conditions. Plasma VTG concentrations were elevated by 8-, 341- and 5,267-fold, in the 25, 50, and 100% effluent treatments respectively, compared with concentrations in control males (27 ng/mL). The resulting plasma VTG concentrations (204, 9,208, and 142,200 ng/mL, for the effluent concentrations of 25, 50, and 100%, respectively) equated to E2 EQ concentrations of 18.5, 26.2, and 36.6 ng/L, respectively, based on the response of males exposed to E2 for 14 days (data not shown). Daily assessments of the estrogenic potency of the effluent in the individual exposure tanks using the rYES produced E2 EQ concentrations of 10.6 ± 0.75, 13.7 ± 0.93, and 17.9 ± 0.94 ng/L (Figure 4). Detectable estrogenic activity (4.9 ± 0.94 ng/L) was also measured in the dilution water control tank and was most likely caused by the excretion of natural estrogens by the mature females that were held with the males in the test vessels. There was no evidence, however, that these concentrations were sufficient to induce a vitellogenic response, and concentrations of plasma VTG in the control males were consistent with those normally measured in adult fathead minnows using the carp VTG ELISA (Panter et al. 2002).
Figure 4.
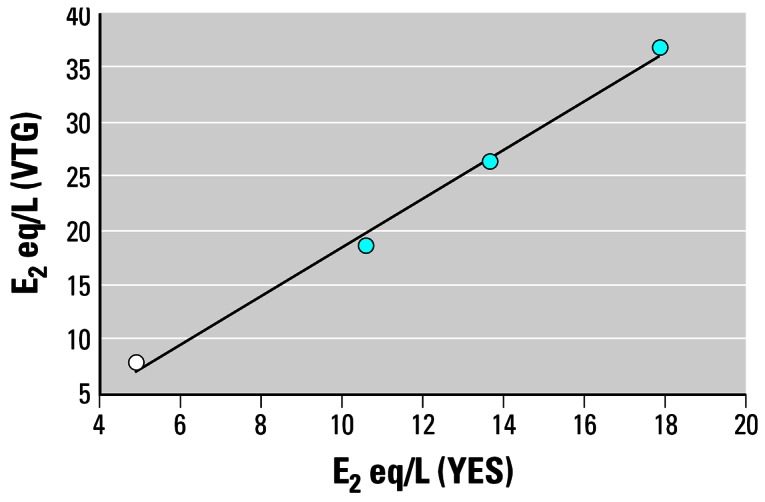
Estradiol equivalent concentrations measured using an in vitro recombinant yeast screen versus a short-term in vivo 14-day vitellogenic induction screen using the fathead minnow for tank water samples receiving either dilution water (○) or graded dilutions of a WwTW effluent (
 ). y = 2.217x – 3.8316; R2 = 0.9941.
). y = 2.217x – 3.8316; R2 = 0.9941.
These results indicate that the rYES underestimates the estrogenic activity by 2-fold compared with the potency of the effluent for VTG induction in vivo. However, in our daily analysis of the estrogenic activity in the effluent holding tank, E2 EQ concentrations were measured at 40 ng/L in the rYES, which is comparable to that obtained based on the VTG response for the 100% effluent exposure (36.6 ng E2 EQ/L). The apparent lower level of estrogenic activity in the fish exposure tanks as measured in the rYES may be a consequence of uptake of the estrogens by the fish and/or degradation of estrogen by microorganisms within the tanks or by the fish themselves. Nevertheless, this study did show that the rYES was reasonably accurate as an indicator of the in vivo estrogenic potency (for VTG induction in relatively short-term exposures) of a WwTW effluent. This finding supports the results of earlier investigations that have shown a good correlation between the induction of VTG in rainbow trout (Pawlowski et al. 2003; Tyler et al. 2005) and channel catfish (Tilton et al. 2002) exposed in situ to WwTW effluents and the estrogenic potency of extracts from the WwTW effluent in the rYES.
It should be noted, however, that deviations in the rYES compared with in vivo induction of VTG have also been reported. In one study using the Japanese medaka, the rYES was found to underestimate the estrogenic activity of a WwTW effluent by 35-fold compared with the in vivo induction of VTG (Huggett et al. 2003). In another investigation, the rYES overpredicted the estrogenic activity of WwTW effluents and three of the five effluents that were estrogenic in the rYES did not induce vitellogenesis in male rainbow trout (Aerni et al. 2004). These data sets indicate that potency estimates derived using the rYES may not always be directly comparable to the in vivo potency of an effluent. Interpretations of the estrogenic potency of chemicals and their mixtures in fish, however, are influenced by the fish species and life stage used as well as by the duration of exposure and the sensitivity of the immunoassay used to quantify concentrations of VTG. Differences in results among the studies reported likely include differences in these parameters rather than discrepancies caused by the rYES alone. Further assessments on the estrogenic potency of WwTW effluents as determined in the rYES versus induction of VTG in vivo would serve to reinforce the utility of the rYES assay for predicting in vivo effects. Collectively, however, the available data indicate that unlike the modeling procedure, the rYES does not produce false negatives. Thus, the rYES has considerable value in the assessment of WwTW as an initial screening system to identify WwTW effluents for subsequent testing in vivo.
The results from the investigations with the rYES demonstrate that the accuracy of the model of CA for predicting estrogenic activity is critically dependent on knowledge and accurate quantification of the chemical composition of the mixture. For synthesized chemical mixtures, the model of CA accurately calculates the estrogenic effects of the mixture. However, for more complex real-world mixtures, the calculations become less accurate, probably because of the presence of other chemicals in the mixture that may interfere with the estrogenic response of the target estrogens and/or because of uncertainties in the measurements of the chemical concentrations themselves in complex mixtures. The CA model is, therefore, currently of limited value in predicting the estrogenic effects of more complex environmental mixtures. It should also be realized that the mixture analyses conducted to date for endocrine-active chemicals have focused on the effects of estrogens only, and then only in simple in vitro assays or on the relatively simple process of induction of VTG in vivo. Applying models to predict mixture effects of estrogens and/or other chemicals that act on different pathways within the endocrine system, on the more integrative processes of growth, development, and reproduction (those with population-level relevance) is arguably not practicable given the complexities of the systems mediating these biological processes.
Given the difficulties highlighted in this study for simple mixtures of estrogens, we propose that an empirical approach, which considers the biological activity of the whole effluent, is a more appropriate way to determine the integrative effects of estrogenic (and other endocrine) chemicals in WwTW effluents. The good correlation observed between the measured E2 EQ concentrations determined using the rYES compared with those derived based on the induction of VTG in male fat-head minnows in a short-term exposure experiment supports the use of the rYES as a rapid and cost-effective assay for quantifying estrogenic activity in WwTW effluents, following an empirical approach.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
This work was sponsored by the U.K. Environment Agency and the University of Exeter.
The work was also sponsored by AstraZeneca, a pharmaceutical company. I.J. and M.G-S. are employed by WRc plc, a research and consulting company. The remaining authors declare they have no competing financial interests.
References
- Aerni H-R, Kobler B, Rutishauser BV, Wettstein FE, Fischer R, Giger W, et al. Combined biological and chemical assessment of estrogenic activities in wastewater treatment plant effluents. Anal Bioanal Chem. 2004;378:688–696. doi: 10.1007/s00216-003-2276-4. [DOI] [PubMed] [Google Scholar]
- Alabaster JS, Calamari D, Dethlefsen V, Konemann H, Lloyd R, Solbe JF. EIFAC working party on water quality criteria for European freshwater fish. Mixtures of toxicants. Environ Topics. 1994;6:145–205. [Google Scholar]
- Ankley GT, Mount DR. Retrospective analysis of the ecological risk of contaminant mixtures in aquatic sediments. Hum Ecol Risk Assess. 1996;2:434–440. [Google Scholar]
- Bailey HC, Miller JL, Miller MJ, Wiborg LC, Deanovic L, Shed T. Joint acute toxicity of diazinon and chlorpyrifos to Ceriodaphnia dubia. Environ Toxicol Chem. 1997;16:2304–2308. [Google Scholar]
- Belfroid AC, van der Horst A, Vethaak AD, Schäfer AJ, Rijs GBJ, Wegener J, et al. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste-water in The Netherlands. Sci Total Environ. 1999;225:101–108. doi: 10.1016/s0048-9697(98)00336-2. [DOI] [PubMed] [Google Scholar]
- Brian JV, Harries CA, Scholze M, Backhaus T, Booy P, Lamoree M, et al. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect. 2005;113:721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargouët M, Perdiz D, Mouatassim-Souali A, Tamisier-Karolak S, Levi Y. Assessment of river contamination by estrogenic compounds in Paris area (France) Sci Total Environ. 2004;324:55–66. doi: 10.1016/j.scitotenv.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Céspedes R, Petrovic M, Ralúda D, Saura Ú, Piña B, Lacorte S, et al. Integrated procedure for determination of endocrine-disrupting activity in surface waters and sediments by use of the biological technique recombinant yeast assay and chemical analysis by LC-ESI-MS. Anal Bioanal Chem. 2004;378:697–708. doi: 10.1007/s00216-003-2303-5. [DOI] [PubMed] [Google Scholar]
- Desbrow C, Routledge EJ, Brighty GC, Sumpter JP, Waldock M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol. 1998;32:1549–1558. [Google Scholar]
- European Inland Fisheries Advisory Commission. 1987. Revised Report on Combined Effects on Freshwater Fish and Other Aquatic Life. EIFAC Technical Paper 37, Rev. 1. Rome:Food and Agriculture Organisation (FAO).
- Harries JE, Janbakhsh A, Jobling S, Matthiessen P, Sumpter JP, Tyler CR. Estrogenic potency of effluent from two sewage treatment works in the United Kingdom. Environ Toxicol Chem. 1999;18:932–937. [Google Scholar]
- Harries JE, Sheahan DA, Jobling S, Matthiessen P, Neall P, Routledge EJ, et al. A survey of estrogenic activity in United Kingdom inland waters. Environ Toxicol Chem. 1996;15:1993–2002. [Google Scholar]
- Huggett DB, Foran CM, Brooks BW, Weston J, Peterson B, Marsh KE, et al. Comparison of in vitro and in vivo bioassays for estrogenicity in effluent from North American municipal wastewater facilities. Toxicol Sci. 2003;72:77–83. doi: 10.1093/toxsci/kfg017. [DOI] [PubMed] [Google Scholar]
- Liney KE, Jobling S, Shears J, Simpson P, Tyler CR. Assessing the sensitivity of different life stages for sexual disruption in roach (Rutilus rutilus) exposed to effluents from wastewater treatment works. Environ Health Perspect. 2005;113:1299–1307. doi: 10.1289/ehp.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–290. [PubMed] [Google Scholar]
- Matthiessen P, Whale G, Rycroft RJ, Sheahan DA. The joint toxicity of pesticide tank-mixes to rainbow trout. Aquat Toxicol. 1988;13:61–76. [Google Scholar]
- Murk AJ, Legler J, van Lipzig MMH, Meerman JHN, Belfroid AC, Spenkelink A, et al. Detection of estrogenic potency in wastewater and surface water with three in vitro bioassays. Environ Toxicol Chem. 2002;21:16–23. [PubMed] [Google Scholar]
- Panter GH, Hutchinson TH, Lange R, Lye CM, Sumpter JP, Zerulla M, et al. Utility of a juvenile fathead minnow screening assay for detecting (anti-)estrogenic substances. Environ Toxicol Chem. 2002;21:319–326. [PubMed] [Google Scholar]
- Pawlowski S, Ternes T, Bonerz M, Kluczka T, van der Burg B, Nau H, et al. Combined in situ and in vitro assessment of the estrogenic activity of sewage and surface water samples. Toxicol Sci. 2003;75:57–65. doi: 10.1093/toxsci/kfg162. [DOI] [PubMed] [Google Scholar]
- Payne J, Rajapakse N, Wilkins M, Kortenkamp A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Environ Health Perspect. 2000;108:983–987. doi: 10.1289/ehp.00108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdom CE, Hardiman PA, Bye VJ, Eno NC, Tyler CR, Sumpter JP. Estrogenic effects of effluents from sewage treatment works. Chem Ecol. 1994;8:275–285. [Google Scholar]
- Rodgers-Gray TP, Jobling S, Morris S, Kelly C, Kirby S, Janbakhsh A, et al. Long-term temporal changes in the estrogenic composition of treated sewage effluent and its biological effects on fish. Environ Sci Technol. 2000;34:1521–1528. [Google Scholar]
- Routledge EJ, Sumpter JP. Estrogenic activity of surfactants and some of their degradation products assessed using a recombinant yeast screen. Environ Toxicol Chem. 1996;15:241–248. [Google Scholar]
- Rutishauser BV, Pesonen M, Escher BI, Ackermann GE, Aerni HR, Suter MJ, et al. Comparative analysis of estrogenic activity in sewage treatment plant effluents involving three in vitro assays and chemical analysis of steroids. Environ Toxicol Chem. 2004;23:857–864. doi: 10.1897/03-286. [DOI] [PubMed] [Google Scholar]
- Scholze M, Boedeker W, Faust M, Backhaus T, Altenburger R, Grimme LH. A general best-fit method for concentration-response curves and the estimation of low-effect concentrations. Environ Toxicol Chem. 2001;20:448–457. [PubMed] [Google Scholar]
- Scientific Committee on Problems of the Environment. 1987. Methods for Assessing the Effects of Mixtures of Chemicals. Chichester, UK:John Wiley.
- Segner H, Navas JM, Schäfers C, Wenzel A. Potencies of estrogenic compounds in in vitro screening assays and in life cycle tests with zebrafish in vivo. Ecotox Environ Saf. 2003;54:315–322. doi: 10.1016/s0147-6513(02)00040-4. [DOI] [PubMed] [Google Scholar]
- Sheahan DA, Brighty GC, Daniel M, Kirby SJ, Hurst MR, Kennedy J, et al. Estrogenic activity measured in a sewage treatment works treating industrial inputs containing high concentrations of alkylphenolic compounds—a case study. Environ Toxicol Chem. 2002;21:507–514. [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”—eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yakou Y, Takahashi A, Higashitani T, Komori K. Comparison between estrogenicities estimated from DNA recombinant yeast assay and from chemical analyses of endocrine disruptors during sewage treatment. Water Sci Technol. 2001;43:125–132. [PubMed] [Google Scholar]
- Thorpe KL, Hutchinson TH, Hetheridge MJ, Sumpter JP, Tyler CR. Development of an in vivo screening assay for estrogenic chemicals using juvenile rainbow trout (Oncorhynchus mykiss) Environ Toxicol Chem. 2000;19:2812–2820. [Google Scholar]
- Thorpe KL, Hutchinson TH, Hetheridge MJ, Scholze M, Sumpter JP, Tyler CR. Assessing the biological potency of binary mixtures of environmental estrogens using vitellogenin induction in juvenile rainbow trout (Oncorhynchus mykiss) Environ Sci Technol. 2001;35:2476–2481. doi: 10.1021/es001767u. [DOI] [PubMed] [Google Scholar]
- Thorpe KL, Cummings RI, Hutchinson TH, Scholze M, Brighty G, Sumpter JP, et al. Relative potencies and combination effects of steroidal estrogens in fish. Environ Sci Technol. 2003;37:1142–1149. doi: 10.1021/es0201348. [DOI] [PubMed] [Google Scholar]
- Tilton F, Benson WH, Schlenk D. Evaluation of estrogenic activity from a municipal wastewater treatment plant with predominantly domestic input. Aquat Toxicol. 2002;61:211–224. doi: 10.1016/s0166-445x(02)00058-9. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Spary C, Gibson R, Shears J, Santos E, Sumpter JP, et al. Accounting for differences in the vitellogenic responses of rainbow trout (Oncorhynchus mykiss) and roach (Rutilus rutilus: Cyprinidae) exposed to oestrogenic effluents from wastewater treatment works. Environ Sci Technol. 2005;39:2599–2607. doi: 10.1021/es0488939. [DOI] [PubMed] [Google Scholar]
- Tyler CR, van Aerle R, Hutchinson TH, Maddix S, Trip H. Development and validation of an anzyme-linked immunosorbent assay (ELISA) to quantify vitellogenin in the fathead minnow (Pimephales promelas) and its application to an in vivo testing system for estrogens in fish early life-stages. Environ Toxicol Chem. 1999;18:337–347. [Google Scholar]
- Van den Belt K, Berckmans P, Vangenechten C, Verheyen R, Witters H. Comparative study on the in vitro/in vivo estrogenic potencies of 17β-estradiol, estrone, 17α-ethynyl-estradiol and nonylphenol. Aquat Toxicol. 2004;66:183–195. doi: 10.1016/j.aquatox.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Walker MK, Cook PM, Butterworth BC, Zabel EW, Peterson RE. Potency of a complex mixture of polychlorinated dibenzo-p-dioxin, dibenzofuran, and biphenyl congeners, compared to 2,3,7,8-tetrachlorodibenzo-p-dioxin in causing fish early life stage mortality. Fundam Appl Toxicol. 1996;30:178–186. doi: 10.1006/faat.1996.0054. [DOI] [PubMed] [Google Scholar]
- Witters HE, Vangenechten C, Berckmans P. Detection of estrogenic activity in Flemish surface waters using an in vitro recombinant assay with yeast cells. Water Sci Technol. 2001;43:117–123. [PubMed] [Google Scholar]