Abstract
Amphibian populations are declining globally at an alarming rate. Pesticides are among a number of proposed causes for these declines. Although a sizable database examining effects of pesticides on amphibians exists, the vast majority of these studies focus on toxicological effects (lethality, external malformations, etc.) at relatively high doses (parts per million). Very few studies focus on effects such as endocrine disruption at low concentrations. Further, most studies examine exposures to single chemicals only. The present study examined nine pesticides (four herbicides, two fungicides, and three insecticides) used on cornfields in the midwestern United States. Effects of each pesticide alone (0.1 ppb) or in combination were examined. In addition, we also examined atrazine and S-metolachlor combined (0.1 or 10 ppb each) and the commercial formulation Bicep II Magnum, which contains both of these herbicides. These two pesticides were examined in combination because they are persistent throughout the year in the wild. We examined larval growth and development, sex differentiation, and immune function in leopard frogs (Rana pipiens). In a follow-up study, we also examined the effects of the nine-compound mixture on plasma corticosterone levels in male African clawed frogs (Xenopus laevis). Although some of the pesticides individually inhibited larval growth and development, the pesticide mixtures had much greater effects. Larval growth and development were retarded, but most significantly, pesticide mixtures negated or reversed the typically positive correlation between time to metamorphosis and size at metamorphosis observed in controls: exposed larvae that took longer to metamorphose were smaller than their counterparts that metamorphosed earlier. The nine-pesticide mixture also induced damage to the thymus, resulting in immunosuppression and contraction of flavobacterial meningitis. The study in X. laevis revealed that these adverse effects may be due to an increase in plasma levels of the stress hormone corticosterone. Although it cannot be determined whether all the pesticides in the mixture contribute to these adverse effects or whether some pesticides are effectors, some are enhancers, and some are neutral, the present study revealed that estimating ecological risk and the impact of pesticides on amphibians using studies that examine only single pesticides at high concentrations may lead to gross underestimations of the role of pesticides in amphibian declines.
Keywords: amphibian declines, amphibians, atrazine, corticosterone, development, endocrine disruption, growth, immunosuppression, mixtures
Increasing evidence demonstrates that chemical environmental contaminants (including many pesticides) can act as endocrine disruptors in humans and wildlife (Cavieres et al. 2002; Choi and Jeung 2002; Colborn 1994; Diel et al. 2004; Duft et al. 2003; Fenner-Crisp 1997; Harvey et al. 2002, 2004; Hayes 1997b, 1998, 2000b, 2005; Hayes et al. 1997; Hofmeister et al. 2004; Kirby et al. 2004; Kleinkauf et al. 2004; Lutz and Kloas 1999; Masutomi et al. 2004; Michallet-Ferrier et al. 2004; Mueller 2004; Nejaty et al. 2001; Noriega and Hayes 2000; Palmer et al. 1998; Rawlings et al. 1998; Sohoni and Sumpter 1998; Sonnenschein and Soto 1998; Uzumcu et al. 2004). The impact of endocrine-disrupting chemicals in the environment is of special concern in amphibians, which are declining globally (Adams 1999; Alford and Richards 1999; Berger et al. 1998; Bishop et al. 1999; Blaustein and Kiesecker 2002; Blaustein and Wake 1990; Bridges and Semlitsch 2005; Burrowes and Green 2004; Carey et al. 1999; Daniels 2003; Davidson et al. 2001, 2002; Delis et al. 1996; Green 2003; Hayes 1997a; Kiesecker et al. 2001; LeNoir et al. 1999; Licht and Grant 1997; Lips et al. 2004; Mazzoni et al. 2003; Muths et al. 2003; Renner 2002; Rollins-Smith et al. 2002; Schmidt 2003; Storfer 2003; Stuart et al. 2004; Wang et al. 2004), but the role of pesticides in this decline is not clear (Bishop et al. 1999; Bridges and Semlitsch 2005; Daniels 2003; Davidson et al. 2001, 2002; Gendron et al. 2003; Hayes 1997a, 1998, 2004, 2005; Hayes et al. 1997, 2002a, 2002b, 2002c, 2006; Kiesecker et al. 2001; LeNoir et al. 1999; Pickford and Morris 1999; Withgott 2002). In addition to having highly permeable skin (which makes amphibians particularly vulnerable to chemical contaminants), amphibians also typically reproduce and pass through critical hormone-regulated developmental stages while in the aquatic environment. Performing these important aspects of the life cycle in habitats contaminated with endocrine-disrupting chemicals may have significant effects on individuals and populations.
Of the known pesticides that act as endocrine disruptors in wildlife, the herbicide atrazine is of special concern because it is a ubiquitous, persistent contaminant of ground-water and surface water that is active at low, ecologically relevant concentrations. As a result, atrazine is also the best-examined endocrine disruptor in amphibians. Atrazine is rarely applied alone in its agricultural use, but rather is used in combination with a number of other pesticides (herbicides, insecticides, and fungicides) that may have their own effects and that may interact in various ways with atrazine.
In the present study, we examined the effects of a realistic pesticide mixture composed of chemicals applied to cornfields in York County, Nebraska. We examined the effects of four herbicides (atrazine, metolachlor, alachlor, and nicosulfuron), three insecticides (cyfluthrin, cyhalothrin, and tebupirimphos), and two fungicides (metalaxyl and propiconizole) alone or in two combinations observed in the wild (Hayes et al. 2002b). We examined size at metamorphosis, time to metamorphosis, and gonadal differentiation in the northern leopard frog (Rana pipiens), a species that is exposed to this mixture in the wild. Because of an unexpected increase in disease contraction, we examined thymus histology as a measure of immunocompetence in R. pipiens. We also examined plasma levels of corticosterone in adult African clawed frogs (Xenopus laevis) exposed to this same mixture to explore a possible mechanism underlying the observed immunosuppressive effects.
Materials and Methods
Materials
Atrazine, alachlor, nicosulfuron, cyfluthrin, λ-cyhalothrin, tebupirimphos, metalaxyl, and propiconizole were purchased from Chem Service, Inc. (Chester, PA) and were ≥ 98% pure (except tebupirimphos, which was 97%). S-metolachlor and the commercial atrazine–metolachlor preparation Bicep II Magnum were gifts from Syngenta Crop Protection U.S. (Research Triangle Park, NC; see below for purity). Except where indicated, all other reagents were obtained from Sigma Chemical Co. (St. Louis, MO).
Experiment 1: Effects of Pesticides on Larval Rana pipiens
Animal care for laboratory studies
Adult R. pipiens for breeding were purchased from Charles D. Sullivan Co. Inc. (Nashville, TN). Frogs were reportedly obtained from populations within 200 miles of Boston, Massachusetts (Sullivan C, personal communication). Fertilized eggs were obtained by injecting three males and three females with a gonadotropin-releasing hormone agonist des-Gly10 [d-His (Bzl)6 luteninizing hormone releasing–hormone ethylamide] as described previously (Hayes et al. 1993) and allowing paired animals to breed. Eggs were maintained in 0.1× Holtfreter’s (i.e., 10%) solution (Holtfreter 1931), and after hatching, the free-swimming larvae (Gosner stage 21; Gosner 1960) were apportioned into rearing tanks (plastic mouse boxes). Larvae (30 per tank) were reared in 4 L of aerated 0.1× Holtfreter’s solution and fed Purina rabbit chow (Purina Mills, St. Louis, MO). Tanks were covered throughout the experiment, and food levels were adjusted as larvae grew to maximize growth. Experiments were conducted at 22–23°C with a 12/12-hr light/dark cycle (lights on at 0600 hr).
Larval laboratory exposures
Larvae were treated by immersion with 0.1 ppb each atrazine, S-metolachlor, alachlor, nicosulfuron, cyfluthrin, λ-cyhalothrin, tebupirimphos, metalaxyl, or propiconizole. These pesticides represented pesticides used on a cornfield in York County, NE (97°22.38 N, 40°55.88 W) (Hayes et al. 2002b) in the years 2000–2001. We also tested a mixture of all nine pesticides (0.1 ppb each or 10 ppb each), which represented the mixture applied to the cornfield in 2000–2001. Further, we examined a two-compound mixture (atrazine + S-metolachlor; tested at 0.1 ppb or 10 ppb each; technical-grade S-metolachlor obtained from Syngenta Crop Protection Inc. was reportedly 88% S-metolachlor/12% R-metolachlor), which represented ecologically relevant concentrations of the two persistent compounds identified in water at the breeding site on 22–23 July 2001 (Hayes et al. 2002b). We also tested the commercial preparation Bicep II Magnum [reported as 33% atrazine, 0.7% atrazine-related products, 26.1% technical-grade S-metolachlor (18.57% S-metolachlor, 2.5% R-metolachlor), 40.2% inert ingredients], a commercial preparation applied to the field twice in 2001. Bicep II Magnum was delivered to tadpoles at two concentrations, one to provide an equivalent of 0.1 ppb atrazine and one to provide 10 ppb atrazine. Comparisons between Bicep II Magnum and the atrazine + S-metolachlor mixture were examined to estimate the potential effects of the solvent used in the Bicep II Magnum mixture.
All pesticides were predissolved in ethanol for a final concentration of 0.0036% ethanol and 0.1 ppb of each pesticide. Controls received an equal amount of ethanol only. Each treatment was replicated 3 times (30 larvae per replicate). Cages were cleaned, water changed, and treatments renewed every 3 days. All treatments were systematically relocated every 3 days to ensure that no treatments or tanks experienced position effects. Animals were exposed throughout the larval period from 2 days posthatching until complete tail reabsorption (TR; Gosner stage 46). In all experiments, all dosing and analyses were conducted blindly in color-coded tanks, and animals were number coded when fixed for examination.
Confirmation of pesticide concentrations
Confirmation of nominal concentrations was conducted by the Iowa Hygienics Laboratory (University of Iowa, Iowa City, Iowa) as described previously (Hayes et al. 2002a). Water samples were extracted in organic solvent and subjected to gas chromatography using a nitrogen phosphorus detector. Analysis was provided for alachlor, atrazine, S-metolachlor, metalaxyl, and propiconizole. Samples were collected just after making the solution (before tadpoles were added) and shipped frozen in chemical-free glassware (Fisher Scientific Co., Houston, TX). Water samples were color coded, and analyses were conducted blindly. The negative control consisted of Holtfreter’s solution only.
General measurements
Upon completion of metamorphosis (TR), each animal was weighed and measured. Animals were euthanized in 0.2% benzocaine (Sigma), assigned a unique identification number, fixed in Bouin’s fixative, and preserved in 70% ethanol until further analysis.
Histological analysis of gonads
All analyses were conducted blindly. Gonads and attached kidneys were dissected under a Nikon SMZ10A dissecting scope fitted with a 0.5× lens (Technical Instruments, Burlingame, CA). Tissues were embedded in paraffin, and histological analysis was conducted as described in Hayes (1995b). Briefly, dissected tissues were dehydrated in graded alcohols and infiltrated with Histoclear (National Diagnostics, Atlanta, GA) followed by paraffin. Serial histological sections were cut at 8 μm using a rotary microtome. Slides were stained in Mallory’s trichrome stain and analyzed using a Nikon Optiphot 2 microscope. Digital images were recorded using a Sony DKC-5000 digital camera.
Histological analysis of thymus
After noting that animals exposed to the pesticide mixture experienced increased disease rates due to the pathogen Chryseobacterium (Flavobacterium) menigosepticum (see “Results”), we examined the thymus histologically as a measure of immunocompetence. The head was dissected (just anterior to the forelegs). Tissues were embedded in paraffin, and histological analysis was conducted as described for gonads except that sections were cut at 4 μm. The number of thymic plaques, maximum transverse cross-sectional area, and cell density were determined using Metamorph software (version 2.75; Universal Imaging Ltd, Buckinghamshire, UK). The effects of all single pesticides were examined (20 animals each), as well as effects of 0.1 ppb Bicep II Magnum and the nine-compound mixture. Animals exposed to 0.1 or 10 ppb atrazine + S-metolachlor were not examined because this analysis was not planned and tissues from these animals were prepared for other analyses.
Experiment 2: Effects of the Pesticide Mixture on Corticosterone Levels in Adult Xenopus laevis
After noting that animals exposed to the nine-compound mixture suffered from thymic damage (increased thymic plaques) and increased disease rates (see “Results”), we examined the effects of the same pesticide mixture on plasma corticosterone levels. We used male X. laevis as a surrogate for this experiment because metamorphic R. pipiens are too small to obtain repeated blood samples and because X. laevis adults are available year-round for such studies.
Adult treatments
Adult males were obtained from a long-term captive colony at University of California, Berkeley. Adults were maintained under the same light and temperature cycles as described for R. pipiens larvae, but animals were housed individually in covered tanks. Animals were acclimated in 0.1× Holtfreter’s solution for 5 days and then exposed to the pesticide mixture (0.1 ppb each compound) or an equal amount of ethanol (0.0036%). Five males were treated with the pesticide mixture and five with ethanol only. Holtfreter’s solution was not aerated, animals were fed Purina trout chow (Purina Mills) daily, and solutions were changed and treatments renewed every 3 days. Animals were treated for 27 days. Blood was collected by cardiac puncture in nonanesthetized animals between 1800 and 2000 hr. Plasma was collected by aspiration after low-speed centrifugation and stored frozen (–80°C) until analysis.
Radioimmunoassay
For corticosterone analysis, plasma was thawed and extracted with diethyl ether and evaporated under nitrogen. All samples were reconstituted in phosphate-buffered saline with gelatin. Hormone radioimmunoassays were conducted as described previously (Hayes and Licht 1992). Corticosterone antiserum was purchased from Endocrine Sciences (Calabasas, CA) and has been validated for several species including X. laevis (Hayes and Licht 1992, 1995; Hayes and Wu 1995; Hayes et al 2002a). Plasma from controls and treated animals was assayed in the same assay at three doses, and the assay was repeated 3 times. Intraassay variation was 1.0%, and interassay variation was 1.4%.
Statistical analyses
Metamorphosis (initiation, total time, and completion), size at metamorphosis [snout-vent length (SVL) and body weight (BW)], and maximum cross-sectional area of the thymus (experiment 1), and hormone levels (experiment 2) were examined using analysis of variance (ANOVA) with replicates (tank) nested within treatment. Statistical groupings were determined using a Tukey post-hoc test. The relationship between time to metamorphosis and size (SVL or BW) at metamorphosis was analyzed by correlational analysis using Spearman’s rank order correlation coefficients followed by Bonferroni probability post-hoc tests. The frequency of thymic plaques, frequency of disease transmission, and mortality were analyzed using a G-test (Sokal and Rohlf 1981) with treatment and tanks as independent variables. Once significant effects were identified, the total G was apportioned into contributions by the individual treatments, first using the results from controls to establish expected frequencies, and then by using the results of the individual chemicals to establish expected frequencies to test if the pesticide mixtures differed from the observed effects of the individual chemicals. In all cases, once statistical significance was obtained using the entire data set, single chemical exposures were compared with ethanol-treated controls without considering the mixtures. All mixtures were then analyzed against ethanol-treated controls. ANOVA and correlational analysis were conducted with the aid of SYSTAT software (version 7; Systat Software Inc., Point Richmond, CA).
Results
Confirmation of pesticide concentrations
The limit of detection for all pesticides analyzed was 0.1 ppb. Alachlor was detected at 0.15 ppb, atrazine at 0.19 ppb, S-metolachlor at 0.22 ppb, metalaxyl at 0.16 ppb, and propiconizole at 0.23 ppb in the nominal 0.1 ppb mixture. No pesticides were detected in the Holtfreter’s control (< 0.1 ppb).
Mortality
With the exception of metalaxyl, no single compound affected mortality (p > 0.05; see sample sizes in Table 1, which reflect the number of larvae surviving to metamorphosis for each treatment). On average, mortality was 4% for animals exposed to single pesticides (range = 0–7.8%), with the highest mortality (7.8%) in S-metolachlor–treated larvae. Larvae treated with metalaxyl, which experienced 35% mortality before reaching metamorphosis, are not included in this analysis because one replicate suffered high mortality (90%) all on a single day, most likely as a result of a mechanical failure in the aeration system. Larvae exposed to the atrazine + S-metolachlor mixture or Bicep II Magnum experienced very low mortality (≤ 10%), but only 65% of the larvae exposed to the 0.1-ppb nine-compound mixture survived to the initiation of metamorphosis [foreleg emergence (FLE), Gosner stage 42]. Animals treated with the nine-compound mixture at 10 ppb all died after the first day of exposure.
Table 1.
Statistics for correlational analysis of time to complete TR and SVL.
| Treatmenta | n | r | Chi-squared | df | p-Value | Figurea |
|---|---|---|---|---|---|---|
| Ethanol | 86 | +0.23 | 4.365 | 1 | 0.040 | 5A |
| Alachlor | 86 | +0.29 | 7.123 | 1 | 0.008 | 5B |
| Atrazine | 86 | −0.19 | 3.061 | 1 | 0.080 | 5J |
| Cyfluthrin | 86 | +0.24 | 4.872 | 1 | 0.027 | 5C |
| λ-Cyhalothrin | 84 | −0.00 | 0.001 | 1 | 0.980 | 5I |
| S-Metolachlor | 83 | +0.29 | 7.140 | 1 | 0.008 | 5F |
| Metalaxyl | 58 | +0.25 | 3.210 | 1 | 0.073 | 5G |
| Nicosulfuron | 85 | +0.29 | 6.785 | 1 | 0.009 | 5D |
| Propiconizole | 88 | −0.14 | 1.581 | 1 | 0.209 | 5H |
| Tebupirimphos | 85 | +0.35 | 10.59 | 1 | 0.001 | 5E |
| 0.1 ppb atrazine + S-metolachlor | 89 | −0.19 | 3.018 | 1 | 0.082 | 5K |
| 10 ppb atrazine + S-metolachlor | 85 | −0.14 | 1.780 | 1 | 0.182 | 5L |
| 0.1 ppb Bicep II | 81 | +0.39 | 11.50 | 1 | 0.001 | 5M |
| 10 ppb Bicep II | 84 | +0.19 | 2.732 | 1 | 0.098 | 5N |
| 0.1 ppb mix | 59 | −0.23 | 2.100 | 1 | 0.147 | 5O |
All treatments were at 0.1 ppb except where indicated for mixtures as described in “Materials and Methods.” Bicep II Magnum (Bicep II) was administered to provide 0.1 ppb atrazine. The nine-compound mixture (mix) was administered to provide 0.1 ppb of all nine pesticides. Sample size (n) represents the number of animals surviving to metamorphosis (of the original 90; 30 animals in each of three replicates).
Indicates the figure number in this article where data are depicted.
Time to metamorphosis
Propiconizole significantly delayed time to initiate metamorphosis (FLE; F = 2.72, df = 10, p = 0.003) and time to complete metamorphosis (TR; F = 2.81, df = 10, p = 0.002) relative to controls (Figure 1). Otherwise, no single compound affected this measure of development, and the rest of the analyses focused on the effects of the three mixtures (atrazine + S-metolachlor, Bicep II Magnum, and the nine-compound mixture).
Figure 1.
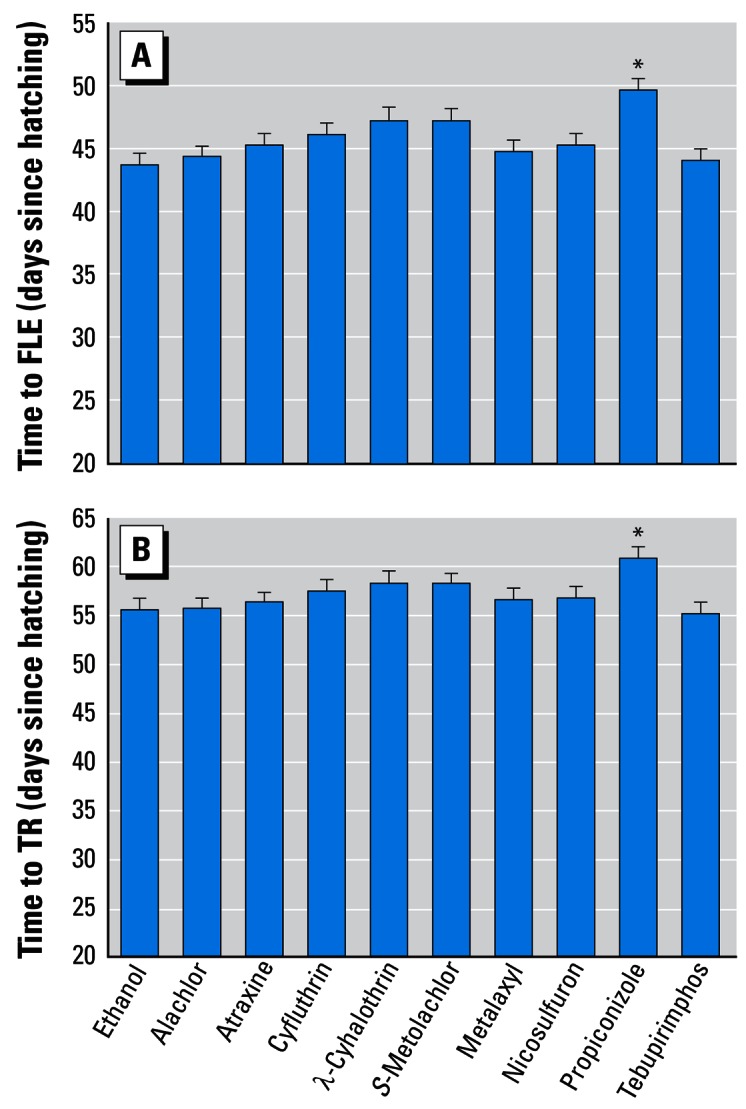
Effect of single pesticides (0.1 ppb each) on time to initiate metamorphosis (FLE; A) and time to complete metamorphosis (TR; B). Error bars show SEM.
*Statistically significant groups (ANOVA, p < 0.05).
Animals exposed to pesticide mixtures at 0.1 ppb had significantly longer larval periods: initiation of metamorphosis (days to FLE) was delayed (F = 37.55, df = 3, p < 0.005; Figure 2), and TR was similarly delayed (F = 29.84, df = 3, p < 0.0001; Figure 2). Larvae exposed to 10 ppb atrazine + S-metolachlor experienced a slight delay in both FLE (p = 0.055) and TR (although not statistically significant, p > 0.05). Larvae exposed to the nine-compound mixture experienced an even greater delay (p < 0.0001). There was no difference in the interval between FLE and TR, however, for any treatment (F = 1.15, df = 3, p = 0.33). No position or tank effects were observed in any of the analyses of the effects of mixtures on time to metamorphosis (p > 0.05).
Figure 2.
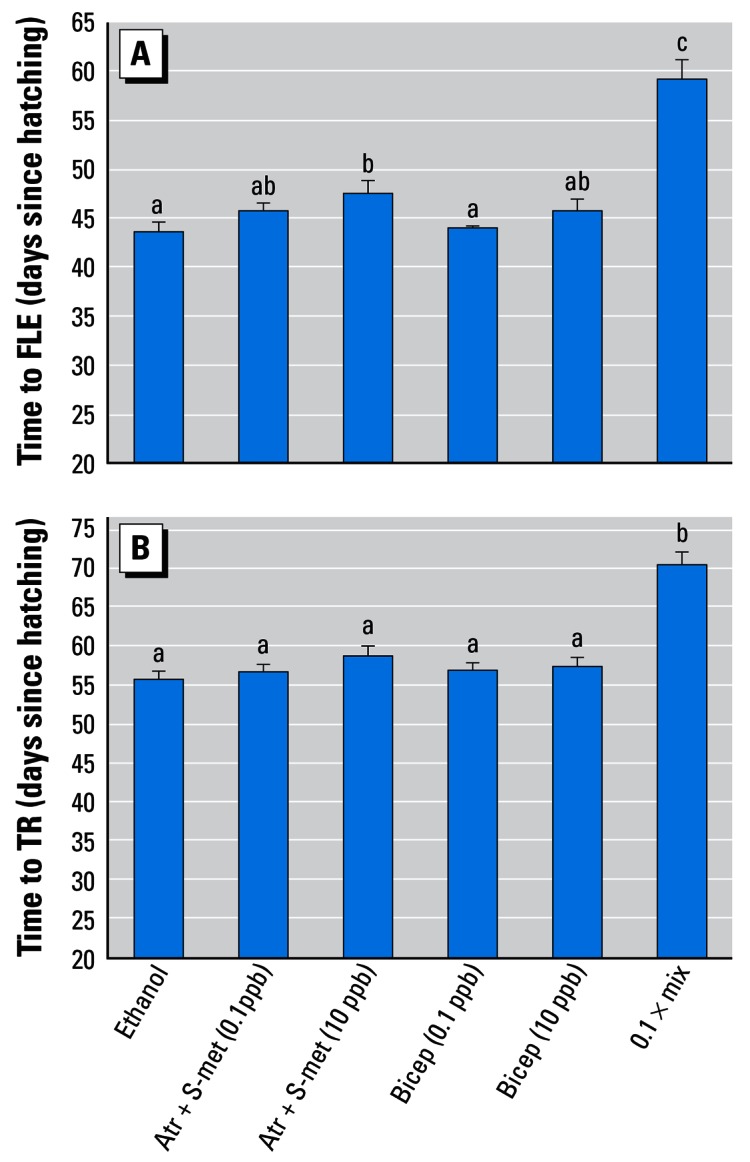
Effect of pesticide mixtures on time to initiate metamorphosis (FLE; A) and time to complete metamorphosis (TR; B). Abbreviations: Atr, atrazine; Bicep, Bicep II Magnum; S-Met, S-metolachlor; Mix, nine-chemical mixture (0.1 ppb each pesticide). Letters above bars indicate statistical groupings. Error bars show SEM.
*Statistically significant groups (ANOVA, p < 0.05).
Size at metamorphosis
For the individual chemicals, there was a significant effect on SVL at metamorphosis (F = 2.1, df = 10, p < 0.05; Figure 3). The smallest animals to metamorphose were those exposed to cyfluthrin, tebupirimphos, or atrazine. There was also a significant effect on BW (F = 2.07, df = 10, p < 0.05; Figure 3).
Figure 3.
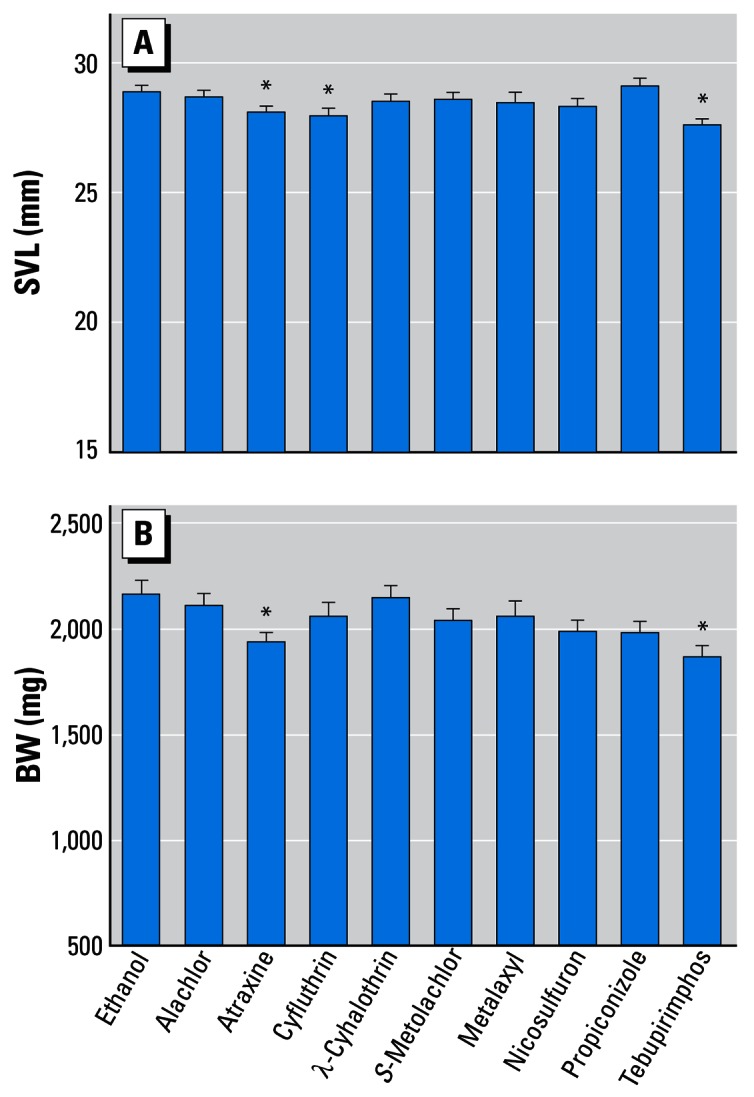
Effect of single pesticides (0.1 ppb each) on SVL (A) and BW (B). Error bars show SEM.
*Statistically significant groups (ANOVA, p < 0.05).
All the mixtures (0.1 ppb each pesticide) retarded growth, resulting in smaller animals (SVL) at metamorphosis (F = 4.03, df = 3, p = 0.008; Figure 4). The mixtures also affected BW (F = 3.86, df = 3, p = 0.01; Figure 4), and in this regard, the atrazine + S-metolachlor mixture had the greatest negative effect, followed by the nine-compound mixture. No tank or position effects were observed (p > 0.05).
Figure 4.
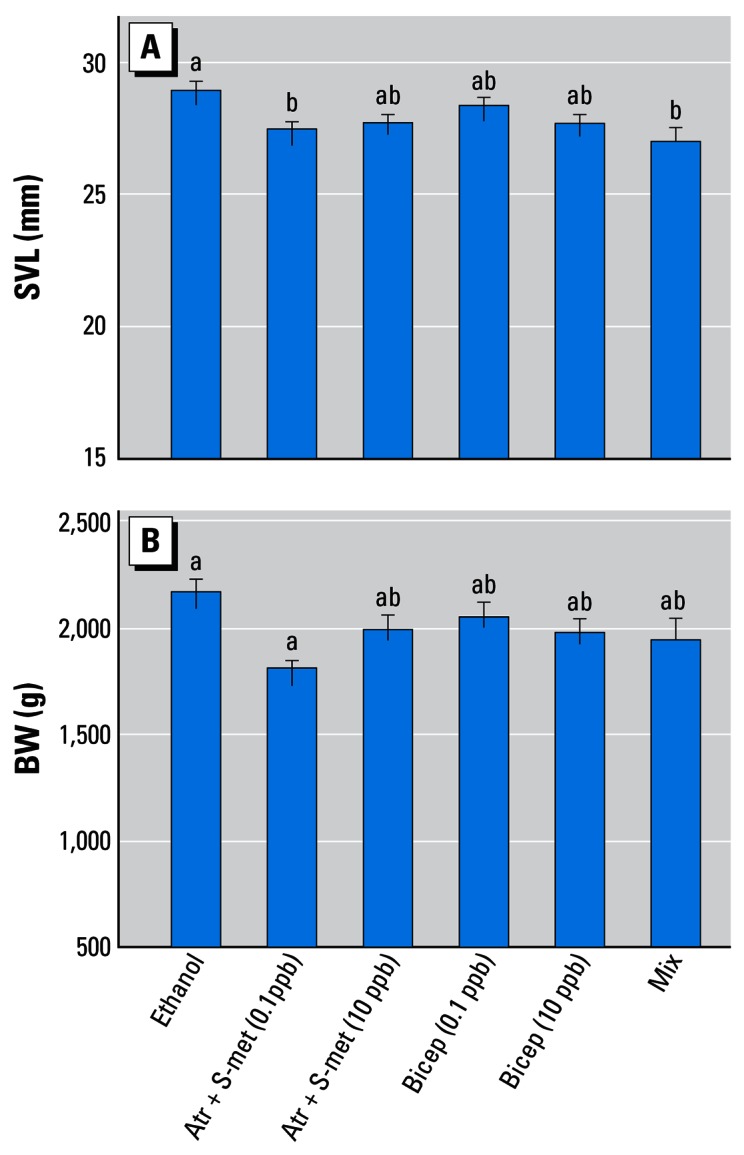
Effect of pesticide mixtures on SVL) (A) and BW(B). Abbreviations: Atr, atrazine; Bicep, Bicep II Magnum; S-met, S-metolachlor; Mix, nine-chemical mixture (0.1 ppb each pesticide). Letters above bars show statistical groupings. Error bars show SEM.
Relationship between time to complete metamorphosis and size at metamorphosis
SVL at metamorphosis was positively correlated with time to complete metamorphosis for controls (Figure 5). Regarding single chemical exposures, all the treatments showed a similar positive significant relationship (p < 0.05; Table 1) with the following exceptions: larvae exposed to metalaxyl showed a positive but nonsignificant relationship between time to complete metamorphosis (TR) and SVL, whereas larvae exposed to atrazine, cyhalothrin or propiconizole showed a negative but nonsignificant relationship between TR and SVL (Figure 5).
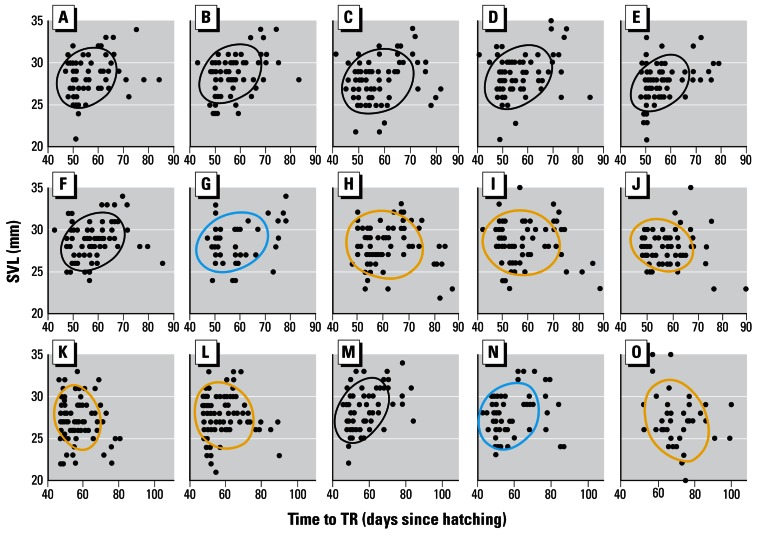
Figure 5.
Correlational analysis of time to complete TR and SVL. Results are shown for single pesticides (0.1 ppb) in A–J and for mixtures (K–O). For the x-axis, the scales for A–J are identical, but note the difference in scale for the mixtures as a result of the delay in metamorphosis in the animals treated with the nine-compound mixture (O). Results are shown for ethanol (A), alachlor (B), cyfluthrin (C), nicosulfuron (D), tebupirimphos (E), S-metolachlor (F), metalaxyl (G), propiconizole (H), λ-cyhalothrin (I), atrazine (J), 0.1 ppb atrazine + S-metolachlor (K), 10 ppb atrazine + S-metolachlor (L), 0.1 ppb Bicep II Magnum (M), 10 ppb Bicep II Magnum (N), and the nine-compound mixture (O). Ellipses show Gaussian bivariate confidence limits. Ellipses are color-coded: black, a positive and significant correlation; blue, a positive but nonsignificant correlation; yellow, a negative but nonsignificant correlation. Significance (p ≤ 0.05) was determined by Bonferroni probabilities and based on Spearmen rank order correlation coefficients. Sample size (as reported in Table 1) cannot be determined from the number of data points due to overlapping data.
For the pesticide mixtures, 0.1 and 10 ppb atrazine + S-metolachlor resulted in a negative but nonsignificant relationship between TR and SVL, whereas 0.1 and 10 ppb Bicep II Magnum exposure resulted in maintenance of the positive relationship between TR and SVL, but the relationship was significant for the 0.1 ppb concentration only (Figure 5). The nine-compound pesticide mixture resulted in a negative but non-significant relationship (Table 1, Figure 5).
For BW, controls also showed a significant positive correlation between time to complete metamorphosis and BW. All single-treatment exposures showed a similar positive significant relationship (p < 0.002 in all cases; Table 2, Figure 6) except nicosulfuron, atrazine, and cyhalothrin, which showed a positive but non-significant relationship between TR and BW at metamorphosis. Regarding pesticide mixtures, a positive relationship was observed with Bicep II Magnum but was significant only for the 0.1 ppb concentration. The relationship was negative but nonsignificant for both 0.1 and 10 ppb atrazine + S-metolachlor and negative and significant for the nine-pesticide mixture (Table 2, Figure 6).
Table 2.
Statistics for correlational analysis of time to complete TR and BW.
| Treatment | n | r | Chi-squared | df | p-Value | Figurea |
|---|---|---|---|---|---|---|
| Ethanol | 86 | +0.41 | 14.61 | 1 | 0.000 | 6A |
| Alachlor | 86 | +0.46 | 18.92 | 1 | 0.000 | 6B |
| Atrazine | 86 | +0.07 | 3.061 | 1 | 0.080 | 6J |
| Cyfluthrin | 86 | +0.34 | 10.03 | 1 | 0.002 | 6C |
| λ-Cyhalothrin | 84 | +0.13 | 1.376 | 1 | 0.241 | 6I |
| S-Metolachlor | 83 | +0.47 | 20.15 | 1 | 0.000 | 6F |
| Metalaxyl | 58 | +0.40 | 9.038 | 1 | 0.003 | 6G |
| Nicosulfuron | 85 | +0.49 | 20.99 | 1 | 0.000 | 6D |
| Propiconizole | 88 | +0.20 | 3.130 | 1 | 0.077 | 6H |
| Tebupirimphos | 85 | +0.58 | 32.77 | 1 | 0.000 | 6E |
| 0.1 ppb atrazine + S-metolachlor | 89 | –0.05 | 0.251 | 1 | 0.616 | 6K |
| 10 ppb atrazine + S-metolachlor | 85 | –0.01 | 0.000 | 1 | 0.960 | 6L |
| 0.1 ppb Bicep | 81 | +0.51 | 20.69 | 1 | 0.000 | 6M |
| 10 ppb Bicep | 84 | +0.33 | 8.143 | 1 | 0.004 | 6N |
| 0.1 ppb mix | 59 | –0.32 | 4.164 | 1 | 0.041 | 6O |
All treatments were at 0.1 ppb except where indicated for mixtures as described in “Materials and Methods.” Bicep II Magnum (Bicep) was administered to provide 0.1 ppb atrazine. The nine-compound mixture (mix) was administered to provide 0.1 ppb of all nine pesticides. Sample size (n) represents the number of animals surviving to metamorphosis (out of the original 90; 30 animals in each of three replicates).
Indicates the figure number in this article where data are depicted.
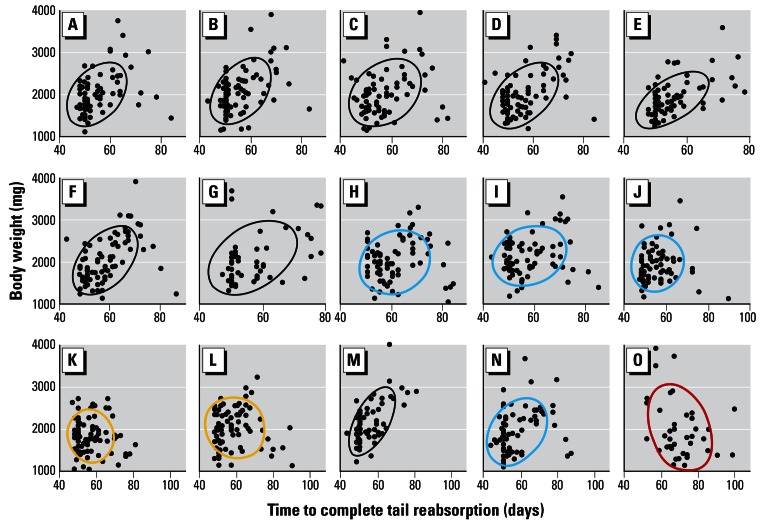
Figure 6.
Correlational analysis of time to complete TR and BW. Results are shown for single pesticides (0.1 ppb) in A–J and for mixtures (K–O). For the x-axis, the scales for the top two rows are identical, but note the difference in scale for the mixtures as a result of the delay in metamorphosis in the animals treated with the nine-compound mixture (O). Results are shown for ethanol (A), alachlor (B), cyfluthrin (C), nicosulfuron (D), tebupirimphos (E), S-metolachlor (F), metalaxyl (G), propiconizole (H), λ-cyhalothrin (I), and atrazine (J), 0.1 ppb atrazine + S-metolachlor (K), 10 ppb atrazine + S-metolachlor (L), 0.1 ppb Bicep II Magnum (M), 10 ppb Bicep II Magnum (N), and the nine-compound mixture (O). Ellipses show Gaussian bivariate confidence limits. Ellipses are color-coded: black, a positive and significant correlation; blue, a positive but nonsignificant correlation; yellow, a negative but nonsignificant correlation; red, a negative and significant correlation. Significance (p ≤ 0.05) was determined by Bonferroni probabilities and based on Spearmen rank order correlation coefficients. Sample size (as reported in Table 2) cannot be determined from the number of data points because of overlapping data.
Gonadal development
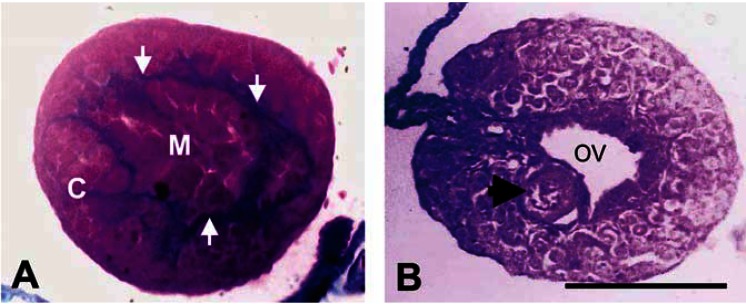
In the population used in the present study, gonadal development was delayed (even in controls) relative to other populations of R. pipiens that have been examined by our laboratory (Hayes et al. 2002a, 2002c) or documented in other published accounts (Merchant-Larios and Villapando 1981; Richards and Nace 1978). Histologically, presumptive males maintained both a cortex and a medulla separated by connective tissue without clear formation of testicular lobules (e.g., undifferentiated), whereas females showed regression of the gonadal medulla and an ovarian vesicle but lacked significant numbers of developing oocytes in the cortical regions of the gonad (Figure 7). Because of the underdeveloped state of the gonads and gametes in this population, assessing the effects of atrazine or pesticide mixtures containing atrazine on sex differentiation of the gonads and gametogenesis (e.g., whether testicular oogenesis occurred) was not possible.
Figure 7.
Histological transverse cross-section (8 μm) of presumptive male (A) and female (B) leopard frog (R. pipiens) at metamorphosis (Gosner stage 46). Gonads are not completely differentiated. Note the intact cortex (C) and medulla (M) separated by blue connective tissue (arrows in A). Also note medullary regression and ovarian vesicle (OV) but absence of significant oogenesis in the female (B). A single oocyte (arrow) is noted in the female. Scale bar = 125 μm.
Flavobacterial response
Seventy percent of the animals exposed to the nine-compound mixture were unable to sit upright. Exposure to the nine-compound pesticide mixture was associated with meningitis, otitis interna, and septicemia due to the gram-negative, water-borne bacteria Chryseobacterium (Flavobacterium) menigosepticum. Diagnosis was based on signalment, clinical signs, necropsy results, histopathologic examination of internal organs with special staining in select cases, and the ante-mortem isolation of organism from the internal lesions of affected animals. Manifestations of disease included anorexia, head tilt, circling, loss of righting response, anisocoria, and death (Figure 8). Morbidity and mortality rates in animals treated with the nine-pesticide mixture were significant (G = 100.12, df = 4, p < 0.001) compared with those in controls or the other mixtures (all of which showed a 0% incidence) and reached 70% of the 59 animals that survived to complete metamorphosis in animals exposed to the pesticide mixture. C. (Flavobacterium) menigosepticum was successfully cultured from animals from all groups (including controls), but no animals from other treatments contracted the disease or suffered the symptoms described above.
Figure 8.
Dorsal (A,C) and frontal (B,D) view of newly metamorphosed (Gosner stage 46) control (A,B) R. pipiens and similar-aged animal exposed to the nine-pesticide mixture (C,D). Control animal is in good body condition as expected. The pesticide-treated animal is in poor body condition because of a generalized gram-negative bacterial infection. The pathogen was identified in control and treated frogs, but only pesticide-exposed animals show signs of disease: head tilt, unilateral extensor muscle rigidity, anisocoria, and intermittent recumbency due to a severe otitis interna and meningitis. This presentation is consistent with C. menigosepticum infection, a stress-induced disease of frogs.
Thymus characteristics
After noting that animals exposed to the nine-compound mixture contracted flavobacterial meningitis (see above “Flavobacterial response”), we examined the condition of the thymus as an estimate of immune function. Exposure to atrazine and S-metolachlor resulted in damage to the thymus as measured by thymic plaques. No other single pesticide produced this effect. The frequency of animals with plaques increased in animals treated with the mixtures (Bicep II Magnum), followed by larvae treated with the nine-compound pesticide mixture (G = 9.4, df = 4, p < 0.05; Figure 9). Larvae treated with atrazine + S-metolachlor (0.1 or 10 ppb) were not examined because this analysis was not planned, and these samples were preserved for another analysis (data not shown).
Figure 9.
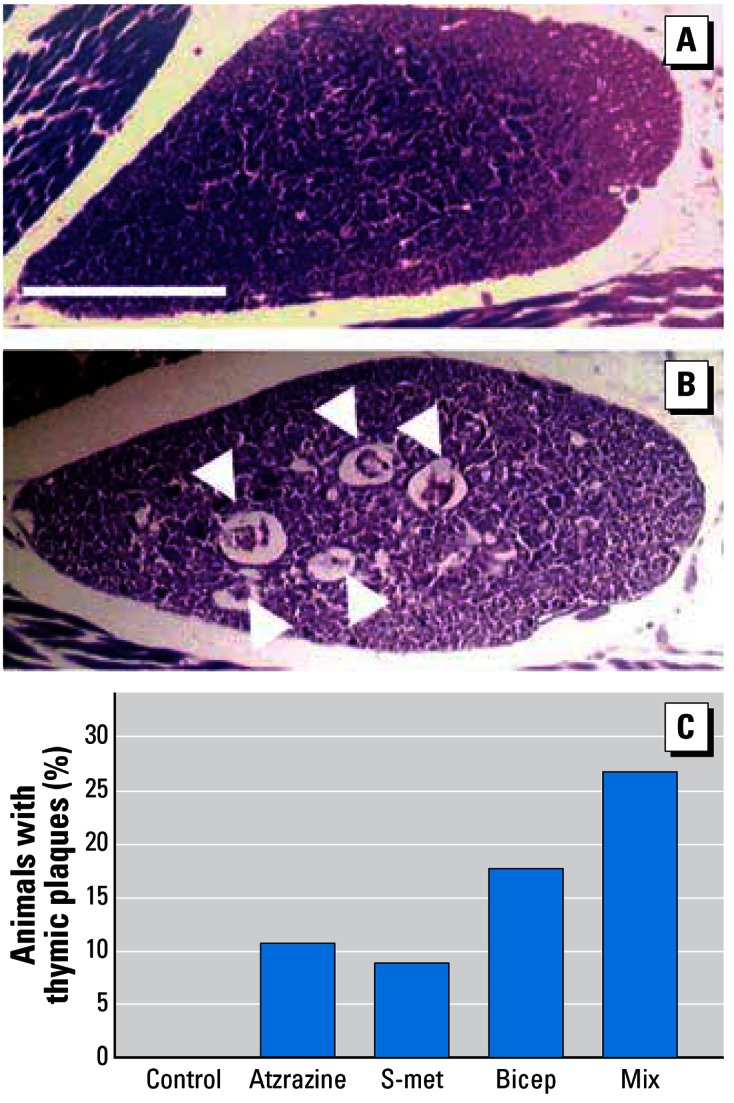
Representative transverse cross-section through the thymus of a control animal (A) and (B) an animal treated with the nine-compound pesticide mixture. (C) The frequency of animals with detectable damage to the thymus. No control animals showed damage to the thymus. Animals exposed to 0.1 ppb atrazine or to S-metolachlor (S-met) had damage as shown in B, with increasing frequencies of damage with exposure to Bicep II Magnum (atrazine + metolachlor, given to concentration of 0.1 ppb atrazine), and maximum damage with exposure to the pesticide mixture (Mix). Histological sections were 4 μm stained in Mallory’s trichrome stain. Scale bar = 0.5 mm for A and B.
Effects of the pesticide mixture on plasma corticosterone
The nine-pesticide mixture had a clear effect on corticosterone levels in male African clawed frogs (X. laevis). Corticosterone levels increased 4-fold in pesticide-exposed males (ANOVA, p < 0.05; Figure 10).
Figure 10.
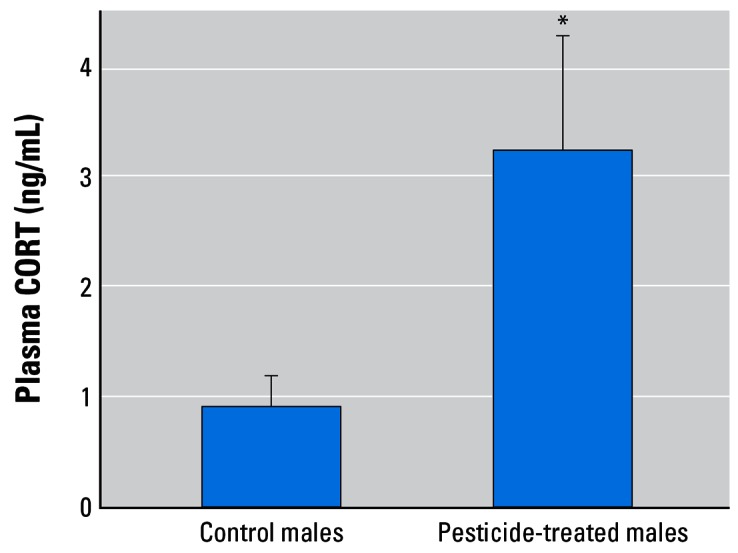
Effect of the pesticide mixture on plasma corticosterone levels in adult male African clawed frogs (X. laevis). Error bars show SEM.
*Statistical significance (p < 0.05).
Discussion
Although a sizable database examining the toxicological effects of pesticides on amphibians exists (Pauli 2004), most of these studies examine acute toxicity, morbidity, and mortality only. Few studies have examined low-concentration effects (especially endocrine disruption) or the effects of pesticide mixtures. In reality, amphibians in the wild (especially in agricultural areas) are exposed to mixtures of pesticides. Further, although brief episodes of high-concentration exposure may occur, prolonged exposures to low concentrations of pesticide mixtures are more common (Battaglin and Goolsby 1999; Burkhart et al. 2003; Capel and Larson 2001; Fischer et al. 1995; Frank and Logan 1988; Frank and Sirons 1979; Frank et al. 1987, 1991; Insensee et al. 1990; Kolpin et al. 1998; Kucklick and Bidleman 1994a, 1994b; Pennington et al. 2001; Solomon et al. 1996; Thurman et al. 1992). Only a few studies have examined the effects of pesticide mixtures on amphibians (Christin et al. 2003; Dawson and Wilke 1991; Gendron et al. 2003; Howe et al. 1998; Mazanti 1999; Mazanti et al. 2003; Relyea 2004), but chemicals were examined at much higher concentrations than those examined here, only toxicity was examined, and the chemical mixture in the present analysis was not examined. Further, fewer than 20 published laboratory studies (Boegi et al. 2002; Hayes 1997a, 1998, 2000, 2004, 2005; Hayes et al. 1997, 2002a, 2002b, 2002c; Lutz and Kloas 1999; Noriega and Hayes 2000; Palmer and Palmer 1995; Palmer et al. 1998; Tavera-Mendoza et al. 2002a, 2002b) and four field studies (du Preez et al. 2005; Hayes et al. 2002b, 2002c; Reeder et al. 1998) have addressed low-concentration, endocrine-disrupting effects of pesticides (single compounds only) on amphibians. A few studies have examined amphibians in the wild (Harris et al. 1998a, 1998b; Ouellet et al. 1997; Sparling et al. 2001), although establishing cause and effect in such studies is difficult. The present study is the first to address endocrine-disrupting effects of low-concentration pesticide mixtures on amphibians in the laboratory.
We demonstrated that a realistic pesticide mixture (based on a mixture applied to an actual field) at low ecologically relevant concentrations can have dramatic effects on amphibian development and growth, and ultimately (we predict) survivorship. We propose here that the lack of examinations of endocrine-disrupting effects of low concentrations of pesticides in amphibians has resulted in underestimates of the impacts of pesticides on wildlife (Hayes et al. 2002a), as similarly suggested by Burkhart et al. (2003). The absence of studies that examine low-concentration effects of pesticide mixtures makes this underestimation even more severe.
Most of the nine pesticides used in the present study have not been examined in amphibians at all: no published studies have addressed the effects of cyfluthrin, cyhalothrin, tebupirimphos, metalaxyl, or propiconizole on amphibians. Here, we show that one of these compounds (propiconizole) retards larval development and delays metamorphosis, and two others (tebupirimphos and cyfluthrin) retard larval growth. In addition to these new data, the present study confirms the retardation of amphibian development (Carr et al. 2003; Rohr and Palmer 2005; Rohr et al. 2004) and growth (Boone and James 2003; Britson and Threlkeld 1998; Carr et al. 2003; Diana et al. 2000) already reported for atrazine. Carr et al. (2003) also showed a reversal of the relationship between time to metamorphosis and size at metamorphosis in their studies, consistent with present results.
The present study is also important because all the pesticides were examined at low ecologically relevant concentrations (0.1 ppb). The few studies that have previously examined the effects of nicosulfuron (Fort et al. 1999), metolachlor (Mazanti 1999; Mazanti et al. 2003; Osano et al. 2002), and alachlor (Howe et al. 1998; Osano et al. 2002) on amphibians have examined concentrations 10,000 times higher than those used in the present study. All but four previous studies (Howe et al. 1998; Mazanti 1999; Mazanti et al. 2003; Relyea 2004) examined single pesticide exposures, and most examined mortality and teratogenesis, with only two studies addressing effects of these pesticide mixtures on larval growth and development (Howe et al. 2004; Relyea 2004). Again, all these previous studies were conducted at concentrations 10,000 times higher than those used in the present study. The exception is atrazine, for which sublethal developmental effects at low concentrations (in the parts per billion range) have been examined by multiple laboratories (Allran and Karasov 2001; Boone and James 2003; Britson and Threlkeld 1998; Carr et al. 2003; Coady et al. 2004, 2005; Diana et al. 2000; du Preez et al. 2005; Goulet and Hontela 2003; Gross et al. 2003; Hayes 2004, 2005; Hayes et al. 2002a, 2002b, 2002c, 2006; Hecker et al. 2004; Howe et al. 1998; Jooste et al. 2005a, 2005b; Miyahara et al. 2003; Reeder et al. 1998; Rohr and Palmer 2005; Rohr et al. 2004; Sullivan and Spence 2003; Tavera-Mendoza et al. 2002a, 2002b).
Atrazine has a number of well-documented adverse effects on amphibian larvae. It is a potent endocrine disruptor that both chemically castrates and feminizes exposed male amphibian larvae and also retards larval development and growth (Carr et al. 2003; du Preez et al. 2005, Hayes 2004, 2005; Hayes et al. 2002a, 2002b, 2002c, 2006; Reeder et al. 1998; Tavera-Mendoza et al. 2002a, 2002b). It also induces edema (Carr et al. 2003), erratic swimming (Carr et al. 2003), and irregular behavioral activity (Rohr and Palmer 2005) and is an immunosuppressant (Christin et al. 2003; Gendron et al. 2003; Kiesecker 2002) in amphibians. The impact of atrazine on amphibian larvae is important both because of the number of documented adverse effects in amphibians and also because atrazine is a ubiquitous, persistent environmental contaminant (Hayes et al. 2006; Solomon et al. 1996): As one of the world’s most commonly applied pesticides, it is the most common contaminant of groundwater and surface water (Hayes et al. 2006). Up to 0.5 million pounds per year are deposited in precipitation in the United States (Miller et al. 2000; Nations and Hallberg 1992; Thurman and Cromwell 2000; Van Dijk and Guicherit 1999), and contamination can spread more than 600 miles from the point of application (Miller et al. 2000; Müller et al. 1997; Thurman and Cromwell 2000).
The present study demonstrates that one of the best-documented effects of atrazine, demasculinization and feminization of male larvae, can vary across populations. In the present population, effects of atrazine on the gonads were not detectable because individuals from the present population do not complete sexual differentiation of the gonads before metamorphosis. In ranids, atrazine induces testicular oogenesis (Hayes et al. 2002b, 2002c), but in the population used in the present study, male gonads were not well differentiated (testicular lobules were not yet developed), and even females lacked significant numbers of oocytes. Although other differences in study design exist (Hayes 2004), this variation in susceptibility may explain some of the disparate findings in the published literature regarding the effects of atrazine on gonadal development in amphibians (Coady et al. 2004, 2005; du Preez et al. 2005; Hayes 2005; Hecker et al. 2004, 2005; Jooste et al. 2005a, 2005b). This finding highlights the importance of understanding population variation when assessing the risk of pesticides to amphibians.
As noted above, retardation of growth and development has been demonstrated for atrazine in previous studies. In the present study, retardation of growth and development was more severe when atrazine was combined with other pesticides (e.g., S-metolachlor), and the nine-pesticide mixture had the most severe impact. The delay in time to initiate and complete metamorphosis was significant. Many amphibians (including leopard frogs) often breed in temporary water sources. In particular, in agricultural areas, where water is manipulated for agricultural purposes, aquatic habitats can be unpredictable from year to year and even day to day (Figure 11). In these habitats, it is important for survivorship that larvae respond to desiccation by metamorphosing rapidly (Denver 1993; Denver et al. 1998). It has already been shown that atrazine alone retards development and prevents the acceleration of metamorphosis induced by pond drying as well as reduces size at metamorphosis (Rohr et al. 2004). As demonstrated by the present study, developing in water sources contaminated with pesticide mixtures (even simply 0.1 ppb atrazine + S-metolachlor) will decrease survivorship because these mixtures delay metamorphosis. Further, as water sources desiccate, pesticide concentrations will increase. Even if larvae metamorphose and escape desiccation, delayed metamorphosis along with decreased size at metamorphosis reduces adult recruitment and the likelihood of reproduction in amphibians (Smith 1987).
Figure 11.
Field 413 (40°55.88 N, 97°22.38 W), York County, Nebraska (A–F). In 2001 frogs bred in the irrigation ditch (A) and were present by the thousands. Even at this time, the water level (and thus temperature and pesticide levels) fluctuated drastically from one day to the next: the photo in B was taken on 22 July 2001 and photos in A and C were taken on 23 July 2001. (B) All the water has evaporated and only the small pool (white glare) remains. In 2004 seven pairs of frogs bred at the same site when the irrigation ditch filled with rainwater and snowmelt (D; arrow indicates where frog clutches were found). (E) One of the seven clutches. (F) The fields around the irrigation ditch were not planted in 2003 or 2004, and the ditch dried up, resulting in 100% failure of the population at this site for the second year since initial collection. (G) A breeding pond (arrow) in Hitchcock County, Nebraska (40°08.433 N, 101°13.804 W) along the Republican River. All the tadpoles died of desiccation at this site, further illustrating the interaction between pesticides that slow development and delay metamorphosis and the impact of agriculture (and irrigation practices) on amphibian populations.
Retardation of growth is also detrimental. Smaller size at metamorphosis limits food availability for newly metamorphosed frogs, which are gape-limited predators (Figure 12A). Further, smaller individuals are more susceptible to predators, which may also themselves be gape-limited predators (e.g., snakes; Figure 12B) (De Vito et al. 1999; Kiesecker and Blaustein 1998; Lardner 1998; Lawler et al. 1999; Nicieza 2000; O’Dwyer et al. 2000; Puttlitz et al. 1999; Relyea 2001a, 2001b, 2003; Skelly 1994; Werner 1986; Wilbur and Collins 1973). Because pesticide mixtures retard growth and size at metamorphosis, exposed amphibians are less likely to find food and more likely to be preyed upon. Also, decreased size at metamorphosis combined with subsequent decreased postmetamorphic growth decreases the chances that amphibians will survive overwintering (Berven and Gill 1983; Smith 1987). Reduced size at metamorphosis also delays reproductive maturity and decreases fecundity (Berven and Gill 1983; Smith 1987; Wilbur et al. 1973). This negative effect is especially true for females, for which size is directly proportional to fecundity (Howard 1981; Shine 1979, 1989), but this is true for males as well. In many species, females prefer larger males as mates, and increased size may be necessary for maintaining territories and fending off rival male suitors during copulation (Balinsky and Balinsky 1954; Howard 1981; Shine 1979, 1989).
Figure 12.

Demonstration of the effects of decreased size at metamorphosis on amphibians. (A) Newly metamorphosed leopard frog (R. pipiens) attempting to ingest a cricket that is too large (frog is 3.3 cm SVL; cricket is 2.1 cm long). (B) A 67-cm-long garter snake (Thamnophis sirtalis) feeding on a newly metamorphosed 3.2-cm-long leopard frog (R. pipiens). Frogs are gape-limited predators and less likely to find appropriate food when size at metamorphosis is reduced by pesticides. Similarly, garter snakes (like all snakes) are gape-limited predators, so smaller frogs are more vulnerable to snake predation.
The alteration of the relationship between time to metamorphosis and size at metamorphosis is even more significant than either measured alone. In amphibians, the larval stage is a period of growth. As nonamniotes, the size of hatching amphibians is limited. The larval stage, during which time amphibians are typically herbivorous, provides a period of growth that allows individuals to both become large enough to be effective predators and to escape predation. As shown in the present study, there is a positive relationship between time to metamorphosis and size at metamorphosis: among controls, larvae that take longer to metamorphosis are larger. However, with exposure to pesticide mixtures, larvae take longer to metamorphosis but do not obtain a size advantage and, in fact, are smaller at metamorphosis than larvae that metamorphose earlier. Interestingly, at least three of the single pesticides tested (propiconizole, λ-cyhalothrin, and atrazine) and potentially metalaxyl had a slight effect on the relationship between time to metamorphosis and size at metamorphosis. Potentially, only these three (or four) pesticides in the mixture produce the additive effects observed with the nine-compound mixture, with no contribution from the other pesticides. Alternatively, pesticides that produce no effects alone may act as “enhancers” that worsen the effects of pesticides that act as “effectors” when the two groups of chemicals are combined.
The present effects of mixtures cannot be assigned to the categories of concentration additive or response additive, as described in Burkhart et al. (2003), and further, the roles of each of the individual pesticides in the effects of the mixture cannot be identified. Although the relative roles of all the individual pesticides in the mixture cannot be discerned from the present study, S-metolachlor does appear to be an effector. In the present study and others cited above, atrazine retarded larval development and growth. Although S-metolachlor had no effect on its own in the present study, the negative effects of atrazine were increased when combined with S-metolachlor. Interestingly, the commercial mixture (Bicep II Magnum) appeared more benign than the pure mixture of atrazine and S-metolachlor. Bicep II Magnum at 0.1 ppb resulted in a positive relationship between time to metamorphosis and size at metamorphosis. There was a concentration effect, however: 10 ppb Bicep II Magnum eliminated the positive relationship. The atrazine + S-metolachlor mixture and Bicep II Magnum differ primarily in the surfactant (inert ingredients) (although the concentration of S-metolachlor was 22% lower in the Bicep II Magnum mixture than in the atrazine + S-metolachlor mixture). These data suggest that the surfactant used in this mixture reduces the effect of the two pesticides. Previous studies have shown that surfactant used in pesticides can have biological activity in amphibian larvae (Relyea 2005).
In addition to the adverse effects on the relationship between time to metamorphosis and size at metamorphosis, the pesticide mixture unexpectedly increased disease rates. Based on the observation that controls tested positive for the flavobacteria but did not display symptoms of disease, the pathogen was otherwise nonvirulent under the present experimental conditions. The increased disease rates were associated with an increased frequency of animals with damage to the thymus. Of all the pesticides tested alone, only atrazine and S-metolachlor increased the frequency of damage to the thymus. Further, atrazine has been shown to increase disease rates and parasite loads in amphibians by several pathogens (Christin et al. 2003; Gendron et al. 2003), including the trematode associated with development of limb deformities (Kiesecker 2002). In the present study, atrazine and S-metolachlor combined (Bicep II Magnum) increased the frequency of animals with thymic plaques relative to either herbicide alone, but disease rates were not increased unless animals were exposed to the nine-pesticide mixture.
Regarding to adverse effects that contribute to amphibian declines, the effects of atrazine on sex differentiation can negatively affect amphibian populations. The effects of the pesticide mixture on growth can have an even more rapid negative effect on populations, as described above. The immunosuppressive effects are likely even more relevant. Most significantly, the nine-pesticide mixture increased plasma corticosterone levels. Corticosterone can produce all the effects observed with the pesticide mixtures, including retarded growth (Hayes 1995a, 1995b; Hayes and Wu 1995; Hayes et al. 1993, 1997), retarded development (Glennemeier et al. 2002a, 2002b; Hayes 1995a, 1995b, 1997b; Hayes and Wu 1995; Hayes et al. 1993, 1997), and immunosuppression (Belden and Kiesecker 2005; Hayes 1995b). Given these adverse effects and the continued increase and use of pesticides in agriculture over the last 50 years, it is likely that pesticides have played and will continue to play a role in amphibian declines. In particular, the effects described here are very important. Pesticide-induced declines in populations as a result of decreased prey availability and increased susceptibility to predators (as a result of decreased size and the negation or reversal of the relationship between time to metamorphosis and size at metamorphosis) may be difficult to discern in the wild. Perhaps more important, emergent diseases caused by agents such as ranavirus (Brunner et al. 2005; Green and Muths 2005; Jancovich et al. 2005; Pearman et al. 2004) and chytrid (Berger et al. 1998; Green and Muths 2005; McCallum 2005; Ouellet et al. 2005; Rollins-Smith et al. 2002; Weldon et al. 2004) are considered major contributors to amphibian declines. Given the present findings with the flavobacteria in the present study, perhaps these diseases are not emergent at all. As suggested by Burkhart et al. (2003), perhaps what is emergent is the inability to mount proper immune responses as a result of pesticide exposure. As Sparling et al. (2003) pointed out, “Unfortunately, almost all research on amphibian population declines has focused on single factors or multiple factors considered individually with little consideration for interactions.” This approach has to change if problems are to be identified and solutions formulated.
Regarding pesticides, the present study demonstrates that examinations of effects of single pesticides are inadequate to assess adverse impacts on amphibian development or to address the role of pesticides in amphibian declines. The examinations needed to characterize pesticide interactions as concentration additive or response additive (Burkhart et al. 2003), to distinguish between effectors and enhancers, and to examine multiple combinations of pesticides at multiple concentrations are difficult to design and carry out and present new challenges to regulators. Such studies are necessary, however, to determine if the net effects of pesticide mixtures are truly due to the sum of the parts or simply some of the parts.
Footnotes
This article is part of the monograph “The Ecological Relevance of Chemically Induced Endocrine Disruption in Wildlife.”
We are grateful to N.B. Carroll, Syngenta Crop Protection U.S., for donating the S-metolachlor and Bicep II Magnum that were used in this study. We also thank Novartis, Syngenta Crop Protection, Ecorisk Inc., and members of the Atrazine Endocrine Ecological Risk Assessment Panel of Ecorisk Inc. for comments, criticisms, and encouragement.
All work was conducted in compliance with animal use protocol R209-0402BCR to T.B.H. We thank H.H. Wheeler and Park Water Co. for funding parts of the work. This work was also funded by a grant from the National Science Foundation (IBN-IOB-0315274). P.C., S.C., D.C., C.H., K.H., V.P.M., Y.M., and M.T. were all funded by the Howard Hughes Biology Scholar’s Program.
References
- Adams M. Correlated factors in amphibian decline: exotic species and habitat change in western Washington. J Wildl Manag. 1999;63:1162–1171. [Google Scholar]
- Alford RA, Richards SJ. Global amphibian declines: a problem in applied ecology. Annu Rev Ecol Syst. 1999;30:133–165. [Google Scholar]
- Allran J, Karasov W. Effects of atrazine on embryos, larvae, and adults of anuran amphibians. Environ Toxicol Chem. 2001;20:769–775. doi: 10.1897/1551-5028(2001)020<0769:eoaoel>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Balinsky B, Balinsky J. On the breeding habits of the South African bullfrog, Pyxicephalus adspersus. S Afr J Sci. 1954;51:55–58. [Google Scholar]
- Battaglin W, Goolsby D. Are shifts in herbicide use reflected in concentration changes in Midwestern Rivers. Environ Sci Technol. 1999;33:2917–2925. [Google Scholar]
- Belden L, Kiesecker J. Glucocorticosteroid hormone treatment of larval treefrogs increases infection by Alaria sp. trematode cercariae. J Parasitol. 2005;91:686–688. doi: 10.1645/GE-397R. [DOI] [PubMed] [Google Scholar]
- Berger L, Speare R, Daszak P, Green D, Cunningham A. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci USA. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berven K, Gill D. Interpreting geographic variation in life-history traits. Am Zool. 1983;23:85–97. [Google Scholar]
- Bishop C, Mahony N, Struger J, Ng P, Petit K. Anuran development, density, and diversity in relation to agricultural activity in the Holland River watershed, Ontario, Canada (1990–1992) Environ Monit Assess. 1999;57:21–43. [Google Scholar]
- Blaustein A, Kiesecker J. Complexity in conservation: lessons from the global decline of amphibian populations. Ecol Lett. 2002;5:597–608. [Google Scholar]
- Blaustein AR, Wake DB. Amphibian declines: judging stability, persistence and susceptibility of populations to local and global extinction. Trends Ecol Evol. 1990;5:203–204. [Google Scholar]
- Boegi C, Levy G, Lutz I, Kloas W. Functional genomics and sexual differentiation in amphibians. Comp Biochem Physiol B Biochem Mol Biol. 2002;133B:559–570. doi: 10.1016/s1096-4959(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Boone M, James S. Interactions of an insecticide, herbicide, and natural stressors in amphibian community mesocosms. Ecol Appl. 2003;13:829–841. [Google Scholar]
- Bridges C, Semlitsch R. Variation in pesticide tolerance of tadpoles among and within species of Ranidae and patterns of amphibian decline. Conserv Biol. 2005;14:1490–1499. [Google Scholar]
- Britson C, Threlkeld S. Abundance, metamorphosis, developmental, and behavioral abnormalities in Hyla chrysoscelis tadpoles following exposure to three agrichemicals and methyl mercury in outdoor mesochosms. Bull Environ Contam Toxicol. 1998;61:154–161. doi: 10.1007/s001289900742. [DOI] [PubMed] [Google Scholar]
- Brunner J, Richards K, Collins J. Dose and host characteristics influence virulence of ranavirus infections. Oecologia (Berlin) 2005;144:399–406. doi: 10.1007/s00442-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Burkhart J, Bidwell J, Fort D, Sheffield S. 2003. Chemical Stressors. In: Amphibian Decline: An Integrated Analysis of Multiple Stressor Effects (Linder G, Krest S, Sparling D, eds). Raleigh, NC:SETAC, 111–128.
- Burrowes P, Green D. Potential causes for amphibian declines in Puerto Rico. Herpetologica. 2004;60:141–154. [Google Scholar]
- Capel P, Larson S. Effect of scale on the behavior of atrazine in surface waters. Environ Sci Technol. 2001;35:648–657. doi: 10.1021/es001220f. [DOI] [PubMed] [Google Scholar]
- Carey C, Cohen N, Rollins-Smith L. Amphibian declines: an immunological perspective. Dev Comp Immunol. 1999;23:459–472. doi: 10.1016/s0145-305x(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Carr J, Gentles A, Smith E, Goleman W, Urquidi L, Thuett K, et al. Response of larval Xenopus laevis to atrazine: assessment of growth, metamorphosis, and gonadal and laryngeal morphology. Environ Toxicol Chem. 2003;22:396–405. [PubMed] [Google Scholar]
- Cavieres M, Jaeger J, Porter W. Developmental toxicity of a commercial herbicide mixture in mice: I. Effects on embryo implantation and litter size. Environ Health Perspect. 2002;110:1081–1085. doi: 10.1289/ehp.021101081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K-C, Jeung E-B. The biomarker and endocrine disruptors in mammals. J Reprod Dev. 2002;49:337–345. doi: 10.1262/jrd.49.337. [DOI] [PubMed] [Google Scholar]
- Christin M-S, Gendron A, Brousseau P, Ménard L, Marcogliese D, Cyr D, et al. Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ Toxicol Chem. 2003;22:1127–1133. [PubMed] [Google Scholar]
- Coady K, Murphy M, Villeneuve D, Hecker M, Jones P, Carr J, et al. Effects of atrazine on metamorphosis, growth, and gonadal development in the green frog (Rana clamitans) J Toxicol Environ Health A. 2004;67:941–957. doi: 10.1080/15287390490443722. [DOI] [PubMed] [Google Scholar]
- Coady KK, Murphy J, Villeneuve DL, Hecker MJ, Carr J, Solomon K, et al. Effects of atrazine on metamorphosis, growth, laryngeal and gonadal development, aromatase activity, and plasma sex steroid concentrations in Xenopus laevis. Ecotoxicol Environ Safety. 2005;62:160–173. doi: 10.1016/j.ecoenv.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Colborn T. The wildlife/human connection: modernizing risk decisions. Environ Health Perspect. 1994;102:55–59. doi: 10.1289/ehp.94102s1255a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R. Impact of tea cultivation on anurans in the western Ghats. 2003;85:1415–1421. [Google Scholar]
- Davidson C, Mahony N, Struger J, Ng P, Pettit K. Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate change hypothesis for California amphibian declines. Conserv Biol. 2002;16:1588–1601. [Google Scholar]
- Davidson C, Shaffer H, Jennings M. Declines of the California red-legged frog: climate, UV-B, habitat, and pesticides hypotheses. Ecol Appl. 2001;11:464–479. [Google Scholar]
- Dawson D, Wilke T. Evaluation of the frog embryo teratogenesis assay: Xenopus (FETAX) as a model system for mixture toxicity hazard assessment. Environ Toxicol Chem. 1991;10:941–948. [Google Scholar]
- Delis P, Mushinsky H, McCoy E. Decline of some west-central Florida anuran populations in response to habitat degradation. Biodivers Conserv. 1996;5:1579–1595. [Google Scholar]
- Denver RJ. Acceleration of anuran amphibian metamorphosis by corticotropin-releasing hormone-like peptides. Gen Comp Endocrinol. 1993;91:38–51. doi: 10.1006/gcen.1993.1102. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Mirhadi N, Phillips M. Adaptive plasticity in amphibian metamorphosis: response of Scaphiopus hammondii tadpoles to habitat desiccation. Ecology. 1998;79:1859–1872. [Google Scholar]
- De Vito J, Chivers D, Kiesecker J, Belden L, Blaustein A. Effects of snake predation on aggregation and metamorphosis of Pacific treefrog (Hyla regilla) larvae. J Herpetol. 1999;33:504–507. [Google Scholar]
- Diana S, Resetarits W, Schaeffer D, Beckmen K, Beasley V. Effects of atrazine on amphibian growth and survival in artificial aquatic communities. Environ Toxicol Chem. 2000;19:2961–2967. [Google Scholar]
- Diel P, Schmidt S, Vollmer G, Janning P, Upmeier A, Michna H, et al. Comparative responses of three rat strains (DA/Han, Sprague-Dawley and Wistar) to treatment with environmental estrogens. Arch Toxicol. 2004;78:183–193. doi: 10.1007/s00204-003-0535-y. [DOI] [PubMed] [Google Scholar]
- Duft M, Schulte-Oehlmann U, Weltje L, Tillman M, Oehlmann J. Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mudsnail Potamopyrgus antipodarum. Aquat Toxicol (Amsterdam) 2003;64:437–449. doi: 10.1016/s0166-445x(03)00102-4. [DOI] [PubMed] [Google Scholar]
- Du Preez LH, Solomon K, Carr J, Giesy J, Gross C, Kendall RJ, et al. Population structure characterization of the clawed frog (Xenopus laevis) in corn-growing versus non-corn-growing areas in South Africa. Afr J Herpetol. 2005;54:61–68. [Google Scholar]
- Fenner-Crisp PA. Endocrine disruptor risk characterization: an EPA perspective. Regul Toxicol Pharmacol. 1997;26:70–73. doi: 10.1006/rtph.1997.1122. [DOI] [PubMed] [Google Scholar]
- Fischer J, Apedaile B, Vanclief L. Seasonal loadings of atrazine and metolachlor to a southeastern Ontario river from surface runoff and groundwater discharge. Water Qual Res J Can. 1995;30:533–553. [Google Scholar]
- Fort D, Rogers R, Copley H, Bruning L, Stover E, Rapaport D. Effect of sulfometuron methyl and nicosulfuron on development and metamorphosis in Xenopus laevis: impact of purity. Environ Toxicol Chem. 1999;18:2934–2940. [Google Scholar]
- Frank R, Logan L. Pesticide and industrial chemical residues at the mouth of the Grand, Saugeen and Thames rivers, Ontario, Canada, 1981–85. Arch Environ Contam Toxicol. 1988;17:741–754. doi: 10.1007/BF01183882. [DOI] [PubMed] [Google Scholar]
- Frank R, Logan L, Clegg B. Pesticide and polychlorinated biphenyl residues in waters at the mouth of the Grand, Saugeen and Thames rivers, Ontario, Canada, 1986–1990. Arch Environ Contam Toxicol. 1991;21:585–595. doi: 10.1007/BF01183882. [DOI] [PubMed] [Google Scholar]
- Frank R, Ripley B, Braun H, Clegg B, Johnson R. Survey of farm wells for pesticides, Ontario, Canada. Arch Environ Contam Toxicol. 1987;16:1–8. [Google Scholar]
- Frank R, Sirons G. Atrazine: its use in corn production and its loss to stream waters in southern Ontario. Sci Total Environ. 1979;12:223–239. [Google Scholar]
- Gendron A, Marcogliese D, Barbeau S, Christin M, Brousseau P, Ruby S, et al. Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia. 2003;135:469–476. doi: 10.1007/s00442-003-1210-y. [DOI] [PubMed] [Google Scholar]
- Glennemeier KA, Denver RJ. Role for corticoids in mediating the response of Rana pipiens tadpoles to intraspecific competition. J Exp Zool. 2002a;292:32–40. doi: 10.1002/jez.1140. [DOI] [PubMed] [Google Scholar]
- Glennemeier KA, Denver RJ. Small changes in whole-body corticosterone content affect larval Rana pipiens fitness components. Gen Comp Endocrinol. 2002b;127:16–25. doi: 10.1016/s0016-6480(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Gosner K. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Goulet B, Hontela A. Toxicity of cadmium, endosulfan, and atrazine in adrenal steroidogenic cells of two amphibian species, Xenopus laevis and Rana catesbeiana. Environ Toxicol Chem. 2003;22:2106–2113. doi: 10.1897/02-255. [DOI] [PubMed] [Google Scholar]
- Green D. The ecology of extinction: population fluctuation and decline in amphibians. Biol Conserv. 2003;111:331–343. [Google Scholar]
- Green D, Muths E. Health evaluation of amphibians in and near Rocky Mountain National Park (Colorado, USA) Alytes (Paris) 2005;22:109–129. [Google Scholar]
- Gross TS, Smith EE, Wiebe E, Sepulveda MS, Carr J, Du Preez LH, et al. 2003. An evaluation of gonadal anomalies and agri-chemical exposures for the cane toad (Bufo marinus) in south Florida, In: 24th Annual Meeting in North America, Society of Environmental Toxicology and Chemistry, 9–13 November 2003, Austin, Texas. Available: http://abstracts.co.allenpress.com/pweb/setac2003 [accessed 30 March 2006].
- Harris M, Bishop C, Struger J, Ripley B, Bogart J. The functional integrity of northern leopard frog (Rana pipiens) and green frog (Rana clamitans) populations in orchard wetlands: II. Effects of pesticides and eutrophic conditions on early life stage development. Environ Toxicol Chem. 1998a;17:1351–1363. [Google Scholar]
- Harris M, Bishop C, Struger J, van den Heauvel M, van Der Kraak G, Dixon D, et al. The functional integrity of northern leopard frog (Rana pipiens) and green frog (Rana clamitans) populations in orchard wetlands: I. Genetics, physiology, and biochemistry of breeding adults and young-of-the-year. Environ Toxicol Chem. 1998b;17:1338–1350. [Google Scholar]
- Harvey P, Darbre P. Endocrine disrupters and human health: could oestrogenic chemicals in body care cosmetics adversely affect breast cancer incidence in women? A review of evidence and call for further research. J Appl Toxicol. 2004;24:167–176. doi: 10.1002/jat.978. [DOI] [PubMed] [Google Scholar]
- Harvey PW, Johnson I. Approaches to the assessment of toxicity data with endpoints related to endocrine disruption. J Appl Toxicol. 2002;22:241–247. doi: 10.1002/jat.854. [DOI] [PubMed] [Google Scholar]
- Hayes T. 2000. Endocrine disruption in amphibians. In: Ecotoxicology of Amphibians and Reptiles (Sparling D, Linder G, Bishop C, eds). Pensacola, FL:SETAC Press, 595–616.
- Hayes TB. Interdependence of corticosterone and thyroid hormones in larval growth and development in the western toad (Bufo boreas). I. Thyroid hormone dependent and independent effects of corticosterone on growth and development. J Exp Zool. 1995a;271:95–102. doi: 10.1002/jez.1402710204. [DOI] [PubMed] [Google Scholar]
- Hayes TB. A histological examination of the effects of corticosterone in larvae of the western toad, Bufo boreas (Anura: Bufonidae) and the oriental fire-bellied toad, Bombina orientalis (Anura: Discoglossidae) J Morphol. 1995b;226:297–307. doi: 10.1002/jmor.1052260306. [DOI] [PubMed] [Google Scholar]
- Hayes TB. 1997a. Steroid-mimicking environmental contaminants: their potential role in amphibian declines. In: Herpetologia Bonnensis (Böhme W, Bischoff W, Ziegler T, eds). Bonn, Germany:SEH, 145–150.
- Hayes TB. Steroids as modulators of thyroid hormone activity in amphibian development. Am Zool. 1997b;37:185–195. [Google Scholar]
- Hayes TB. Endocrine disruptors in amphibians: potential impacts and the usefulness of amphibian screens for detecting endocrine disrupting compounds. Sci J (Kagaku) 1998;68:557–568. [Google Scholar]
- Hayes TB. There is no denying this: defusing the confusion about atrazine. Bioscience. 2004;54:1138–1149. [Google Scholar]
- Hayes TB. Welcome to the revolution: integrative biology and assessing the impact of endocrine disruptors on environmental and public health. J Integr Comp Biol. 2005;45:321–329. doi: 10.1093/icb/45.2.321. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Chan R, Licht P. Interactions of temperature and steroids in growth, development, and metamorphosis in a toad (Bufo boreas) J Exp Zool. 1993;266:206–215. doi: 10.1002/jez.1402660306. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, et al. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci USA. 2002a;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environ Health Perspect. 2002b;111:568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Feminization of male frogs in the wild. Nature. 2002c;419:895–896. doi: 10.1038/419895a. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Licht P. Gonadal involvement in sexual size dimorphism in the African bullfrog (Pyxicephalus adspersus) J Exp Zool. 1992;264:120–135. [Google Scholar]
- Hayes TB, Licht P. Factors influencing testosterone metabolism by anuran larvae. J Exp Zool. 1995;271:112–119. doi: 10.1002/jez.1402710206. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Stuart AA, Mendoza M, Collins A, Noriega N, Vonk A, et al. Characterization of atrazine-induced gonadal malformations in African clawed frogs (Xenopus laevis) and comparisons with effects of an androgen antagonist (cyproterone acetate) and exogenous estrogen (17β-estradiol): support for the demasculinization/feminization hypothesis. Environ Health Perspect. 2006;114(suppl 1):134–141. doi: 10.1289/ehp.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Wu T-H. Interdependence of corticosterone and thyroid hormones in larval growth and development in the western toad (Bufo boreas). II. Regulation of corticosterone and thyroid hormones. J Exp Zool. 1995;271:103–111. doi: 10.1002/jez.1402710205. [DOI] [PubMed] [Google Scholar]
- Hayes TB, Wu T-H, Gill T. Similarities in effects of DDT and corticosterone in anuran larvae: is DDT a stressor or corticosterone mimic? Environ Toxicol Chem. 1997;16:1948–1953. [Google Scholar]
- Hecker MJ, Giesy JP, Jones P, Jooste AM, Carr J, Solomon KR, et al. Plasma sex steroid concentrations and gonadal aromatase activities in African clawed frogs (Xenopus laevis) from South Africa. Environ Toxicol Chem. 2004;23:1996–2007. doi: 10.1897/03-450. [DOI] [PubMed] [Google Scholar]
- Hecker M, Kim W, Park J-W, Murphy M, Villeneuve D, Coady K, et al. Plasma concentrations of estradiol and testosterone, gonadal aromatase activity and ultrastructure of the testis in Xenopus laevis exposed to estradiol or atrazine. Aquat Toxicol (Amsterdam) 2005;72:383–396. doi: 10.1016/j.aquatox.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Hofmeister M, Bonefeld-Jorgensen E. Effects of the pesticides prochloraz and methiocarb on human estrogen receptor alpha and beta mRNA levels analyzed by on-line RT-PCRE. Toxicology In Vitro. 2004;18:427–433. doi: 10.1016/j.tiv.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Holtfreter J. Uber die Aufzucht isolierter Teile des Amphibian Keimes II. Arch F Ent Mech. 1931;124:404–465. doi: 10.1007/BF00652482. [DOI] [PubMed] [Google Scholar]
- Howard R. Sexual dimorphism in bullfrogs. Ecology. 1981;62:303–310. [Google Scholar]
- Howe C, Berrill M, Pauli B, Helbing C, Werry K, Veldhoen N. Toxicity of glyphosate-based pesticides to four North American frog species. Environ Toxicol Chem. 2004;23:1928–1938. doi: 10.1897/03-71. [DOI] [PubMed] [Google Scholar]
- Howe G, Gillis R, Mowbray R. Effect of chemical synergy and larval stage on the toxicity of atrazine and alachlor to amphibian larvae. Environ Toxicol Chem. 1998;17:519–525. [Google Scholar]
- Insensee A, Nash R, Helling C. Effect of conventional vs. no-tillage on pesticide leaching to shallow groundwater. J Environ Qual. 1990;19:434–440. [Google Scholar]
- Jancovich J, Davidson E, Parameswaran N, Mao J, Chinchar V, Collins J, et al. Evidence for emergence of an amphibian iridoviral disease because of human-enhanced spread. Mol Ecol. 2005;14:213–224. doi: 10.1111/j.1365-294X.2004.02387.x. [DOI] [PubMed] [Google Scholar]
- Jooste A, Du Preez L, Carr J, Giesy JP, Gross T, Kendall R, et al. Response to comment on “Gonadal development of larval male Xenopus laevis exposed to atrazine in outdoor microcosms. Environ Sci Technol. 2005a;39:7759–7760. [Google Scholar]
- Jooste AM, Du Preez LH, Carr J, Giesy JP, Gross C, Kendall RJ, et al. Gonadal development of larval male Xenopus laevis exposed to atrazine in outdoor microcosms. Environ Sci Technol. 2005b;39:5255–5261. doi: 10.1021/es048134q. [DOI] [PubMed] [Google Scholar]
- Kiesecker J. Synergism between trematode infection and pesticide exposure: a link to amphibian limb deformities in nature? Proc Natl Acad Sci USA. 2002;99:9900–9904. doi: 10.1073/pnas.152098899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesecker J, Blaustein A. Effects of introduced bullfrogs and smallmouth bass on mirohabitat use, growth, and survival of native red-legged frogs (Rana aurora) Conserv Biol. 1998;12:776–787. [Google Scholar]
- Kiesecker J, Blaustein A, Belden L. Complex causes of amphibian population declines. Nature (Lond) 2001;410:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- Kirby M, Allen Y, Dyer R, Feist S, Katsiadaki I, Matthiessen P, et al. Surveys of plasma vitellogenin and intersex in male flounder (Platichthys flesus) as measures of endocrine disruption by estrogenic contamination in United Kingdom estuaries: temporal trends, 1996 to 2001. Environ Toxicol Chem. 2004;23:748–758. doi: 10.1897/03-166. [DOI] [PubMed] [Google Scholar]
- Kleinkauf A, Macfarlane C, Yeates S, Simpson M, Leah R. A biomarker approach to endocrine disruption in flounder-estrogen receptors, hepatocyte proliferation, and sperm motility. Ecotoxicol Environ Safety. 2004;58:324–334. doi: 10.1016/j.ecoenv.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Kolpin D, Barbash J, Gilliom R. Occurrence of pesticides in shallow groundwater of the United States: initial results from the National Water-Quality Assessment Program. Environ Sci Technol. 1998;32:558–566. [Google Scholar]
- Kucklick J, Bidleman T. Organic contaminants in Winyah Bay South Carolina. I: Pesticides and polycyclic aromatic hydrocarbons in subsurface and microlayer waters. Mar Environ Res. 1994a;37:63–78. [Google Scholar]
- Kucklick J, Bidleman T. Organic contaminants in Winyah Bay, South Carolina. II: Using natural fluorescence to follow atrazine levels and river mixing. Mar Environ Res. 1994b;37:79–91. [Google Scholar]
- Lardner B. Plasticity or fixed adaptive traits? Strategies for predation avoidance in Rana arvalis tadpoles. Oecologia. 1998;117:119–126. doi: 10.1007/s004420050639. [DOI] [PubMed] [Google Scholar]
- Lawler S, Dritz D, Strange T, Holyoak M. Effects of introduced mosquitofish and bullfrogs on the threatened California red-legged frog. Conserv Biol. 1999;13:613–622. [Google Scholar]
- LeNoir J, McConnell L, Fellers G, Cahill T, Seiber J. Summertime transport of current-use pesticides from California’s central valley to the Sierra Nevada mountain range, USA. Environ Toxicol Chem. 1999;18:2715–2722. [Google Scholar]
- Licht L, Grant K. The effects of ultraviolet radiation on the biology of amphibians. Am Zool. 1997;37:137–145. [Google Scholar]
- Lips K, Mendelson JI, Munoz-Alonso A, Canseco-Marquez L, Mulcahy D. Amphibian population declines in montane southern Mexico: resurveys of historical localities. Biol Conserv. 2004;119:555–564. [Google Scholar]
- Lutz I, Kloas W. Amphibians as a model to study endocrine disruptors: I. Environmental pollution and estrogen receptor binding. Sci Total Environ. 1999;225:49–57. doi: 10.1016/s0048-9697(99)80016-3. [DOI] [PubMed] [Google Scholar]
- Masutomi N, Shibutani M, Takagi H, Uneyama C, Lee K-Y, Hirose M. Alteration of pituitary hormone-immunoreactive cell populations in rat offspring after maternal dietary exposure to endocrine-active chemicals. Arch Toxicol. 2004;78:232–240. doi: 10.1007/s00204-003-0528-x. [DOI] [PubMed] [Google Scholar]
- Mazanti L. 1999. The Effects of Atrazine, Metolachlor and Chlorpyrifos on the Growth and Survival of Larval Frogs under Laboratory and Field Conditions. College Park, MD:University of Maryland, 146.
- Mazanti L, Sparling D, Rice C, Bialek K, Stevenson C, Teels B. 2003. Synergistic effects of a combined exposure to herbicides and an insecticide in Hyla versicolor In: Multiple Stressor Effects in Relation to Declining Amphibian Populations (Linder G, Krest S, Sparling D, Little E, eds). ASTM STP 1443. West Conshohocken, PA:American Society for Testing Materials, 111–129.
- Mazzoni R, Cunningham A, Apolo A, Perdomo E, Speranza G. Emerging pathogen of wild amphibians in frogs (Rana catesbeiana) farmed for international trade. Emerg Infect Dis. 2003;9:995–998. doi: 10.3201/eid0908.030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H. Inconclusiveness of chytridiomycosis as the agent in widespread frog declines. Conserv Biol. 2005;19:1421–1430. [Google Scholar]
- Merchant-Larios H, Villapando I. Ultrastructural events during early gonadal development in Rana pipiens and Xenopus laevis. Anat Rec. 1981;199:349–360. doi: 10.1002/ar.1091990305. [DOI] [PubMed] [Google Scholar]
- Michallet-Ferrier P, Ait-Aissa S, Balaguer P, Dominik J, Haffner G, Pardos M. Assessment of estrogen (ER) and aryl hydrocarbon receptor (AhR) mediated activities in organic sediment extracts of the Detroit River, using in vitro bioassays based on human MELN and teleost PLHC-1 cell lines. J Great Lakes Res. 2004;30:82–92. [Google Scholar]
- Miller S, Sweet C, Depinto J, Hornbuckle K. Atrazine and nutrients in precipitation: results from the Lake Michigan mass balance study. Environ Sci Technol. 2000;34:55–61. doi: 10.1021/es991463b. [DOI] [PubMed] [Google Scholar]
- Miyahara M, Oka T, Mitsui N, Sagoe C, Kashiwagi A, Shinkai T, et al. 2003. Evaluation of atrazine on Xenopus laevis in a partial life test [Abstract]. In: 6th Annual Meeting of Japan Society of Endocrine Disruptor Research, 2–3 December 2003, Sendai, Japan. Tsukuba City, Japan:Japan Society of Endocrine Disrupters Research, 259.
- Mueller S. Xenoestrogens: mechanisms of action and detection methods. Anal Bioanal Chem. 2004;378:582–587. doi: 10.1007/s00216-003-2238-x. [DOI] [PubMed] [Google Scholar]
- Müller S, Berg M, Ulrich M, Schwarzenbach RP. Atrazine and its primary metabolites in Swiss lakes: input characteristics and long-term behavior in the water column. Environ Sci Technol. 1997;31:2104–2113. [Google Scholar]
- Muths E, Corn P, Pessier A, Green D. Evidence for disease-related amphibian decline in Colorado. Biol Conserv. 2003;110:357–365. [Google Scholar]
- Nations B, Hallberg G. Pesticides in Iowa precipitation. J Environ Qual. 1992;21:486–492. [Google Scholar]
- Nejaty H, Lacey M, Whitehead SA. Differing effects of endocrine-disrupting chemicals on basal and FSH-stimulated progesterone production in rat granulosa-luteal cells. Exp Biol Med. 2001;226:570–576. doi: 10.1177/153537020122600610. [DOI] [PubMed] [Google Scholar]
- Nicieza A. Interacting effects of predation risk and food availability on larval anuran behavior and development. Oecologia. 2000;123:497–505. doi: 10.1007/s004420000343. [DOI] [PubMed] [Google Scholar]
- Noriega N, Hayes TB. DDT congener effects on secondary sex coloration in the reed frog Hyperolius argus: a partial evaluation of the Hyperolius argus estrogen screen. Comp Biochem Physiol B Biochem Mol Biol. 2000;126B:231–237. doi: 10.1016/s0305-0491(00)00201-7. [DOI] [PubMed] [Google Scholar]
- O’Dwyer T, Buttemer W, Priddel D. Inadvertent translocation of amphibians in the shipment of agricultural produce into New South Wales: its extent and conservation implications. Pac Conserv Biol. 2000;6:40–45. [Google Scholar]
- Osano O, Admiraal W, Otieno D. Developmental disorders in embryos of the frog Xenopus laevis induced by chloroacetanilide herbicides and their degradation products. Environ Toxicol Chem. 2002;21:375–379. doi: 10.1897/1551-5028(2002)021<0375:ddieot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Bonin M, Rodrigue J, DesGranges J, Lair J. Hindlimb deformities (ectromelia, ectrodactyly) in free-living anurans from agricultural areas. J Wildl Dis. 1997;33:95–104. doi: 10.7589/0090-3558-33.1.95. [DOI] [PubMed] [Google Scholar]
- Ouellet M, Mikaelian I, Pauli B, Rodrigue J, Green D. Historical evidence of widespread chytrid infection in North American amphibian populations. Conserv Biol. 2005;19:1431–1440. [Google Scholar]
- Palmer B, Huth L, Pieto D, Selcer K. Vitellogenin as a bio-marker for xenobiotic estrogens in an amphibian model system. Environ Toxicol Chem. 1998;17:30–36. [Google Scholar]
- Palmer B, Palmer S. Vitellogenin induction by xenobiotic estrogens in the red-eared turtle and African clawed frog. Environ Health Perspect. 1995;103:19–25. doi: 10.1289/ehp.95103s419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli B. 2004. Reptile and Amphibian Toxicological Literature Database Ottawa, Ontario, CN:Environment Canada. Available: http://www.cwsscf.ec.gc.ca/publications/AbstractTemplate.cfm?lang=e&id=321 [accessed 29 March 2006].
- Pearman P, Garner T, Straub M, Greber U. Response of the Italian agile frog (Rana latastei) to a Ranavirus, frog virus 3: a model for viral emergence in naive populations. J Wildl Dis. 2004;40:660–669. doi: 10.7589/0090-3558-40.4.660. [DOI] [PubMed] [Google Scholar]
- Pennington P, Daugomah J, Colbert A, Fulton M, Key P, Thompson B, et al. Analysis of pesticide runoff from mid-Texas estuaries and risk assessment implication for marine phytoplankton. J Environ Sci Health B. 2001;36:1–14. doi: 10.1081/pfc-100000912. [DOI] [PubMed] [Google Scholar]
- Pickford D, Morris I. Effects of endocrine-disrupting contaminants on amphibian oogenesis: methoxychlor inhibits progesterone-induced maturation of Xenopus laevis oocytes in vitro. Environ Health Perspect. 1999;107:285–292. doi: 10.1289/ehp.99107285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttlitz M, Chivers D, Kiesecker J, Blaustein A. Threat-sensitive predator avoidance by larval Pacific treefrogs (Amphibia, Hylidae) Ethology. 1999;105:449–456. [Google Scholar]
- Rawlings N, Cook S, Waldbillig D. Effects of the pesticides carbofuran, chlorpyrifos, dimethoate, lindane, triallate, trifluralin, 2,3-D, and pentachlorophenol on metabolic endocrine and reproductive endocrine system in ewes. J Toxicol Environ Health A. 1998;54:21–36. doi: 10.1080/009841098159006. [DOI] [PubMed] [Google Scholar]
- Reeder A, Foley G, Nichols D, Hansen L, Wikoff B, Faeh S, et al. Forms and prevalence of intersexuality and effects of environmental contaminants on sexuality in cricket frogs (Acris crepitans) Environ Health Perspect. 1998;106:261–266. doi: 10.1289/ehp.98106261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relyea R. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology. 2001a;82:523–540. [Google Scholar]
- Relyea R. The relationship between predation risk and antipredator responses in larval anurans. Ecology. 2001b;67:434–441. [Google Scholar]
- Relyea R. Predator cues and pesticides: a double dose of danger for amphibians. Ecol Appl. 2003;13:1515–1521. [Google Scholar]
- Relyea R. Growth and survival of five amphibian species exposed to combinations of pesticides. Environ Toxicol Chem. 2004;23:1737–1742. doi: 10.1897/03-493. [DOI] [PubMed] [Google Scholar]
- Relyea R. The lethal impact of Roundup on aquatic and terrestrial amphibians. Ecol Appl. 2005;15:1118–1124. [Google Scholar]
- Renner R. Amphibian declines: conflict brewing over herbicide links to frog deformities. Science. 2002;298:938. doi: 10.1126/science.298.5595.938. [DOI] [PubMed] [Google Scholar]
- Richards C, Nace G. Gynogenetic and hormonal sex reversal used in tests of the XX-XY hypothesis of sex determination in Rana pipiens. Growth. 1978;42:319–331. [Google Scholar]
- Rohr J, Elskus A, Shepherd B, Crowley P, McCarthy T, Niedzwiecki J, et al. Multiple stressors and salamanders: effects of an herbicide, food limitation, and hydroperiod. Ecol Appl. 2004;14:1028–1040. [Google Scholar]
- Rohr J, Palmer B. Aquatic herbicide exposure increases salamander desiccation risk eight months later in a terrestrial environment. Environ Toxicol Chem. 2005;24:1253–1258. doi: 10.1897/04-448r.1. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith L, Doersam J, Longcore J, Taylor S, Shamblin J, Carey C, et al. Antimicrobial peptide defenses against pathogens associated with global amphibian declines. Dev Comp Immunol. 2002;26:63–72. doi: 10.1016/s0145-305x(01)00041-6. [DOI] [PubMed] [Google Scholar]
- Schmidt BR. Count data, detection probabilities, and the demography, dynamics, distribution, and decline of amphibians. C R Biol. 2003;326:S119–S124. doi: 10.1016/s1631-0691(03)00048-9. [DOI] [PubMed] [Google Scholar]
- Shine R. Sexual selection and sexual dimorphism in the amphibia. Copeia. 1979;1979:297–306. [Google Scholar]
- Shine R. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Q Rev Biol. 1989;64:419–461. doi: 10.1086/416458. [DOI] [PubMed] [Google Scholar]
- Skelly D. Activity level and the susceptibility of anuran larvae to predation. Anim Behav. 1994;47:465–468. [Google Scholar]
- Smith D. Adult recruitment in chorus frogs: effects of size and date at metamorphosis. Ecology. 1987;68:344–350. [Google Scholar]
- Sohoni P, Sumpter J. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158:327–339. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- Sokal R, Rohlf F. 1981. Biometry: The Principles and Practice of Statistics in Biological Research. New York:W.H. Freeman.
- Solomon K, Baker D, Richards R, Dixon K, Klaine S, LaPoint T, et al. Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem. 1996;15:31–76. doi: 10.1002/etc.2050. [DOI] [PubMed] [Google Scholar]
- Sonnenschein C, Soto A. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol. 1998;65:143–150. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- Sparling D, Fellers G, McConnell L. Pesticides and amphibian population declines in California, USA. Environ Toxicol Chem. 2001;20:1591–1595. doi: 10.1897/1551-5028(2001)020<1591:paapdi>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sparling DW, Krest SK, Linder G. 2003. Multiple stressors and declining amphibian populations: an integrated analysis of cause-effect to support adaptive resource management. In: Amphibian Decline: An Integrated Analysis of Multiple Stressor Effects (Linder G, Krest SK, Sparling DW, eds). Pensacola, FL:SETAC Press, 1–7.
- Storfer A. Amphibian declines: future directions. Divers Distrib. 2003;9:151–163. [Google Scholar]
- Stuart S, Chanson J, Cox N, Young B, Rodrigues A, Fischman D, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Sullivan K, Spence K. Effects of sublethal concentrations of atrazine and nitrate on metamorphosis of the African clawed frog. Environ Toxicol Chem. 2003;22:627–635. [PubMed] [Google Scholar]
- Tavera-Mendoza L, Ruby S, Brousseau P, Fournier M, Cyr D, Marcogliese D. Response of the amphibian tadpole (Xenopus laevis) to atrazine during sexual differentiation of the testis. Environ Toxicol Chem. 2002a;21:527–531. doi: 10.1897/1551-5028(2002)021<0527:rotatx>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Tavera-Mendoza L, Ruby S, Brousseau P, Fournier M, Cyr D, Marcogliese D. Response of the amphibian tadpole Xenopus laevis to atrazine during sexual differentiation of the ovary. Environ Toxicol Chem. 2002b;21:1264–1267. [PubMed] [Google Scholar]
- Thurman E, Cromwell A. Atmospheric transport, deposition, and fate of triazine herbicides and their metabolites in pristine areas at Isle Royale National Park. Environ Sci Technol. 2000;34:3079–3085. [Google Scholar]
- Thurman E, Goolsby D, Meyer M, Mills M, Pomes M, Kolpin D. A reconnaissance study of herbicides and their metabolites in surface water of the midwestern United States using immunoassay and gas chromatography/mass spectrometry. Environ Sci Technol. 1992;26:2440–2447. [Google Scholar]
- Uzumcu M, Suzuki H, Skinner M. Effect of the anti-androgenic endocrine disruptor vinclozolin on embryonic testis cord formation and postnatal testis development and function. Reprod Toxicol. 2004;18:765–774. doi: 10.1016/j.reprotox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Van Dijk H, Guicherit R. Atmospheric dispersion of current-use pesticides: a review of the evidence from monitoring studies. Water Air Soil Pollut. 1999;115:21–70. [Google Scholar]
- Wang X-M, Zhang K-J, Wang Z-H, Ding Y-Z, Wu W, Huang S. The decline of the Chinese giant salamander Andrias davidianus and implications for its conservation. Oryx. 2004;36:197–202. [Google Scholar]
- Weldon C, du Preez L, Hyatt A, Muller R, Speare R. Origin of the amphibian chytrid fungus. Emerg Infect Dis. 2004;10:2100–2105. doi: 10.3201/eid1012.030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner E. Amphibian metamorphosis: growth rate, predation risk, and the optimal size at transformation. Am Nat. 1986;128:319–341. [Google Scholar]
- Wilbur H, Collins J. Ecological aspects of amphibian metamorphosis. Science. 1973;182:1305–1313. doi: 10.1126/science.182.4119.1305. [DOI] [PubMed] [Google Scholar]
- Withgott J. Amphibian declines: ubiquitous herbicide emasculates frogs. Science. 2002;296:447–448. doi: 10.1126/science.296.5567.447a. [DOI] [PubMed] [Google Scholar]