Abstract
Significantly higher frequencies of tumor necrosis factor alpha- and interleukin-2-secreting human T-lymphotropic virus type 1 (HTLV-1)-specific CD4+ T cells were present in the peripheral blood mononuclear cells of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients than in those of asymptomatic carriers with similar provirus loads. The data suggest that HTLV-1-specific CD4+ T cells play a role in the pathogenesis of HAM/TSP.
HTLV-1 (human T-lymphotropic virus type 1) is the causative agent of adult T-cell leukemia/lymphoma and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (46) and is distantly related to human immunodeficiency virus type 1 (HIV-1). However, whereas infection with HIV-1 results in disease in almost all of those infected, infection with HTLV-1 usually results in persistent asymptomatic carriage; less than 7% will develop either adult T-cell leukemia/lymphoma or HAM/TSP. The cause of these differing outcomes is unknown. Despite the fact that HTLV-1 causes disabling and fatal diseases, there is no vaccine and no satisfactory treatment for either the malignancy or the inflammatory diseases.
HAM/TSP is characterized by a chronic, progressive, inflammatory, demyelinating myelopathy that resembles progressive spinal forms of multiple sclerosis. The pathogenesis of this disease is unclear but is thought to involve the host's immune response to the virus (5, 22). The lesions in the central nervous system (CNS) contain infiltrates of inflammatory cells. Until now, most work on the cell-mediated immune response to HTLV-1 has focused on the cytotoxic T-lymphocyte (CTL) response. CTL responses are critical to clearing virus infections by the specific lysis of infected cells and secretion of antiviral cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (33).
In addition to CTLs, acquired immunity is also critically dependent on the generation of virus-specific CD4+ T-helper responses. By secreting cytokines upon recognition of cognate antigen, the CD4+ T-helper cell is involved in the coordination of the immune response to the invading pathogen. Activated T-helper cells can produce interleukin-2 (IL-2) and IFN-γ, which augment CTL effector functions (Th1), and IL-4, IL-5, IL-6, and IL-10, which promote B-cell and antibody responses (Th2). T-helper cells can also activate professional antigen-presenting cells by secretion of cytokines or upregulation of CD40L on the cell surface (10, 28, 43). Several observations on HTLV-1 demonstrate the potential importance of CD4+ T cells in the cellular immune response to the virus. (i) CD4+ T cells are the main subset of cells infected with HTLV-1 in vivo (18, 42). (ii) Infected CD4+ T cells spontaneously secrete proinflammatory, neurotoxic cytokines such as TNF-α and IFN-γ (17, 19, 38), and high levels of these cytokines have been demonstrated in the serum, cerebrospinal fluid, and spinal cord lesions of HAM/TSP patients (30, 36, 48). (iii) HTLV-1 infection in CD4+ T cells can impair T-helper function (41, 51). (iv) CD4+ CD45RO+ T cells are the predominant infiltrating subset (65%) of total CD3+ T cells in newer CNS lesions of HAM/TSP patients (21). Others have also detected more CD4+ T cells in the CNS lesions of HAM/TSP patients with a short history of disease (1, 47) than in patients with a long history of disease (when CD8+ T cells predominate) (47).
We recently reported that IFN-γ-secreting, HTLV-1-specific CD4+ T cells were significantly more frequent in HAM/TSP patients than in asymptomatic carriers (ACs) (16). This observation suggested that these HTLV-1-specific CD4+ T cells could be involved in the initiation and pathogenesis of HAM/TSP. According to the bystander damage hypothesis (11, 20), the inflammatory and neurotoxic cytokines TNF-α and IFN-γ could cause the nonspecific cell death and inflammation seen in HAM/TSP patients.
In this study, we tested the hypotheses that (i) HAM/TSP patients and ACs with similar proviral loads would differ in the frequencies of TNF-α- and IL-2-secreting HTLV-1-specific CD4+ T cells and (ii) there is a correlation between the proviral load and the frequency of Th1-type CD4+ T cells in either HAM/TSP patients or ACs.
We therefore used a flow cytometric assay with intracellular cytokine staining (16, 17) to detect IFN-γ-, TNF-α-, and IL-2-positive, HTLV-1-specific CD4+ T cells. The cells were stimulated with overlapping peptides (20 amino acids, overlapping by 14 or 15) corresponding to all of the major proteins of HTLV-1. Peptides were grouped into one pool and added to cell culture medium to achieve a final concentration of 1 μg of each peptide per ml prior to incubation at 37°C. This strategy (2, 6, 27, 32) should detect the total T-helper type 1 response to all potential epitopes of HTLV-1. We studied HTLV-1-infected ACs and patients with HAM/TSP attending the HTLV-1 clinic at St. Mary's Hospital, London, England. The diagnosis of HAM/TSP was made in accordance with World Health Organization criteria (40). Control samples were obtained from healthy volunteers in our laboratory. Peripheral blood mononuclear cells (PBMCs) were isolated by standard density gradient centrifugation and cryopreserved until use.
Nonspecific cytokine production by PBMCs was induced by a combination of 0.1 ng of phorbol myristate acetate (PMA) per ml (Sigma) and 0.5 μg of A23187 (Sigma) per ml, or Staphylococcus enterotoxin B (SEB) at 5 μg/ml was added to the culture medium. Twenty nanomolar concanamycin A (a potent inhibitor of perforin activity) was added to the culture medium to prevent CTL-mediated lysis via the perforin-dependent cytotoxic pathway (18). Ten micrograms of brefeldin A (Sigma) per ml was added for the last 5 h of culture to inhibit protein secretion. HTLV-1 DNA was quantified by real-time PCR with a LightCycler (Roche, Mannheim, Germany) (45). HTLV-1 primers SK43 and SK44 (31) were used. The β-globin copy number of each sample was similarly quantified with primers PC03 and PC04 (44). Standard curves were generated for both PCRs with genomic DNA from C10-PBL cells, which contain one Tax copy per cell.
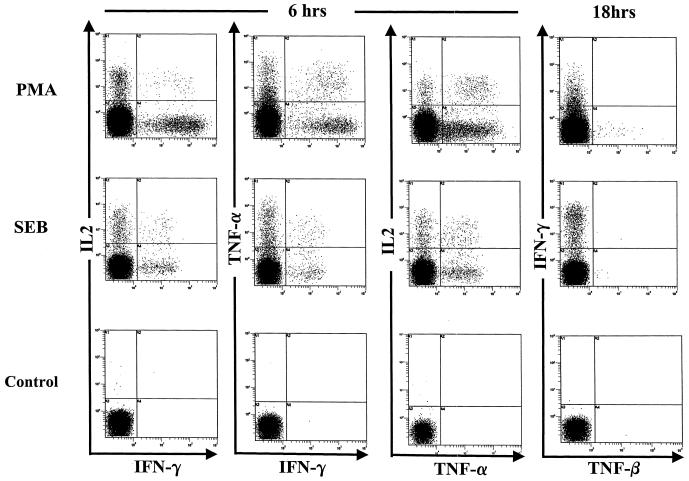
We have previously published data on the flow cytometric detection of IFN-γ-positive, CD4+ T cells in HTLV-1-infected patients (16). We established that our 6-h flow cytometric assay could also be used to detect the cytokines TNF-α, TNF-β (lymphotoxin), and IL-2. As positive controls, we used the nonspecific T-cell stimulators PMA-A23187 and SEB. Figure 1 illustrates the cytokine characterization of CD4+ T cells after 6 and 18 h of in vitro culture with brefeldin A inhibition of protein secretion. The results showed that IFN-γ, TNF-α, and IL-2 protein expression could be detected after 6 h. TNF-β was only detectable after 18 h of culture and only from a small percentage of CD4+ T cells. Isotype controls showed that the staining was specific (data not shown). Similar data were obtained from three uninfected controls, two HAM/TSP patients, and two ACs.
FIG. 1.
Th1-type cytokine functional characterization of CD4+ T cells. Staining for intracellular IFN-γ, TNF-α, TNF-β, and IL-2 was conducted following stimulation with SEB or with PMA and A23187 (a calcium ionophore) for 6 or 18 h of in vitro culture of PBMCs. Brefeldin A was added for the last 5 h to inhibit protein secretion. TNF-β was only detected after 18 h of culture and only at a low frequency. Cells were gated on the CD4hi population for SEB stimulation and controls or on all lymphocytes for PMA stimulation.
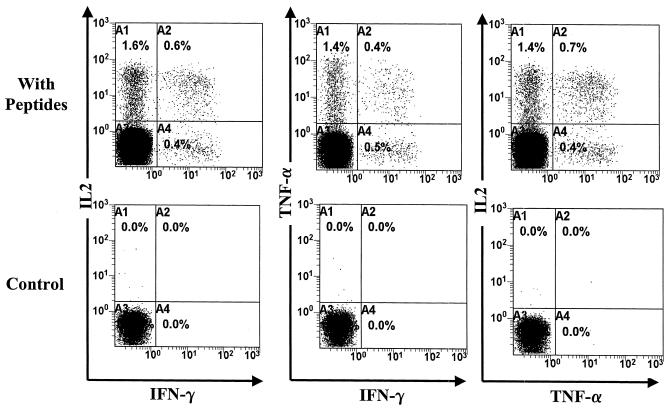
We applied the 6-h assay to our cohort of HAM/TSP patients and ACs with similar proviral loads (Table 1). We initially also stained for TNF-β-positive, HTLV-1-specific CD4+ T cells in two HAM/TSP patients (TAQ and TAL) and two ACs (HT and HBI) but did not detect any positive cells. We therefore focused only on TNF-α and IL-2 staining for the other seven subjects in each group. Figure 2 shows the flow cytometric data from a representative HAM/TSP patient (TBO). We characterized the cytokine expression of HTLV-1-specific CD4+ T cells in nine HAM/TSP patients and nine ACs with similar proviral loads. The data (Table 1) show that there were large populations of hitherto undetected TNF-α- and IL-2-positive CD4+ T cells that are HTLV-1 specific in HAM/TSP patients but not in ACs. The differences were statistically significant for all three cytokines (IFN-γ, P = 0.0003; TNF-α, P = 0.00056; IL-2, P = 0.0003 [Mann-Whitney, two-tailed P values]). There were also small numbers of cells that coexpressed two cytokines, but consistent with previous reports (9, 26), these were much less frequent than single-cytokine expression in the one cell. Triple-cytokine secretors were not tested for, but these would be expected to be even less common.
TABLE 1.
HTLV-specific CD4+ T-cell frequencies in HTLV-1-infected subjectsa
| Subject | Proviral load (% PBMC) | Total IFN-γ+ (% CD4+) | Total TNF-α+ (% CD4+) | Total IL-2+ (% CD4+) | Total Th1+ (% CD4+)b |
|---|---|---|---|---|---|
| TAA | 8.65 | 0.30 | 0.68 | 0.57 | 1.04 |
| TAE | 2.10 | 0.50 | 0.60 | 0.82 | 1.28 |
| TBO | 9.05 | 0.91 | 1.47 | 2.03 | 2.69 |
| TAY | 1.50 | 0.10 | 0.21 | 0.24 | 0.45 |
| TAZ | 10.10 | 0.46 | 0.22 | 0.51 | 0.86 |
| TAU | 5.40 | 0.46 | 0.22 | 0.40 | 0.81 |
| TBP | 14.50 | 0.38 | 0.18 | 0.30 | 0.62 |
| TAQ | 1.50 | 0.41 | 0.08 | 0.10 | 0.56 |
| TAL | 4.60 | 0.55 | 0.09 | 0.32 | 0.92 |
| Median | 5.40 | 0.46 | 0.22 | 0.40 | 0.86 |
| HAI | 14.84 | 0.19 | 0.23 | 0.26 | 0.52 |
| HBA | 0.50 | 0.05 | 0 | 0.02 | 0.06 |
| HBF | 4.00 | 0.02 | 0 | 0.02 | 0.04 |
| HBE | 6.90 | 0.20 | 0 | 0.09 | 0.29 |
| HAE | 0.03 | 0.04 | 0 | 0.03 | 0.06 |
| HT | 0.60 | 0.13 | 0 | 0 | 0.13 |
| HBI | 1.00 | 0.17 | 0.03 | 0.04 | 0.22 |
| HS | 5.80 | 0.06 | 0.09 | 0.09 | 0.16 |
| HBK | 4.60 | 0.07 | 0.11 | 0.15 | 0.26 |
| Median | 4.00 | 0.06 | 0 | 0.04 | 0.16 |
| Mann-Whitney P value | 0.1903 | 0.0003c | 0.00056c | 0.0003c | <0.0001c |
Subject codes beginning with T indicate a HAM/TSP patient, while H indicates an AC. Mann-Whitney two-tailed P values show comparisons between HAM/TSP and AC. groups. Total IFN-γ frequency = mean total IFN-γ+ of two stainings (similarly for other cytokines).
Total Th1 frequency = spIFN-γ + spTNF-α + spIL-2 + dpIFN-γ-TNF-α + dpTNFα-IL-2 + dpIFN-γ-IL-2 (no triple-positive data available; therefore, this represents an approximate value). A value of 0% denotes “not detected.” sp = single positive; dp = double positive; e.g., spIFN-γ denotes IFNγ+ IL-2− TNFα−. The data on IFN-γ-confirm and extend our previous work (16).
Significant difference.
FIG. 2.
High frequencies of TNF-α- and IL-2-positive CD4+ T cells in HAM/TSP patients after 6 h of in vitro culture with HTLV-1 peptides. Data on TBO, a patient with HAM/TSP, are shown. Cells were gated on the CD4hi population. The total number of events collected was 200,000 for the peptide-stimulated culture and 100,000 for the control (no peptides).
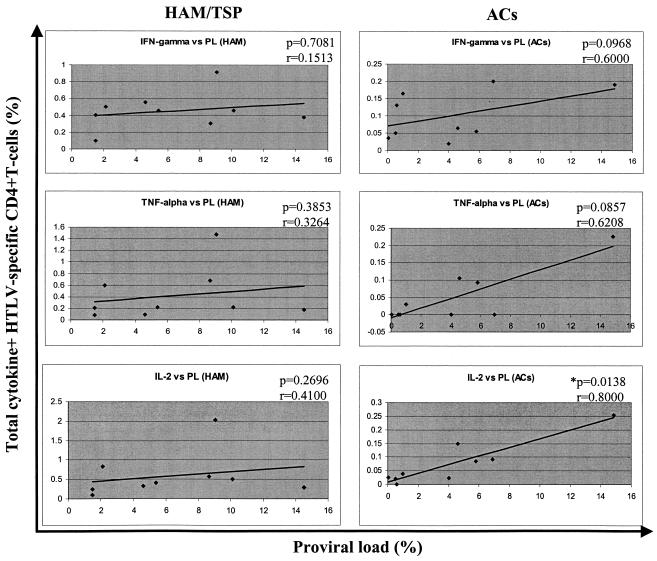
We tested for correlations between individual cytokines and proviral loads in both HAM/TSP subjects and ACs by nonparametric statistical analysis (Spearman rank correlation). The data are shown in Fig. 3. There was a significant correlation between the frequency of total IL-2-positive HTLV-1-specific CD4+ T cells in ACs and the proviral load (two-tailed P = 0.0138, r = 0.8000). There was a tendency to a positive correlation between proviral load and both IFN-γ and TNF-α in the ACs, but this did not reach statistical significance. The data on HAM/TSP patients showed no significant correlation between the proviral load and the specific-cytokine frequency for the three cytokines tested (Fig. 3).
FIG. 3.
Correlation between frequency of single-cytokine-positive, HTLV-1-specific CD4+ T cells and proviral load (PL). There is a significant positive correlation between IL-2-positive HTLV-1-specific CD4+ T-cell frequency and proviral load in ACs. There is a trend toward a positive correlation for all three cytokines in ACs, but this does not reach significance for IFN-γ and TNF-α. Data were analyzed by Spearman rank correlation. The solid line represents a least-squares regression line. The asterisk indicates a significant correlation.
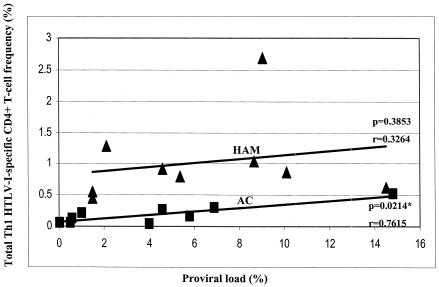
The analysis of T-cell responses with a single cytokine such as IFN-γ may not be representative of the entire Th1-type response. This may account for the conflicting reports of correlation and noncorrelation between antigen loads and cytokine responses, particularly for HIV (6, 12, 15, 39). We therefore estimated the total frequency of Th1-type HTLV-1-specific CD4+ T cells in these subjects (Table 1) and tested for a correlation with proviral load; the data are shown in Fig. 4. We show that the estimated total frequency did not correlate with the proviral load in HAM/TSP patients but there was a significant positive correlation in ACs (HAM/TSP patients, two-tailed P = 0.3853, r = 0.3264; ACs, two-tailed P = 0.0214, r = 0.7615).
FIG. 4.
Correlation between total Th1-type, HTLV-1-specific CD4+ T-cell frequency and proviral load. The total Th1 frequency correlates positively with the proviral load in ACs but not in HAM/TSP patients. Analysis was done by Spearman rank correlation. The solid line represents a least-squares regression line. The asterisk indicates a significant correlation.
The proviral load in HTLV-1 infection has been shown to correlate with the risk of inflammatory diseases such as HAM/TSP (34, 50). There is typically a strong CTL response to the virus in both HAM/TSP patients and ACs (5). The CTL response has been shown to exert significant selection pressure on the Tax protein in ACs (37) but not in HAM/TSP patients. Recent work has suggested that CTLs exert a beneficial effect by lightening the proviral load and protecting against HAM/TSP (24, 25). However, there is also evidence that CTLs contribute to the inflammation seen in HAM/TSP. HTLV-1-specific CTLs are found at higher frequency in cerebrospinal fluid than in peripheral blood, implying either that they preferentially migrate into the CNS or that they selectively expand in the CNS (or both) (29, 35). Also, some of these clones secrete inflammatory cytokines, chemokines, and matrix metalloproteinases (7). It is possible to reconcile these apparently contradictory data with the concept of a threshold proviral load (3). In this model, in an individual with a proviral load below the threshold, sufficient T-cell receptors on HTLV-1-specific CTLs are engaged to ensure efficient cell killing (protective) but insufficient to elicit production of inflammatory cytokines such as TNF-α and IFN-γ. As the proviral load increases, specific CTLs commence cytokine production, in addition to their cytotoxic function (which itself may be pathogenic as well as protective). Indeed, it has been shown that above a threshold proviral load of ∼1% of PBMCs, the risk of HAM/TSP rises exponentially (34).
However, in acquired immunity, the CD4+ T-helper cell also plays a critical role in the immune response. In HTLV-1, little is known about the role of the CD4+ T-helper cell. In this study, we used overlapping peptide libraries of all major HTLV-1 proteins to quantify the Th1 CD4+ T-cell response to the virus in both HAM/TSP patients and infected ACs. Production of IFN-γ is frequently used as a marker of Th1 CD4+ T cells in ELISpot or intracellular cytokine staining assays. However, certain Th1 cells produce IL-2 or TNF-α but not IFN-γ. Previously, we showed that HAM/TSP patients have a predominance of Th1-type HTLV-1-specific CD4+ T cells (16). In this study, we wished to determine whether there are similarly large populations of other Th1-type cytokine (TNF-α, TNF-β, and IL-2)-secreting, HTLV-1-specific CD4+ T cells in HAM/TSP patients compared to ACs with similar proviral loads. These cytokines may play an important role both in the immune response to HTLV-1 and in the pathogenesis of inflammatory neurological disease. A heavy proviral load has been shown to be associated with increased risk and severity of HAM/TSP (34, 50). Therefore, to elucidate a potential mechanism of pathogenesis of HAM/TSP, we selected a group of patients and HTLV-1-infected ACs with similar loads so that any observed differences would not reflect proviral load differences (Table 1).
We show that total TNF-α- and IL-2-positive, HTLV-1-specific CD4+ T cells were significantly more frequent in HAM/TSP patients than in ACs. TNF-α is a powerful proinflammatory cytokine and has been implicated in many inflammatory disorders (13). It also plays a central role in coordination of the inflammatory response (8, 14). The discovery of numerous TNF-α+, HTLV-1-specific CD4+ T cells in patients with inflammatory CNS disease, but not in ACs, supports the hypothesis that bystander damage is inflicted by invading CD4+ T cells in the early, active lesions of HAM/TSP. IL-2 has an important role in lymphocyte proliferation and in arming effector T cells, including both T-helper cells and CTLs. IL-2 has also recently been shown to rescue secondary expansion and function in CTLs depleted of CD4+ T-cell help (23). The high frequency of IFN-γ+, TNF-α+, and IL-2+, HTLV-1-specific CD4+ T cells raises the possibility that such cells contribute to the pathogenesis of inflammatory CNS disease by forming self-perpetuating inflammatory lesions. The observation that these cells are found in abundance in patients with HAM/TSP but not in ACs with similarly high proviral loads suggests that the high Th1-cell frequency is specifically associated with the disease HAM/TSP.
Analyses of total IFN-γ-positive, virus-specific T cells have been reported in several articles (6, 12, 15, 39). Our results show that other Th1 cytokines also need to be examined if a more complete picture of CD4+ T-helper responses in other chronic infections with viruses such as HIV, cytomegalovirus, Epstein-Barr virus, and hepatitis B or C virus is to be obtained. Similarly, detection of IFN-γ alone may give an incomplete measure of virus-specific CD8+ T-cell frequency.
We observed positive correlations between the estimated total Th1 (and total IL-2-positive) CD4+ T-cell frequencies and the proviral load in ACs but not in HAM/TSP patients. There are several interesting implications. The frequency of the Th1 response rises as the proviral load increases in ACs but not in HAM/TSP patients. Yet the proviral loads are not dissimilar between the two groups. This suggests that there are fundamental qualitative (functional), as well as quantitative, differences in the CD4+ T-cell response to HTLV-1.
What might such qualitative differences be? It is not possible to reach a robust conclusion about the efficiency or effectiveness of the antiviral T-cell response from the frequency of such cells in the circulation (4, 6, 49). However, the greater frequency of HTLV-1-specific CD4+ T cells in patients with HAM/TSP and a given proviral load implies either that their CD4+ T cells proliferate faster (or die more slowly) than those of ACs or that more HTLV-1 antigen is expressed in HAM/TSP patients than in ACs with a similar proviral load (or both).
In conclusion, we show here that there are significantly higher frequencies of TNF-α- and IL-2-positive, HTLV-1-specific CD4+ T cells in HAM/TSP patients than in HTLV-1-infected ACs with similar proviral loads. These results confirm and extend our previous work on IFN-γ-positive, HTLV-1-specific CD4+ T cells (16) with another assay. The relative importance of these three cytokines in the pathogenesis of HAM/TSP is unknown. There were positive correlations between the estimated total Th1 (and total IL-2 response) and proviral load in ACs but not in patients with HAM/TSP. The data suggest that there are quantitative and qualitative differences in the T-helper cell populations between HAM/TSP patients and ACs. We have preliminary evidence that the HTLV-1 antigens recognized by CD4+ T cells in HAM/TSP patients and ACs do not differ (unpublished data); however, it remains possible that the epitopes within these antigens differ systematically. The data presented in this report have important implications for the understanding of the pathogenesis of HAM/TSP disease, the cellular immune response to HTLV-1, and possible therapeutic approaches.
Acknowledgments
We thank the staff and patients of St. Mary's Hospital, London, England.
This work was funded by the Wellcome Trust.
REFERENCES
- 1.Akizuki, S. 1989. The neuropathology of human T cell lymphotropic virus type I-associated myelopathy, p. 253-260. In M. Osame (ed.), HTLV-I and the nervous system. Alan R. Liss, Inc., New York, N.Y.
- 2.Appay, V., L. Papagno, C. A. Spina, P. Hansasuta, A. King, L. Jones, G. S. Ogg, S. Little, A. J. McMichael, D. D. Richman, and S. L. Rowland-Jones. 2002. Dynamics of T cell responses in HIV infection. J. Immunol. 168:3660-3666. [DOI] [PubMed] [Google Scholar]
- 3.Asquith, B., and C. R. Bangham. 2000. The role of cytotoxic T lymphocytes in human T-cell lymphotropic virus type 1 infection. J. Theor. Biol. 207:65-79. [DOI] [PubMed] [Google Scholar]
- 4.Bangham, C. R. 2002. Genetics and dynamics of the immune response to HTLV-I. Gann Monogr. Cancer Res. 50:149-170. [Google Scholar]
- 5.Bangham, C. R. 2000. The immune response to HTLV-I. Curr. Opin. Immunol. 12:397-402. [DOI] [PubMed] [Google Scholar]
- 6.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biddison, W. E., R. Kubota, T. Kawanishi, D. D. Taub, W. W. Cruikshank, D. M. Center, E. W. Connor, U. Utz, and S. Jacobson. 1997. Human T cell leukemia virus type I (HTLV-I)-specific CD8+ CTL clones from patients with HTLV-I-associated neurologic disease secrete proinflammatory cytokines, chemokines, and matrix metalloproteinase. J. Immunol. 159:2018-2025. [PubMed] [Google Scholar]
- 8.Brennan, F. M., D. Chantry, A. Jackson, R. Maini, and M. Feldmann. 1989. Inhibitory effect of TNF-α antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet ii:244-247. [DOI] [PubMed]
- 9.Bucy, R. P., L. Karr, G. Q. Huang, J. Li, D. Carter, K. Honjo, J. A. Lemons, K. M. Murphy, and C. T. Weaver. 1995. Single cell analysis of cytokine gene coexpression during CD4+ T-cell phenotype development. Proc. Natl. Acad. Sci. USA 92:7565-7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella, M., D. Scheidegger, K. Palmer-Lehmann, P. Lane, A. Lanzavecchia, and G. Alber. 1996. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184:747-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daenke, S., and C. R. Bangham. 1994. Do T cells cause HTLV-1-associated disease?: a taxing problem. Clin. Exp. Immunol. 96:179-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalod, M., M. Dupuis, J. C. Deschemin, D. Sicard, D. Salmon, J. F. Delfraissy, A. Venet, M. Sinet, and J. G. Guillet. 1999. Broad, intense anti-human immunodeficiency virus (HIV) ex vivo CD8+ responses in HIV type 1-infected patients: comparison with anti-Epstein-Barr virus responses and changes during antiretroviral therapy. J. Virol. 73:7108-7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinarello, C. A. 2003. Cytokines: interleukin-1 and tumour necrosis factor in inflammation, p. 108-111. In D. A. Warrell (ed.), Oxford textbook of medicine, 4th ed., vol. 1. Oxford University Press, Oxford, England.
- 14.Fong, Y., K. J. Tracey, L. L. Moldawer, D. G. Hesse, K. B. Manogue, J. S. Kenney, A. T. Lee, G. C. Kuo, A. C. Allison, S. F. Lowry, et al. 1989. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1β and interleukin 6 appearance during lethal bacteremia. J. Exp. Med. 170:1627-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. deq Uiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 16.Goon, P. K., E. Hanon, T. Igakura, Y. Tanaka, J. N. Weber, G. P. Taylor, and C. R. Bangham. 2002. High frequencies of Th1-type CD4+ T cells specific to HTLV-1 Env and Tax proteins in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. Blood 99:3335-3341. [DOI] [PubMed] [Google Scholar]
- 17.Hanon, E., P. Goon, G. P. Taylor, H. Hasegawa, Y. Tanaka, J. N. Weber, and C. R. Bangham. 2001. High production of interferon gamma but not interleukin-2 by human T-lymphotropic virus type I-infected peripheral blood mononuclear cells. Blood 98:721-726. [DOI] [PubMed] [Google Scholar]
- 18.Hanon, E., S. Hall, G. P. Taylor, M. Saito, R. Davis, Y. Tanaka, K. Usuku, M. Osame, J. N. Weber, and C. R. Bangham. 2000. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood 95:1386-1392. [PubMed] [Google Scholar]
- 19.Higuchi, M., K. Nagasawa, T. Horiuchi, M. Oike, Y. Ito, M. Yasukawa, and Y. Niho. 1997. Membrane tumor necrosis factor-alpha (TNF-α) expressed on HTLV-I-infected T cells mediates a costimulatory signal for B cell activation—characterization of membrane TNF-α. Clin. Immunol. Immunopathol. 82:133-140. [DOI] [PubMed] [Google Scholar]
- 20.Ijichi, S., S. Izumo, N. Eiraku, K. Machigashira, R. Kubota, M. Nagai, N. Ikegami, N. Kashio, F. Umehara, and I. Maruyama. 1993. An autoaggressive process against bystander tissues in HTLV-I-infected individuals: a possible pathomechanism of HAM/TSP. Med. Hypotheses 41:542-547. [DOI] [PubMed] [Google Scholar]
- 21.Iwasaki, Y., Y. Ohara, I. Kobayashi, and S. Akizuki. 1992. Infiltration of helper/inducer T lymphocytes heralds central nervous system damage in human T-cell leukemia virus infection. Am. J. Pathol. 140:1003-1008. [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson, S. 2002. Immunopathogenesis of human T cell lymphotropic virus type I-associated neurologic disease. J. Infect. Dis. 186(Suppl. 2):S187-S192. [DOI] [PubMed] [Google Scholar]
- 23.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 24.Jeffery, K. J., A. A. Siddiqui, M. Bunce, A. L. Lloyd, A. M. Vine, A. D. Witkover, S. Izumo, K. Usuku, K. I. Welsh, M. Osame, and C. R. Bangham. 2000. The influence of HLA class I alleles and heterozygosity on the outcome of human T cell lymphotropic virus type I infection. J. Immunol. 165:7278-7284. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery, K. J., K. Usuku, S. E. Hall, W. Matsumoto, G. P. Taylor, J. Procter, M. Bunce, G. S. Ogg, K. I. Welsh, J. N. Weber, A. L. Lloyd, M. A. Nowak, M. Nagai, D. Kodama, S. Izumo, M. Osame, and C. R. Bangham. 1999. HLA alleles determine human T-lymphotropic virus-I (HTLV-I) proviral load and the risk of HTLV-I-associated myelopathy. Proc. Natl. Acad. Sci. USA 96:3848-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karulin, A. Y., M. D. Hesse, M. Tary-Lehmann, and P. V. Lehmann. 2000. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J. Immunol. 164:1862-1872. [DOI] [PubMed] [Google Scholar]
- 27.Kern, F., N. Faulhaber, C. Frommel, E. Khatamzas, S. Prosch, C. Schonemann, I. Kretzschmar, R. Volkmer-Engert, H. D. Volk, and P. Reinke. 2000. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur. J. Immunol. 30:1676-1682. [DOI] [PubMed] [Google Scholar]
- 28.Koch, F., U. Stanzl, P. Jennewein, K. Janke, C. Heufler, E. Kampgen, N. Romani, and G. Schuler. 1996. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J. Exp. Med. 184:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota, R., S. S. Soldan, R. Martin, and S. Jacobson. 2002. Selected cytotoxic T lymphocytes with high specificity for HTLV-I in cerebrospinal fluid from a HAM/TSP patient. J. Neurovirol. 8:53-57. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda, Y., and M. Matsui. 1993. Cerebrospinal fluid interferon-gamma is increased in HTLV-I-associated myelopathy. J. Neuroimmunol. 42:223-226. [DOI] [PubMed] [Google Scholar]
- 31.Kwok, S., G. Ehrlich, B. Poiesz, R. Kalish, and J. J. Sninsky. 1988. Enzymatic amplification of HTLV-I viral sequences from peripheral blood mononuclear cells and infected tissues. Blood 72:1117-1123. [PubMed] [Google Scholar]
- 32.Maecker, H. T., H. S. Dunn, M. A. Suni, E. Khatamzas, C. J. Pitcher, T. Bunde, N. Persaud, W. Trigona, T. M. Fu, E. Sinclair, B. M. Bredt, J. M. McCune, V. C. Maino, F. Kern, and L. J. Picker. 2001. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J. Immunol. Methods 255:27-40. [DOI] [PubMed] [Google Scholar]
- 33.McMichael, A. J. 2003. Principles of immunology, p. 131-144. In D. A. Warrell (ed.), Oxford textbook of medicine, 4th ed., vol. 1. Oxford University Press, Oxford, England.
- 34.Nagai, M., K. Usuku, W. Matsumoto, D. Kodama, N. Takenouchi, T. Moritoyo, S. Hashiguchi, M. Ichinose, C. R. Bangham, S. Izumo, and M. Osame. 1998. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 4:586-593. [DOI] [PubMed] [Google Scholar]
- 35.Nagai, M., Y. Yamano, M. B. Brennan, C. A. Mora, and S. Jacobson. 2001. Increased HTLV-I proviral load and preferential expansion of HTLV-I Tax-specific CD8+ T cells in cerebrospinal fluid from patients with HAM/TSP. Ann. Neurol. 50:807-812. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura, S., I. Nagano, M. Yoshioka, S. Shimazaki, J. Onodera, and K. Kogure. 1993. Detection of tumor necrosis factor-alpha-positive cells in cerebrospinal fluid of patients with HTLV-I-associated myelopathy. J. Neuroimmunol. 42:127-130. [DOI] [PubMed] [Google Scholar]
- 37.Niewiesk, S., S. Daenke, C. E. Parker, G. Taylor, J. Weber, S. Nightingale, and C. R. Bangham. 1994. The transactivator gene of human T-cell leukemia virus type 1 is more variable within and between healthy carriers than patients with tropical spastic paraparesis. J. Virol. 68:6778-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishiura, Y., T. Nakamura, K. Ichinose, S. Shirabe, A. Tsujino, H. Goto, T. Furuya, and S. Nagataki. 1996. Increased production of inflammatory cytokines in cultured CD4+ cells from patients with HTLV-I-associated myelopathy. Tohoku J. Exp. Med. 179:227-233. [DOI] [PubMed] [Google Scholar]
- 39.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 40.Osame, M. 1990. HTLV, p. 191-197. In W. A. Blattner (ed.), Human retrovirology: HTLV. Raven Press, New York, N.Y.
- 41.Popovic, M., N. Flomenberg, D. J. Volkman, D. Mann, A. S. Fauci, B. Dupont, and R. C. Gallo. 1984. Alteration of T-cell functions by infection with HTLV-I or HTLV-II. Science 226:459-462. [DOI] [PubMed] [Google Scholar]
- 42.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seiki, M., A. Hikikoshi, T. Taniguchi, and M. Yoshida. 1985. Expression of the pX gene of HTLV-I: general splicing mechanism in the HTLV family. Science 228:1532-1534. [DOI] [PubMed] [Google Scholar]
- 45.Tosswill, J. H., G. P. Taylor, J. P. Clewley, and J. N. Weber. 1998. Quantification of proviral DNA load in human T-cell leukaemia virus type I infections. J. Virol. Methods 75:21-26. [DOI] [PubMed] [Google Scholar]
- 46.Uchiyama, T. 1997. Hum. T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 47.Umehara, F., S. Izumo, M. Nakagawa, A. T. Ronquillo, K. Takahashi, K. Matsumuro, E. Sato, and M. Osame. 1993. Immunocytochemical analysis of the cellular infiltrate in the spinal cord lesions in HTLV-I-associated myelopathy. J. Neuropathol. Exp. Neurol. 52:424-430. [DOI] [PubMed] [Google Scholar]
- 48.Umehara, F., S. Izumo, A. T. Ronquillo, K. Matsumuro, E. Sato, and M. Osame. 1994. Cytokine expression in the spinal cord lesions in HTLV-I-associated myelopathy. J Neuropathol. Exp. Neurol. 53:72-77. [DOI] [PubMed] [Google Scholar]
- 49.Wodarz, D., S. E. Hall, K. Usuku, M. Osame, G. S. Ogg, A. J. McMichael, M. A. Nowak, and C. R. Bangham. 2001. Cytotoxic T-cell abundance and virus load in human immunodeficiency virus type 1 and human T-cell leukaemia virus type 1. Proc. R. Soc. Lond. B Biol. Sci. 268:1215-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamano, Y., M. Nagai, M. Brennan, C. A. Mora, S. S. Soldan, U. Tomaru, N. Takenouchi, S. Izumo, M. Osame, and S. Jacobson. 2002. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8+ T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood 99:88-94. [DOI] [PubMed] [Google Scholar]
- 51.Yarchoan, R., H. G. Guo, M. Reitz, Jr., A. Maluish, H. Mitsuya, and S. Broder. 1986. Alterations in cytotoxic and helper T cell function after infection of T cell clones with human T cell leukemia virus, type I. J. Clin. Investig. 77:1466-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]