Abstract
Orf virus (ORFV; Parapoxvirus ovis) was used to develop a novel vector system for the generation of effective and safe live vaccines. Based on the attenuated ORFV strain D1701-V, recombinants were produced that express the glycoproteins gC (D1701-VrVgC) or gD (D1701-VrVgD) of the alphaherpesvirus of swine, pseudorabies virus (PRV). Expression of gC and gD was also demonstrated on the surface of recombinant virus-infected murine cells that do not produce infectious ORFV. Single or combined immunization with the ORFV recombinants protected different mouse strains of a host species nonpermissive for ORFV against a fulminant, lethal PRV challenge infection equal to immunization with PRV live vaccine. Most notably, even a single immunization with D1701-VrVgC was protective, whereas two applications of D1701-VrVgD were required for immune protection. The higher protective capacity of D1701-VrVgC correlated with the induction of a strong specific humoral immune response. This suggestion was supported by transfer experiments using sera from recombinant-immunized mice, which resulted in partial gC but not gD antibody-mediated protection of the naïve recipients. Remarkably, immunization of different immune-deficient mice demonstrated that the application of the PRV gC-expressing recombinant controlled the challenge infection in the absence of either CD4+ or CD8+ T cells, B cells, or an intact perforin pathway. In contrast, D1701-VrVgD-immunized mice lacking CD4+ T cells exhibited reduced protection, whereas animals lacking CD8+ T cells, B cells, or perforin resisted the challenge infection. The present study demonstrates the potential of these new vector vaccines to efficiently prime both protective humoral and cell-mediated immune mechanisms in a host species nonpermissive for the vector virus.
Vaccines based on live virus are excellent inducers of long-term immunity by eliciting protective humoral and cell-mediated immune responses against the inserted antigen. To this end, poxviruses are one of the most versatile expression systems for foreign antigens and have been considered as vectors for human and veterinary live vaccines (37, 41). Long-term immunity induced by vaccinia virus (VACV) or other poxviruses, however, might result in unsuccessful revaccination or reduced protection against VACV-encoded foreign antigens (5, 23, 48). For safety reasons, different strategies are used to develop attenuated, host-restricted, or replication-deficient poxviruses, which retain their ability to activate the host's immune response (for review, see references 38 and 39). The host range-restricted attenuated VACV strain MVA (modified VACV Ankara) was found to induce lower levels of VACV-neutralizing antibodies than wild-type VACV (45) and is currently widely used as a vector vaccine. It cannot grow in human cells (8) and is propagated on primary chicken embryo fibroblasts (CEFs). Avipoxvirus vectors, which show an abortive replication in mammalian cells, are also produced in CEFs (42). However, products from CEFs do not represent optimal safety profiles due to different adventitious contaminants, in contrast to production in permanent cell lines.
Recently, the genus Parapoxvirus (PPV) of the family Poxviridae, and in particular the type species Orf virus (ORFV), has been proposed as candidate for novel vector vaccines. Arguments in favor of an ORFV vector include the very restricted host range (sheep and goats), its tropism restricted to the skin, the lack of systemic infection, a short-term vector-specific protective immunity, and the exceptionally strong stimulation of fast innate cellular immune mechanisms at the site of infection (for review, see references 4 and 47). In addition to cytokines, chemokines, and alpha/beta interferon (IFN-α/β) as part of the host's inflammatory response against the infection, major histocompatibility complex class II-positive dendritic cells accumulate in the infected skin, which represent professional antigen-presenting cells for the subsequent induction of a specific immune response (4, 13). CD4+ T cells dominate the local accumulation of B and T cells and were found to be of importance for the development of ORFV-specific antibodies (24). It is worthwhile to stress the short-lived duration of ORFV-specific immunity, which allows frequent reinfections (14). A most important feature of ORFV in the context with its use as a vaccine is the absence of systemic virus spread, even in immunocompromised individuals or after intravenous injection of high virus doses (4, 18, 47, 59). Occasional transmission of wild-type ORFV to humans often remains unrecognized (4, 14).
A prime candidate for use as a recombinant vector is the highly attenuated, cell culture-adapted ORFV strain D1701, which is almost apathogenic in sheep (31). This attenuated virus strain possesses various immunostimulatory properties (for review, see reference 4). After adaptation of D1701 on the nonruminant Vero cell line, a new variant (D1701-V) was obtained without altered immunogenic properties and also lacking pathogenicity, even in immunosuppressed sheep (4, 50).
To investigate the immunogenicity of recombinant ORFV against a clinically relevant pathogen, the Alphaherpesvirus of swine, Pseudorabies virus (PRV; Herpesvirus suis type 1) was chosen. The neurotropic PRV has a broad host range with a high mortality, including rodents, which are used as models to investigate the role of viral proteins in neurotropism and neurovirulence of PRV (for review, see reference 10). Moreover, mice are commonly used to investigate immunorelevant virus components in the PRV-specific immune response as well as to evaluate the immunogenicity and protective capacity of vaccines against lethal PRV infection. Among the 10 different PRV glycoproteins, particularly the glycoproteins gB, gC, and gD are important for the antiviral humoral and cellular immune responses (33, 58). Several reports demonstrated some protective effect after passive immunization with anti-gC and anti-gD antibodies and the immunogenic relevance of gC and gD by using recombinant VACVs or glycoprotein-encoding plasmid DNA for immunization (12, 16, 17, 27, 32, 46).
The present study describes the generation of ORFV recombinants expressing the PRV glycoproteins gC and gD, which are correctly processed in ORFV permissive and nonpermissive cell cultures. The attenuated ORFV strain D1701-VrV was used as parental virus for recombinant construction, in which the Escherichia coli lacZ gene replaces the virus-encoded VEGF-E gene, which is a functional homologue of the mammalian vascular endothelial growth factor and represents an important virulence factor of ORFV (34, 50, 51). The results presented demonstrate the powerful potential of this vector system to protect against a fulminant, lethal herpesvirus infection. Even single immunization with a mixture of both recombinants or with the gC-expressing D1701-VrV recombinant alone protected mice against a lethal PRV challenge infection. Experiments using different immune-deficient mice revealed that the induced PRV glycoprotein gC-specific humoral response is necessary, although not sufficient to control the challenge infection. Moreover, it was found that the ORFV recombinant-induced immune mechanisms are able to compensate for the lack of either B cells, CD4+ or CD8+ T cells, or perforin.
MATERIALS AND METHODS
Cells and virus.
The attenuated ORFV strain D1701 (31), originally propagated in the bovine kidney cell line BKKL-3A, was adapted to the simian cell line Vero (D1701-V) and propagated as described recently (6). After appearance of cytopathogenic effect (CPE), cells were harvested after trypsin treatment (0.125 mg/ml; Difco, Augsburg, Germany) and centrifuged at 30,000 × g, and the resulting pellet was sonified. Titration and plaque purification of virus were performed with Vero cells in six-well plates with 0.9% (wt/vol) agarose (SeaPlaque agarose; BMA, Rockland, Maine) in minimal essential medium. The recombinant ORFV D1701-VrV contains the functional E. coli lacZ gene replacing the viral VEGF-E gene, which is present in two copies due to its location in the inverted terminal repeats of the D1701 genome (Fig. 1A and B) After removal of the VEGF-E gene, a lacZ gene cassette was inserted into the EcoRV site of plasmid pdV550 (Fig. 1C) (50).
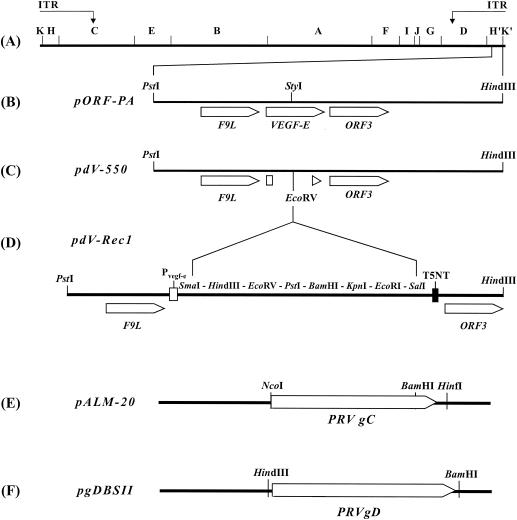
FIG. 1.
Construction of the ORFV recombinants (A) The map locations of HindIII fragments and of the inverted terminal repeats (ITR) of the genome of ORFV strain D1701-V are depicted. (B) The PstI-HindIII fragment containing the VEGF-E and adjacent genes was cloned as plasmid pORF-PA. (C) The singular StyI restriction site in pORF-PA was used to delete the VEGF-E gene by a bidirectional Bal31 digest that resulted in plasmid pdV-550. (D) A synthetic linker covering the indicated restriction sites was inserted into the EcoRV site. The obtained plasmid, pdV-Rec1, contains the early promoter of VEGF-E (Pvegf-e) and the original early transcription stop motif T5NT. (E) The NcoI-HinfI fragment of plasmid pALM-20 containing the complete PRV gC gene was blunt-end ligated into the EcoRV site of pDV-Rec1, resulting in plasmid pdV-gC. (F) The use of the HindIII-BamHI fragment of plasmid pgDBSII allowed cloning of the complete gD gene of PRV in pdV-Rec1 to obtain plasmid pdVgD.
Construction of recombinant transfer plasmids.
For insertion into the VEGF-E gene locus of ORFV D1701-V, the PRV glycoprotein genes were cloned into plasmid pdV-Rec1, which was obtained by linker insertion of a multiple cloning site into pdV550 (Fig. 1D). The complete coding sequence of the PRV gC gene was excised from plasmid pALM-20 (generously provided by L. W. Enquist, Princeton University, Princeton, N.J.) as a 1.47-kbp NcoI-HinfI fragment (Fig. 1E). After fill-in reaction with Klenow polymerase, this fragment was blunt end ligated into the single EcoRV site of pdVRec1, resulting in plasmid pdVgC. The complete PRV gD gene was obtained from plasmid pgDBSII (kindly provided by A. Jestin, AFSSA, Ploufragan, France) by HindIII-BamHI digestion (Fig. 1F) and subsequently ligated into the HindIII and BamHI sites of pdVRec1, resulting in plasmid pdVgD. The correct construction of all plasmids was verified with restriction enzyme digests and DNA sequencing.
Generation and selection of recombinant viruses.
For the generation of ORFV recombinants expressing PRV gC (D1701-VrVgC) or gD (D1701-VrVgD), Vero cells were infected with D1701-VrV at a multiplicity of infection (MOI) of 0.1 50% tissue culture infectious dose (TCID50) and were transfected 2 h later with 2 μg of the recombinant transfer plasmid (pdVgC or pdVgD) and 4 μl of SuperFect transfection reagent according to the recommendations of the manufacturer (Qiagen, Hilden, Germany). Four days later, the cells were lysed by multiple freeze-thawing cycles and plaque titrated on Vero cells. Using a 1% SeaPlaque agarose overlay containing 300 μg of Bluo-Gal per ml (Invitrogen Life Technologies, Karlsruhe, Germany), white virus plaques could be identified after successful exchange of the lacZ gene cassette in D1701-VrV. Immunostaining of virus plaques with a polyclonal goat anti-PRV serum (diluted 1:500), peroxidase-conjugated antigoat immunoglobulin G (IgG) antibodies (diluted 1:500; Dianova, Hamburg, Germany), and the Vector-VIP substrate kit for peroxidase (Vector Laboratories, Burlingame, Calif.) confirmed expression of the foreign genes. After three to four consecutive rounds of plaque purification, virus stocks were prepared in Vero cells. For immunization, virus stocks were briefly sonicated, and cell debris was removed by centrifugation before virus titration.
DNA analyses.
Viral DNA of the recombinant viruses was prepared according to the alkaline lysis procedure followed by phenol extraction as described in reference 19. Restriction endonuclease analysis of the purified viral DNA and Southern blot hybridization were performed as described previously (6) with PRV glycoprotein gene-specific or lacZ gene-specific, radioactively labeled probes.
RNA isolation and Northern blot hybridization.
For enrichment of viral early RNA, cells were pretreated with 100 μg of cycloheximide (CH) per ml for 1 h before infection (MOI of 10). After an additional 8 h in the presence of CH, total cellular RNA was prepared with TriZOL reagent (Invitrogen Life Technologies, Leek, The Netherlands). RNA (10 μg) was separated in formaldehyde-containing agarose gels and transferred onto nylon membranes (Hybond N+; Amersham Biosciences, Freiburg, Germany). Hybridization using PRV glycoprotein gene-specific or ORFV ANK3 gene-specific probes was performed overnight at 45°C in a mixture containing 3× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]), 1.0% nonfat dried milk, 2.0% sodium dodecyl sulfate (SDS), 0.5% (vol/vol) diethyl-pyrocarbonate (DEPC; Serva, Heidelberg, Germany), 7.0% (wt/vol) dextran sulfate, and 60% deionized formamide (Invitrogen Life Technologies).
Western blot analysis.
Cells infected at an MOI of 10 were harvested, collected by centrifugation, and lysed in a mixture of 12.5 mM Tris-HCl (pH 6.8), 2.5% SDS (wt/vol), 10% sucrose (wt/vol), 0.02% bromphenol blue (wt/vol), and 5% 2-mercaptoethanol (vol/vol). Total cell proteins were resolved by electrophoresis in an SDS-10% ProSieve 50 (BMA) gel and electroblotted onto a polyvinylidene difluoride Western blotting membrane (Roche Applied Sciences, Mannheim, Germany) for 30 min at 100 V in a mixture of 25 mM Tris, 192 mM glycine, and 20% (vol/vol) methanol (pH 8.6). The membranes were blocked in TBST buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% Tween 20), containing 5% nonfat dried milk for 1 h and then incubated with primary antibody for 2 h at room temperature. To this end, undiluted PRV gC-specific mouse monoclonal hybridoma supernatant A18b containing 1% nonfat dried milk, the polyclonal PRV gD-specific rabbit antiserum 016/00 (diluted 1:2,500 in TBST containing 1% nonfat dried milk), or the monoclonal antibody (MAb) 4D9 (diluted 1:100) directed against the 39K major envelope protein of ORFV (7) was used. After being washed with TBST, the membranes were incubated for 1 h with a 1:100 dilution of species-specific secondary anti-IgG (H+L) antibodies conjugated to peroxidase (Dianova) followed by staining with phosphate-buffered saline (PBS) containing 20% methanol, 0.02% diaminobenzidine, 0.06% chloronaphthol, and 0.006% H2O2.
Fluorescent analysis of protein surface expression.
For flow cytometric analyses using the FACSCalibur fluorescence-activated cell sorter (FACS) (Becton Dickinson, Heidelberg, Germany), cells were harvested 24 h after infection (MOI of 10) and immunostained with the PRV gC-specific MAb A18b or with the 1:100-diluted PRV gD-specific antiserum 016/00 and 1:100-diluted species-specific secondary anti-IgG (H+L) antibodies conjugated to fluorescein isothiocyanate (Dianova). All incubations and washing steps were performed at 4°C in PBS containing 3% fetal bovine serum.
Mice.
Wild-type BALB/c, C57BL/6, and 129/Sv/Ev mice and mutant CD4−/− 129/Sv/Ev, B-cell deficient C57BL/6 (μMT), CD8−/− C57BL/6, or perforin−/− C57BL/6 (PKOB) mice were used. All of these mice were kindly provided by R. M. Zinkernagel (Institute for Experimental Immunology, University of Zurich, Zurich, Switzerland) and bred at the Federal Research Center for Virus Diseases of Animals, Tuebingen, Germany. Mice of both sexes were challenge infected at 8 to 10 weeks of age.
Immunization and challenge infection of mice.
Mice were injected intramuscularly (i.m.) with 107 TCID50s of the ORFV recombinants expressing the PRV glycoproteins in a total volume of 0.2 ml (0.1 ml for each hind leg). Immunization was repeated at 2-week intervals, and 2 weeks after the last immunization, mice were bled from the retroorbital plexus for serum collection. Immediately thereafter, the BALB/c mice were challenge infected intraperitoneally (i.p.) with 102 PFU (corresponding to 30 50% lethal doses [LD50s]) of the highly virulent PRV strain NIA-3, and the C57BL/6 and 129/Sv/Ev mice were infected with 103 PFU (corresponding to 25 LD50s). The LD50 was determined for each wild-type mouse strain (four animals per group) by i.p. infection using serial dilutions of PRV NIA-3 ranging from 105 to 3.3 PFU, and was calculated as described previously (2, 53). As negative controls, mice were immunized with 107 TCID50s of the parental ORFV D1701-VrV, and as positive controls, mice were immunized with 107 PFU of the PRV live vaccine Begonia (Intervet International BV, Boxmeer, The Netherlands).
Antibody analyses.
Sera from immunized and control mice were analyzed for PRV-specific IgG1 and IgG2a antibodies by enzyme-linked immunosorbent assay (ELISA) using PRV-coated microtiter plates and substrate of the commercially available Checkit Aujeszkytest II (Intervet, Unterschleissheim, Germany). The plates were incubated with serial twofold dilutions (starting with a 1:80 dilution) of each serum for 90 min at 37°C, washed as recommended, and incubated with 1:5,000-diluted biotinylated goat anti-mouse IgG1 or biotinylated goat anti-mouse IgG2a antibody (Southern Biotechnology, Birmingham, Ala.) for 90 min at 37°C. The plates were washed and incubated with a 1:2,000 dilution of peroxidase-conjugated avidin (BD PharMingen) for 1 h at 37°C. Antibody titers were expressed as the reciprocal of a serum dilution exhibiting an at least twofold increase in optical density over that of the negative control serum that was obtained from D1701-VrV-immunized mice. The standard deviation was calculated from the mean titer of sera obtained from the individual mice (eight animals per group immunized separately with the individual ORFV recombinants and five animals per group vaccinated with PRV live vaccine or with a combination of both recombinants).
Serum neutralizing antibodies directed against PRV were determined by a complement-independent neutralization assay. Sera were inactivated at 56°C for 30 min, and twofold dilutions of the sera (starting with a dilution of 1:20) were incubated in triplicates together with 50 PFU of PRV strain NIA-3 for 90 min at 37°C in flat-bottom 96-well plates. Thereafter, Vero cells were added, and 3 days later, the serum neutralizing antibody titer was determined as the highest serum dilution resulting in 100% reduction of CPE.
RESULTS
In vitro characterization of D1701 recombinants in ORFV permissive cells.
Virus recombinants were selected and plaque purified as described in Materials and Methods. The correct substitution of both copies of lacZ by the PRV gC and gD genes, respectively, was verified by Southern blot hybridization with probes specific for lacZ, gC, or gD (data not shown). Specific transcription of the PRV glycoprotein genes, which are controlled by the strong early VEGF-E promoter, was examined by Northern blot analysis of total RNA isolated 8 h postinfection (p.i.) from CH-treated cells. The results demonstrated specific transcription of early mRNA of the expected size for gC (Fig. 2A lane 1) as well as for gD (Fig. 2B, lane 2). To control for comparable levels of viral gene expression in the cells infected with the individual recombinants, the transcription rate of the early ORFV gene ANK-3 (49) was tested. As shown in Fig. 2C, cells infected with D1701-VrVgC (lane 1), D1701-VrVgD (lane 2), or the parental virus, D1701-VrV (lane 3), synthesized comparable amounts of viral mRNA. In addition, each PRV glycoprotein gene-specific probe detected a transcript approximately 3.8 kb in size, which was not found in uninfected cells (lane 4). This can be explained by partial read-through of the early transcription stop motif (T5NT) of the original VEGF-E by using another stop motif 1.65 kb downstream, as also found for the ANK-3 gene. In addition, Northern blot analysis also demonstrated substantial foreign gene expression at later times p.i. in the absence of CH (data not shown), which is also found for the VEGF-E gene of D1701-V (M. Henkel and H.-J. Rziha, unpublished data).
FIG. 2.
Expression of PRV gC and gD in ORFV recombinant-infected cells. Vero cells were infected (MOI of 10) with D1701-VrVgC (lanes 1), D1701-VrVgD (lanes 2), D1701-VrV (lanes 3), and PRV Begonia (lanes 5) or mock infected (lanes 4). For Northern blot hybridization using radioactively labeled probes specific for PRV gC (A), PRV gD (B) and the ORFV early gene ANK3 (C), total RNA was isolated from CH-treated Vero cells. The sizes of the respective specific transcripts are indicated in kilobase pairs to the left. Cell lysates were obtained 24 h p.i., and Western blot analysis was performed with the gC-specific MAb A18b (D), the gD-specific rabbit serum 016/00 (E), or the ORFV 39K-specific MAb 4D9 (F). The apparent molecular mass of the detected proteins is indicated in kilodaltons.
Translation of the PRV glycoproteins was tested in cells infected with each D1701-VrV recombinant by Western blot analysis. Using the gC-specific MAb A18b, all known processing products of gC, the 58-kDa precursor, the 74-kDa pre-Golgi form, and the 92-kDa mature form could be demonstrated in D1701-VrVgC-infected cells (Fig. 2D, lane 1) as in PRV-infected cells (Fig. 2D, lane 5). Similarly, cells infected with D1701-VrVgD exhibited authentic expression of gD compared to PRV-infected cells. The gD-specific polyclonal antiserum detected the precursor (45 kDa) and the mature glycosylated form (60 kDa) of gD (Fig. 2E). Comparable synthesis of both forms of the ORFV major envelope protein (39 and 31 kDa) was found in Vero cells infected with the recombinant viruses and the parental D1701-VrV (Fig. 2F), which indicated similar viral protein synthesis. Finally, flow cytometry demonstrated surface expression of gC or gD on Vero cells infected with the individual D1701-VrV recombinants, but an approximately 10-fold-higher synthesis of gC than of gD (Fig. 3A and D). This is in agreement with the Western blot results, although a comparable amount of the specific transcripts seemed to be synthesized in the productively infected Vero cells (Fig. 2), and both recombinants grew to equal virus titers (data not shown). From the experiments done, the reasons for a lower level of gD synthesis in permissive Vero cells compared to the level of gC synthesis remain obscure and need further investigation.
FIG. 3.
Surface expression of PRV gC and gD on ORFV recombinant-infected cells. Flow cytometry of the indicated cells 24 h after infection with D1701-VrVgC (A to C) or D1701-VrVgD (D to F). Nonfixed infected cells (dark lines) or noninfected cells (bright lines) were stained 24 h after infection with the antigen-specific antibodies. The diagrams show the number of counted cells exhibiting specific fluorescence intensities.
Expression of gC and gD in D1701-VrV recombinant-infected cells nonpermissive for ORFV.
Since one objective of this study was to evaluate the efficiency of the D1701-VrV recombinants in mice, a nonpermissive host for ORFV, the foreign gene expression and production of infectious progeny were tested in mouse cell lines. Northern and Western blot analyses of 3T3 and L929 cells infected with D1701-VrVgC3 or D1701-VrVgD showed the correct expression of both PRV glycoproteins (data not shown). In contrast to the permissive Vero cells, surface expression of comparable amounts of gC and gD was found by flow cytometry in both mouse cell lines infected with the individual recombinant virus (Fig. 3B, C, E, and F).
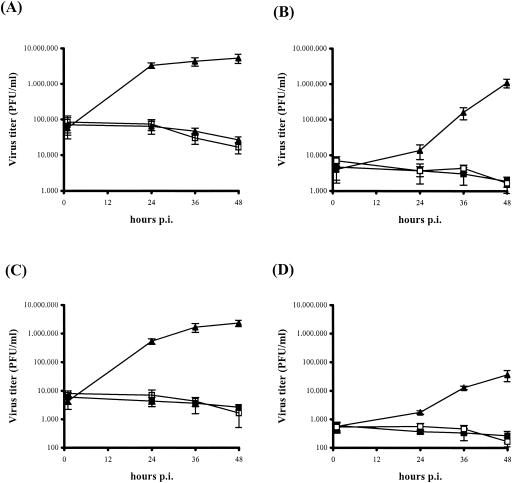
ORFV production was tested by titration experiments (single-step virus growth curve) in 3T3 and L929 cells infected with a D1701-VrVgC3 MOI of 1.0 or 10.0. Production of infectious progeny was detectable in neither cell lysates (Fig. 4A and C) nor supernatants of both infected mouse cell lines (Fig. 4B and D), whereas high titers of the recombinant viruses were produced in Vero cells (Fig. 4, solid triangles) indistinguishable from those with the parental D1701-VrV (data not shown). Collectively, the results demonstrate the in vitro expression of the inserted PRV glycoproteins also on the surface of infected cells nonpermissive for ORFV.
FIG. 4.
Single-step growth curve of D1701-VrVgC. Vero cells (solid triangles), 3T3 cells (open squares), or L929 cells (solid squares) were infected with a MOI of 10 (A and B) or 1.0 (C and D) and harvested at the indicated times after infection. Cell lysates (A and C) and supernatants (B and D) were separately titrated on Vero cells in triplicate to determine infectious virus progeny. Bars indicate standard deviations.
PRV-specific serum antibody response in immunized mice.
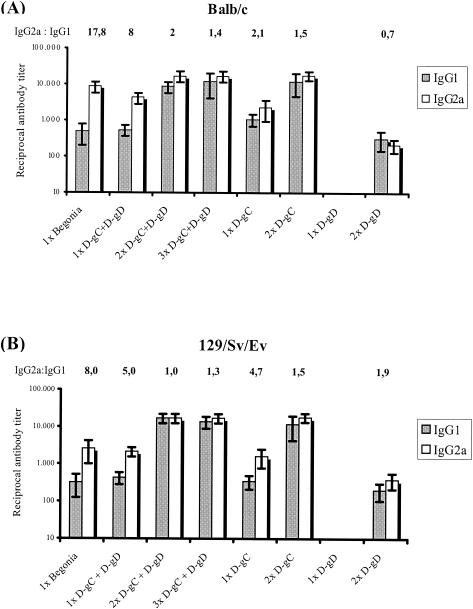
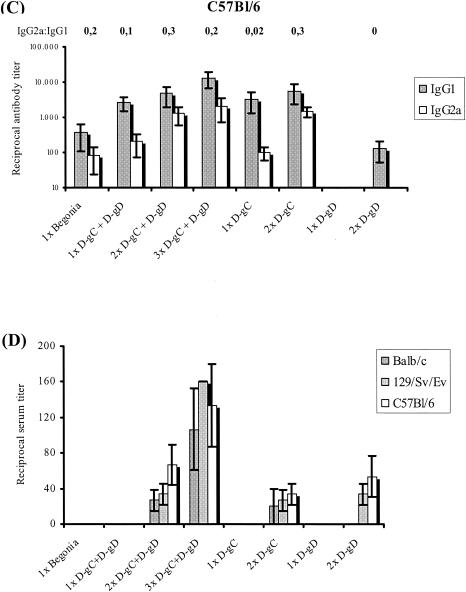
To evaluate the immunogenicity of the PRV gC- and gD-expressing ORFV recombinants, three different mouse strains, BALB/c, 129/Sv/Ev, and C57BL/6, were immunized i.m. up to three times at 2-week intervals. Groups of animals were vaccinated with D1701-VrVgC and D1701-VrVgD alone or in combination at 107 TCID50s per dose. As controls, animals were immunized with the same dose of a PRV live vaccine (Begonia), with the parental D1701-VrV, or with PBS. PRV-specific serum antibodies of IgG1 and IgG2a subclasses were determined by ELISA. As shown in Fig. 5A immunization of BALB/c mice with the PRV live vaccine induced higher specific IgG2a than IgG1 serum antibody titers, very similar to the results found after a single combined immunization with D1701-VrVgC plus D1701-VrVgD. A second combined immunization increased the specific IgG1 and IgG2a antibody titers 16-fold and 4-fold, respectively, whereas a third immunization did not change that picture (Fig. 5A). Essentially the same results were found in the various immunization regimens in 129/Sv/Ev mice (Fig. 5B). In contrast, significantly lower titers of IgG2a serum antibodies to PRV were found after all immunizations of C57BL/6 mice, resulting in a slight prevalence of the specific IgG1 subclass (Fig. 5C).
FIG. 5.
PRV-specific serum antibody response in immunized mice. Sera from immunized BALB/c (A), 129/Sv/Ev (B), and C57BL/6 (C) mice were taken 2 weeks after the final vaccination. PRV-specific IgG1 (shaded columns) and IgG2a (white columns) antibodies were determined by subclass-specific ELISA. The mice were immunized as indicated with D1701-VrVgC (D-gC) and D1701-VrVgD (D-gD) alone or in combination as well as with the PRV live vaccine Begonia. The ratios of IgG2a to IgG1 subclasses are given above the columns. Bars indicate standard deviations. (D) PRV-neutralizing antibody titers of the different sera. Note the different titer scale from those of panels A to C.
Individual immunizations with both D1701-VrV recombinants were performed to determine their contribution to the antigen-specific immune response. The ELISA results demonstrated that the specific IgG1 and IgG2a serum antibody titers induced by D1701-VrVgC in the different mouse strains were nearly identical to those found after the first and second immunizations with the combination of both recombinants (Fig. 5). In contrast, no specific serum antibodies were detectable after a single application of D1701-VrVgD, and after the second immunization, approximately 30- to 85-fold-lower titers of PRV-specific IgG1 and IgG2a antibodies were induced in all mouse strains (Fig. 5A to C). Due to the generally low induction of specific antibodies by D1701-VrVgD, no antigen-specific IgG2a serum antibodies were detectable after the second immunization of C57BL/6 mice (Fig. 5C). These results suggest a prevalence of a PRV gC-specific antibody response after simultaneous immunization with D1701-VrVgC and D1701-VrVgD.
Finally, sera were tested for the presence of complement-independent PRV-neutralizing antibodies (Fig. 5D). All sera obtained after the first immunization with either the PRV live vaccine or with each D1701-VrV recombinant alone or in combination did not contain PRV-neutralizing activity (cutoff, dilution of 1:20). After booster immunization, the sera of all three mouse strains exhibited low titers of PRV-neutralizing antibodies. A third combined immunization with both recombinants slightly increased the titers of PRV-neutralizing serum antibodies (Fig. 5D). In conclusion, these results indicate that D1701-VrVgC and D1701-VrVgD were both able to induce comparable amounts of antigen-specific neutralizing antibodies in serum despite their clearly different capabilities of inducing antigen-specific IgG antibodies.
Protective capacity of D1701-VrVgC3 and D1701-VrVgD.
The protection experiments were performed with a 25- to 30-fold LD50 of PRV for challenge infection of the different mouse strains (eight animals per group) and are summarized in Table 1. All nonimmunized (PBS) or D1701-VrV-immunized animals succumbed to challenge infection within 72 to 96 h, whereas a single application of the PRV live vaccine (Begonia) protected all wild-type mouse strains. Even a single i.m. application of the combination of D1701-VrVgC and D1701-VrVgD mediated 100% protection to C57BL/6 or 129/Sv/Ev mice, and seven of eight BALB/c mice were protected (Table 1). The same protection rates were found after a single immunization with D1701-VrVgC alone. Using D1701-VrVgD, seven of eight C57BL/6 or 129/Sv/Ev mice, but only one of eight immunized BALB/c mice, survived the challenge infection (Table 1). Thus, priming of BALB/c mice with D1701-VrVgD was much less protective than with D1701-VrVgC. Two separate immunizations with each recombinant protected all animals of each wild-type mouse strain, except for one BALB/c mouse immunized with D1701-VrVgD (Table 1). All protected animals were observed over a period of up to 9 weeks and did not show clinical symptoms at any time after challenge infection. Interestingly, BALB/c mice were also completely protected against a 10-fold-higher dose of challenge virus after two immunizations with D1701-VrVgC alone or with a combination of both recombinants, but only 50% of BALB/c mice immunized twice with D1701-VrVgD survived this higher infectious dose (data not shown). The data show that already a single application of the new ORFV recombinants could mediate protection of the different mouse strains against a lethal PRV infection, whereby the recombinant D1701-VrVgC appeared to have a higher protective capacity.
TABLE 1.
Protection of different immunized mouse strains against lethal PRV challenge infection
| Immunization (no. of vaccinations) | No. of survivors/total in mouse strain
|
||
|---|---|---|---|
| BALB/c | C57BL/6 | 129/Sv/Ev | |
| D1701-VrVgC + D1701-VrVgD (1) | 7/8 | 8/8 | 8/8 |
| D1701-VrVgC (1) | 7/8 | 8/8 | 8/8 |
| D1701-VrVgD (1) | 1/8 | 7/8 | 7/8 |
| D1701-VrVgC + D1701-VrVgD (2) | 5/5 | 6/6 | 5/5 |
| D1701-VrVgC (2) | 8/8 | 8/8 | 8/8 |
| D1701-VrVgD (2) | 7/8 | 8/8 | 8/8 |
| D1701-VrV (2) | 0/5 | 0/5 | 0/5 |
| Begonia (1) | 5/5 | 5/5 | 5/5 |
Serum transfer experiments.
Since a strong antibody response was induced by the gC recombinant, the role of antigen-specific serum antibodies in protection against lethal challenge infection was further scrutinized. Therefore, sera from BALB/c mice immunized twice with either D1701-VrVgC or D1701-VrVgD were used for passive immunization of naïve recipients. Twenty-four hours after intravenous transfer of 0.1, 0.3, or 0.5 ml of serum, the antigen-specific antibody titers of the recipients were determined immediately before challenge infection (data not shown). Control animals received 0.5 ml of serum from mice immunized twice with D1701-VrV, and all died between 72 and 96 h after challenge infection (Fig. 6). Transfer of 0.1 ml of serum from D1701-VrVgC-immunized mice (DgC serum) did not protect against challenge infection, although the specific serum antibody titers (1:530 titer of IgG1, 1:1,200 titer of IgG2a) were comparable to those of BALB/c mice after one active immunization with D1701-VrVgC mediating protection (Fig. 5 and 6). However, two BALB/c mice died 30 to 50 h later compared to the controls (Fig. 6A, squares). After passive immunization with 0.3 ml of DgC serum, the recipients exhibited higher serum antibody titers (1:1,920 titer of IgG1, 1:2,970 titer of IgG2a) than mice after a single immunization with live D1701-VrVgC; nevertheless, only one of six animals was protected. However, again two animals survived 30 to 50 h longer than the controls (Fig. 6A, circles). Finally, compared to actively single-immunized BALB/c mice, approximately threefold-higher antigen-specific antibody titers were found in the sera of animals passively immunized with 0.5 ml of DgC serum, but again only a low level of protection against the PRV challenge infection (two of six mice) was obtained. Notably, the death of four mice of this group was significantly delayed (Fig. 6A, triangles). These results indicate that PRV gC-specific serum antibodies alone are not sufficient for protection. Passive immunization with sera from D1701-VrVgD-immunized mice did not result in detectable antibody titers: none of the animals was protected against challenge infection, and the time to death of only two animals, each receiving 0.3 or 0.5 ml of serum, was prolonged by 50 h (Fig. 6B).
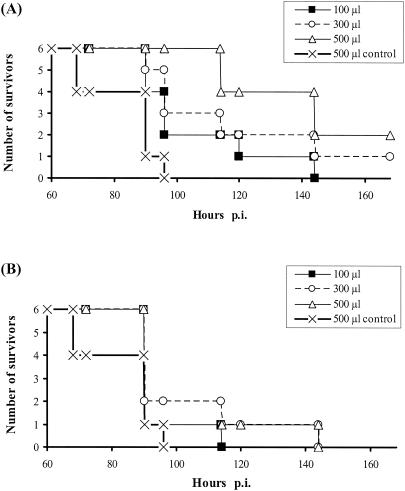
FIG. 6.
PRV challenge infection of mice after intravenous transfer of recombinant ORFV-immune sera. Sera were obtained from BALB/c mice after twofold immunization with each ORFV recombinant alone and passively transferred to naïve recipients. The survival times of individual animals are depicted after transfer of the indicated volumes of D1701-VrVgC immune sera (A) or D1701-VrVgD immune sera (B). Mice receiving serum from twofold D1701-VrV-immunized animals served as a control. Challenge infection with 300 LD50s of PRV was performed 24 h after serum transfer.
Immunization of immune-deficient mice.
To further elucidate the role of the humoral response in controlling the challenge virus, experiments with B-cell-deficient μMT mice were performed. Due to the finding that the protection rate after combined immunizations equaled that after immunization with D1701-VrVgC alone, in the following experiments, mice were only immunized with the individual recombinants. Protection of five of eight μMT mice was found after single administration of D1701-VrVgC or D1701-VrVgD, respectively (data not shown), whereas after two immunizations with each recombinant alone, seven of eight mice were protected (Table 2). With the PRV live vaccine, four of five μMT mice were protected (Table 2). These results demonstrate that the Ig-deficient mice are still capable of controlling challenge infection after the different immunizations.
TABLE 2.
ORFV recombinant-mediated protection of immune-deficient mice
| Mouse strain | No. of survivors/total (%)
|
||
|---|---|---|---|
| D1701-VrVgCa | D1701-VrVgDa | Begonia%b | |
| μMT | 7/8 (87.5) | 7/8 (87.5) | 4/5 (80.0) |
| CD4−/− | 8/8 (100) | 4/8 (50.0) | 2/4 (50.0) |
| CD8−/− | 8/8 (100) | 6/6 (100) | 3/3 (100) |
| PKOB | 8/8 (100) | 8/8 (100) | 5/5 (100) |
Two immunizations.
Single vaccination.
It has been reported that successful protection of mice against lethal PRV infection might depend on IFN-γ-producing CD4+ T cells (52). To investigate whether similar immune effector mechanisms might be responsible for the protective effect of the new PRV glycoprotein-expressing ORFV recombinants, 129/Sv/Ev CD4−/− knockout mice were investigated. The specific antibody response was generally lower in CD4−/− mice after immunization with the ORFV recombinants than in wild-type 129/Sv/Ev mice (Fig. 5). Single immunization with D1701-VrVgC induced two- to threefold-lower PRV-specific IgG1 and IgG2a serum antibody levels than in wild-type mice, and antigen-specific serum antibodies were not detectable even after two immunizations with D1701-VrVgD. PRV live vaccination (Begonia) of the CD4−/− mice induced no detectable IgG1 and 16-fold-lower titers of specific IgG2a serum antibodies compared to those in wild-type mice. Furthermore, none of the sera obtained from the different immunizations exhibited PRV-neutralizing activity (data not shown). After different single immunizations, CD4−/− mice were clearly less protected against challenge infection than were the congenic 129/Sv/Ev mice. Single administration of D1701-VrVgC protected 62.5% of the CD4-deficient animals, but single administration of D1701-VrVgD protected only 25% (data not shown), and only two of four PRV live-vaccinated mice survived the challenge infection. However, a booster immunization with D1701-VrVgC protected all CD4−/− mice against challenge infection, but only 50% of mice survived the challenge infection after two administrations of the D1701-VrVgD recombinant (Table 2). Altogether, protection of CD4−/− mice by a single application of D1701-VrVgC was reduced, which appeared to correlate with the reduced induction of specific antibodies in serum, but could be compensated for by a second immunization. This was not the case with D1701-VrVgD, although partial protection of the CD4−/− mice could be achieved.
The reduced protection of CD4-deficient mice mediated by the ORFV recombinants might be a consequence of a reduced capacity of cytolytic T-cell effector mechanisms in those animals. Therefore, challenge experiments with perforin knockout (PKOB) and CD8-deficient mice (which had been immunized twice with each recombinant alone) were performed. The results, however, demonstrated 100% protection of PKOB or CD8−/− mice after administration of D1701-VrVgC or D1701-VrVgD, as well as of the PRV live vaccine (Table 2). Therefore, these results indicate that after immunization with the new ORFV recombinants, the control of the PRV challenge infection can be maintained in the absence of CD4+ or CD8+ T cells, and the perforin pathway seems to be not required for immune protection.
DISCUSSION
Like other poxviruses, ORFV possesses properties suitable for the development of virus vector vaccines (i.e., the stable incorporation of foreign DNA, easy propagation, and inexpensive production). In addition, the restricted virus host range in vivo, as well as its immunomodulatory properties (11), makes ORFV an interesting candidate for novel virus vector vaccines. So far, however, the potential of recombinant ORFV to induce protective immunity against microbial infections has not been investigated. In this report, for the first time, we describe the development of novel ORFV recombinants for vaccination against a fulminant herpesvirus infection and demonstrate their protective capacity in mice as a nonpermissive host species for ORFV replication. A single application of the individual PRV glycoprotein expressing ORFV recombinants alone or in combination conferred solid protection against PRV infection to the majority of animals of the different mouse strains tested. Also using a 10-fold-higher infectious dose of 300 LD50s, 75 and 25% of the BALB/c mice immunized with the recombinants expressing glycoprotein gC or gD, respectively, survived the challenge infection. Most importantly, even a single vaccination with D1701-VrVgC gave protection from lethal challenge infection equal to that provided by vaccination with the PRV live vaccine. In contrast, twofold vaccination with D1701-VrVgD was required for solid protection. In addition, we found high titers of gC-specific serum IgG antibodies in D1701-VrVgC-immunized animals, but significantly lower titers of gD-specific antibodies after administration of D1701-VrVgD. This indicated an important role for the vector-induced humoral immunity in protection against PRV infection and might explain the lower protective capacity of the recombinant expressing glycoprotein gD.
PRV glycoprotein gC represents one of the major viral proteins that trigger the humoral immune response in mice and swine, and gC-specific MAbs can be sufficient to protect against PRV infection (1, 27). This has been attributed to the neutralizing activity of antibodies specific for the heparin binding domain of this glycoprotein, which is necessary for host cell attachment (40). However, despite nondetectable PRV-neutralizing activity in sera of mice after a single vaccination with the ORFV gC recombinant, the majority of animals were protected against PRV infection. Even after a second application, the gC-specific neutralizing antibody titers were low, coinciding with observations made after vaccination of mice with a PRV live vaccine (9), a gC-specific DNA vaccine (17), or purified glycoprotein gC (29). In the present study, intravenous transfer of sera obtained from mice immunized twice with D1701-VrVgC resulted in delayed mortality, but could not solidly protect the recipient mice, even when serum antibody titers before PRV challenge infection were higher than those found in mice protected after a single active immunization with D1701-VrVgC. This is in contrast to the protection of mice after transfer of sera obtained from mice immunized with inactivated PRV (53). In that study, however, serum administration at the same site immediately before i.p. challenge infection might result in rapid inactivation of the virus, but this does not entirely reflect the in vivo situation of an actively immunized host. Furthermore, neutralizing antibodies directed against other components of the virus might contribute to the protective capacity of the sera from mice immunized with whole virus particles. In conclusion, our results indicate that gC-specific antibodies induced by the ORFV recombinant play an important role early after PRV infection, but are not solely responsible for solid long-term protection. This is corroborated by the observation that even B-cell-deficient mice were protected after vaccination with the gC recombinant, which indicates that cellular mechanisms of immunity can counterbalance the lack of specific antibodies.
The importance of cell-mediated immunity in protection against virus infections is generally acknowledged. In herpesvirus infections, including PRV, the development of antigen-specific CD4+ effector T cells has been frequently reported to be important for protective immunity in mice (2, 15, 26, 56). Using CD4-deficient mice, we also found that the lack of antigen-primed CD4+ T cells in PRV Begonia-immunized mice results in impaired resistance against PRV infection. The PRV-specific immunity induced by a single administration of the ORFV recombinants expressing PRV glycoprotein gC or gD was less protective in CD4-deficient mice than that in congenic wild-type animals. Thus, both glycoprotein-specific vector vaccines obviously enable priming of antigen-specific CD4+ T cells, participating in protection against PRV challenge infection. Most notably, however, a second immunization with D1701-VrVgC, but not with D1701-VrVgD, induced substantial immune serum antibodies independent of T-helper cells (only twofold-lower titers than in congenic mice), and subsequently all CD4−/− mice were protected against challenge infection. The generation of antiviral serum IgG antibodies with the help of CD4− CD8− T cells has been reported in CD4−/− mice (44, 54). However, as discussed above, transfer of the induced gC antibodies alone was not sufficient for solid protection, and, therefore, the presence of immune T cells seems to be required for full protective capacity of immune serum antibodies, as also reported for herpes simplex virus type 2 (HSV-2) infection (36).
The D1701-VrVgD recombinant exhibits a rather limited capability to induce gD-specific humoral immunity. The approximately 10-fold-lower translation (Fig. 2D and E) and surface expression (Fig. 3A and D) of gD compared to gC in Vero cells productively infected with the recombinants might explain the reduced specific humoral response. However, comparable levels of synthesis of both PRV glycoproteins were demonstrated in the recombinant-infected murine cells that do not support production of infectious ORFV. Although not proven in vivo, it seems unlikely that substantially smaller amounts of gD than of gC are synthesized in mice immunized with each recombinant. Thus, the protective capacity of D1701-VrVgD seems to be primarily dependent on the induction of cell-mediated immune mechanisms.
So far, the effector functions of the vector vaccine-induced CD4+ T cells remain to be clarified. Besides providing help to antigen-specific B cells, they might also be important for the generation of PRV-specific CD8+ cytotoxic T lymphocytes (CTLs). Recently it has been reported that CD4+ T cells are dispensable for primary differentiation of virus antigen-specific CD8+ CTLs, but secondary CTL expansion upon reencounter with antigen is wholly dependent on the presence of T-helper cells during the priming process (20). However, although CTLs are generated in PRV-vaccinated mice and swine, CD8+ T cells have been shown to be dispensable for protection against subsequent lethal PRV infection, at least in the murine model (2, 61). Similarly, our results using CD8 knockout mice indicate that effector functions of antigen-primed CD8+ T cells are of minor importance as well for the ORFV vector vaccine-mediated protection against PRV infection. In addition, the complete protection of perforin knockout mice or of NK cell-depleted mice (data not shown) after twofold vaccination with the ORFV recombinants argues against the necessity of NK cell-mediated cytolysis of infected target cells for protection. Collectively, these observations are in agreement with the general concept that lymphocyte-mediated cytotoxicity particularly is important for the elimination of noncytolytic viruses prior to their release from infected cells, whereas cytopathic viruses, like herpesviruses, are controlled most efficiently by antiviral interleukins or IFNs (21, 60).
Previous studies have shown that a functional IFN-γ system is important for protective PRV-specific immunity induced by PRV vaccines (52, 53) and that IFN-γ secretion by antigen-specific CD4+ T-helper cells reduces morbidity and mortality as well of HSV-infected mice (15, 26, 36, 56). In the presence of the Th1 cytokine IFN-γ, an enhanced expression of inducible nitric oxide synthetase (iNOS) by macrophages has been reported to improve destruction of phagocytically ingested virus particles (22, 25, 36). Characteristic for a Th1-type immune response is the IFN-γ-induced expression of Igs of the IgG2a isotype (57). In immunized C57BL/6 mice, we observed an inverse relationship between antigen-specific IgG1 and IgG2a isotypes as compared to that in the immunized BALB/c and 129/Sv/Ev mice. However, that should not be misinterpreted as a shift towards a Th2-type immune response. In C57BL/6 mice, the gene for IgG2a is deleted and the isotype IgG2c is expressed (35), and commercial anti-IgG2a antibodies do not cross-react substantially with IgG2c (28). Interestingly, we have found that in BALB/c mice, the ORFV recombinants are less protective than in the other mouse strains tested. BALB/c mice have been shown to exhibit higher susceptibility to microbial infections, including herpesvirus infections, due to being less competent to elicit Th1-controlled cellular immunity than, for example, C57BL/6 mice (3). Altogether, the cytokine secretion pattern of the vector vaccine-induced PRV-specific T cells, particularly in the absence of CD4+ T cells, and the importance of IFN-γ now need to be investigated to elucidate additional immune effectors stimulated by the protective ORFV recombinants.
Importantly, preliminary experiments indicate that the ORFV recombinants elicit their protective immunogenicity even with preexisting immunity to the viral vector. Prime immunization of BALB/c mice with the parental ORFV recombinant D1701-VrV did not impair the development of a gC-specific humoral immune response and protection against PRV infection after subsequent application of the recombinant D1701-VrVgC (unpublished observations). This might be due to the short-lived ORFV-specific adaptive immunity even in sheep, where productive replication of the virus occurs (14). Thus, it seems likely that in a nonpermissive host species, the induced ORFV-specific immunity does not prevent vector-encoded foreign gene expression. Hence, induction of immunity to different pathogens by sequential immunization with diverse ORFV vector vaccines should be possible. In this regard ORFV vector vaccines might be superior to recombinants based on VACV, where with preexisting VACV-specific immunity, a limited foreign antigen-specific primary immune response was found in murine and primate model systems (23, 43, 45, 48, 55). In addition, propagation of recombinants based on host range-restricted and replication-deficient VACV and avipoxvirus strains requires cumbersome preparation of CEFs or complementing cell lines (39). VACV strain MVA was found to regain growth in Vero cells (30), but the safety of that Vero cell-derived virus variant remains to be shown. In contrast, ORFV recombinants merge the advantage of a natural restricted host range with the ability to be maintained in a permanent cell line, which meet the criteria of state-of-the-art production of biologicals. Current studies shall substantiate the capability of the ORFV recombinants to induce an antigen-specific memory immune response. In addition to protection against lethal infection with a cytolytic herpesvirus, the ORFV vector system was successfully used to protect rats, also nonpermissive for ORFV, against challenge infection with the neurotropic, noncytolytic Borna disease virus even 8 months after the last immunization (unpublished data). This indicates a more common applicability of ORFV recombinants to control acute or persistent virus infections requiring different immune defense mechanisms (21, 60). Therefore, studies are needed to dissect the immunostimulatory properties of ORFV in more detail.
Acknowledgments
The polyclonal PRV gD-specific rabbit antiserum 016/00 was generously provided by T. C. Mettenleiter, Federal Research Centre for Virus Diseases in Animals, Insel Riems, Germany. The excellent technical assistance of B. Bauer is greatly acknowledged.
Part of this work was supported by the EU grant QLK2-1999-00459.
REFERENCES
- 1.Ben Porat, T., J. M. DeMarchi, B. Lomniczi, and A. S. Kaplan. 1986. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology 154:325-334. [DOI] [PubMed] [Google Scholar]
- 2.Bianchi, A. T., H. W. Moonen-Leusen, F. J. van Milligen, H. F. Savelkoul, R. J. Zwart, and T. G. Kimman. 1998. A mouse model to study immunity against pseudorabies virus infection: significance of CD4+ and CD8+ cells in protective immunity. Vaccine 16:1550-1558. [DOI] [PubMed] [Google Scholar]
- 3.Brenner, G. J., N. Cohen, and J. A. Moynihan. 1994. Similar immune response to nonlethal infection with herpes simplex virus-1 in sensitive (BALB/c) and resistant (C57BL/6) strains of mice. Cell. Immunol. 157:510-524. [DOI] [PubMed] [Google Scholar]
- 4.Büttner, M., and H. J. Rziha. 2002. Parapoxviruses: from the lesion to the viral genome. J. Vet. Med. B Infect. Dis. Vet. Public Health 49:7-16. [DOI] [PubMed] [Google Scholar]
- 5.Cooney, E. L., A. C. Collier, P. D. Greenberg, R. W. Coombs, J. Zarling, D. E. Arditti, M. C. Hoffman, S. L. Hu, and L. Corey. 1991. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet 337:567-572. [DOI] [PubMed] [Google Scholar]
- 6.Cottone, R., M. Büttner, B. Bauer, M. Henkel, E. Hettich, and H. J. Rziha. 1998. Analysis of genomic rearrangement and subsequent gene deletion of the attenuated Orf virus strain D1701. Virus Res. 56:53-67. [DOI] [PubMed] [Google Scholar]
- 7.Czerny, C.-P., R. Waldmann, and T. Scheubeck. 1997. Identification of three distinct antigenic sites in parapoxviruses. Arch. Virol. 142:807-821. [DOI] [PubMed] [Google Scholar]
- 8.Drexler, I., K. Heller, B. Wahren, V. Erfle, and G. Sutter. 1998. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J. Gen. Virol. 79:347-352. [DOI] [PubMed] [Google Scholar]
- 9.Eloit, M., D. Fargeaud, R. L'Haridon, and B. Toma. 1988. Identification of the pseudorabies virus glycoprotein gp50 as a major target of neutralizing antibodies. Arch. Virol. 99:45-56. [DOI] [PubMed] [Google Scholar]
- 10.Enquist, L. W., P. J. Husak, B. W. Banfield, and G. A. Smith. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347. [DOI] [PubMed] [Google Scholar]
- 11.Fachinger, V., T. Schlapp, W. Strube, N. Schmeer, and A. Saalmuller. 2000. Poxvirus-induced immunostimulating effects on porcine leukocytes. J. Virol. 74:7943-7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerdts, V., A. Jons, and T. C. Mettenleiter. 1999. Potency of an experimental DNA vaccine against Aujeszky's disease in pigs. Vet. Microbiol. 66:1-13. [DOI] [PubMed] [Google Scholar]
- 13.Haig, D. M., C. J. McInnes, D. L. Deane, H. W. Reid, and A. A. Mercer. 1997. The immune and inflammatory response to Orf virus. Comp. Immunol. Microbiol. Infect. Dis. 20:197-204. [DOI] [PubMed] [Google Scholar]
- 14.Haig, D. M., and A. A. Mercer. 1998. Ovine diseases: Orf. Vet. Res. 29:311-326. [PubMed] [Google Scholar]
- 15.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Differential roles of B cells and IFN-gamma-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845-853. [DOI] [PubMed] [Google Scholar]
- 16.Ho, T. Y., C. Y. Hsiang, C. H. Hsiang, and T. J. Chang. 1998. DNA vaccination induces a long-term antibody response and protective immunity against pseudorabies virus in mice. Arch. Virol. 143:115-125. [DOI] [PubMed] [Google Scholar]
- 17.Hong, W., S. Xiao, R. Zhou, L. Fang, Q. He, B. Wu, F. Zhou, and H. Chen. 2002. Protection induced by intramuscular immunization with DNA vaccines of pseudorabies in mice, rabbits and piglets. Vaccine 20:1205-1214. [DOI] [PubMed] [Google Scholar]
- 18.Hussain, K. A., and D. Burger. 1989. In vivo and in vitro characteristics of contagious ecthyma virus isolates: host response mechanism. Vet. Microbiol. 19:23-36. [DOI] [PubMed] [Google Scholar]
- 19.Inoshima, Y., A. Morooka, K. Murakami, and H. Sentsui. 2000. Simple preparation of parapoxvirus genome DNA for endonuclease analysis. Microbiol. Immunol. 44:69-72. [DOI] [PubMed] [Google Scholar]
- 20.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 21.Kagi, D., and H. Hengartner. 1996. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr. Opin. Immunol. 8:472-477. [DOI] [PubMed] [Google Scholar]
- 22.Karupiah, G., Q. W. Xie, R. M. Buller, C. Nathan, C. Duarte, and J. D. MacMicking. 1993. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 261:1445-1448. [DOI] [PubMed] [Google Scholar]
- 23.Kundig, T. M., C. P. Kalberer, H. Hengartner, and R. M. Zinkernagel. 1993. Vaccination with two different vaccinia recombinant viruses: long-term inhibition of secondary vaccination. Vaccine 11:1154-1158. [DOI] [PubMed] [Google Scholar]
- 24.Lloyd, J. B., H. S. Gill, D. M. Haig, and A. J. Husband. 2000. In vivo T-cell subset depletion suggests that CD4+ T-cells and a humoral immune response are important for the elimination of Orf virus from the skin of sheep. Vet. Immunol. Immunopathol. 74:249-262. [DOI] [PubMed] [Google Scholar]
- 25.MacLean, A., X. Q. Wei, F. P. Huang, U. A. Al Alem, W. L. Chan, and F. Y. Liew. 1998. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J. Gen. Virol. 79:825-830. [DOI] [PubMed] [Google Scholar]
- 26.Manickan, E., R. J. Rouse, Z. Yu, W. S. Wire, and B. T. Rouse. 1995. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J. Immunol. 155:259-265. [PubMed] [Google Scholar]
- 27.Marchioli, C., R. J. Yancey, Jr., J. G. Timmins, L. E. Post, B. R. Young, and D. A. Povendo. 1988. Protection of mice and swine from pseudorabies virus-induced mortality by administration of pseudorabies virus-specific mouse monoclonal antibodies. Am. J. Vet. Res. 49:860-864. [PubMed] [Google Scholar]
- 28.Martin, R. M., J. L. Brady, and A. M. Lew. 1998. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 212:187-192. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda, T. A., S. Katayama, N. Okada, T. Okabe, and N. Sasaki. 1992. Protection from pseudorabies virus challenge in mice by a combination of purified gII, gIII and gVI antigens. J. Vet. Med. Sci. 54:447-452. [DOI] [PubMed] [Google Scholar]
- 30.Mayr, A. 2001. Altered strain of the modified vaccinia virus Ankara (MVA). WO 01/68820 A1. World Intellectual Property Organization, Geneva, Switzerland.
- 31.Mayr, A., M. Herlyn, H. Mahnel, A. Danco, A. Zach, and H. Bostedt. 1981. Control of contagious ecthyma in sheep by means of a new parenteral cell culture derived live vaccine. J. Med. Vet. B 28:535-549. [PubMed] [Google Scholar]
- 32.Mengeling, W. L., S. L. Brockmeier, and K. M. Lager. 1994. Evaluation of a recombinant vaccinia virus containing pseudorabies (PR) virus glycoprotein genes gp50, gII, and gIII as a PR vaccine for pigs. Arch. Virol. 134:259-269. [DOI] [PubMed] [Google Scholar]
- 33.Mettenleiter, T. C. 1996. Immunobiology of pseudorabies (Aujeszky's disease). Vet. Immunol. Immunopathol. 54:221-229. [DOI] [PubMed] [Google Scholar]
- 34.Meyer, M., M. Clauss, A. Lepple-Wienhues, J. Waltenberger, H. G. Augustin, M. Ziche, C. Lanz, M. Büttner, H. J. Rziha, and C. Dehio. 1999. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 18:363-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgado, M. G., P. Cam, C. Gris-Liebe, P. A. Cazenave, and E. Jouvin-Marche. 1989. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 8:3245-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison, L. A., L. Zhu, and L. G. Thebeau. 2001. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J. Virol. 75:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moss, B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA 93:11341-11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss, B., M. W. Carroll, L. S. Wyatt, J. R. Bennink, V. M. Hirsch, S. Goldstein, W. R. Elkins, T. R. Fuerst, J. D. Lifson, M. Piatak, N. P. Restifo, W. Overwijk, R. Chamberlain, S. A. Rosenberg, and G. Sutter. 1996. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv. Exp. Med. Biol. 397:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ober, B. T., P. Bruhl, M. Schmidt, V. Wieser, W. Gritschenberger, S. Coulibaly, H. Savidis-Dacho, M. Gerencer, and F. G. Falkner. 2002. Immunogenicity and safety of defective vaccinia virus Lister: comparison with modified vaccinia virus Ankara. J. Virol. 76:7713-7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ober, B. T., B. Teufel, K.-H. Wiesmüller, G. Jung, E. Pfaff, A. Saalmüller, and H. J. Rziha. 2000. The porcine humoral immune response against pseudorabies virus specifically targets attachment sites on glycoprotein gC. J. Virol. 74:1752-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paoletti, E. 1996. Applications of pox virus vectors to vaccination: an update. Proc. Natl. Acad. Sci. USA 93:11349-11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paoletti, E., J. Taylor, B. Meignier, C. Meric, and J. Tartaglia 1995. Highly attenuated poxvirus vectors: NYVAC, ALVAC and TROVAC. Dev. Biol. Stand. 84:159-163. [PubMed] [Google Scholar]
- 43.Perkus, M. E., A. Piccini, B. R. Lipinskas, and E. Paoletti. 1985. Recombinant vaccinia virus: immunization against multiple pathogens. Science 229:981-984. [DOI] [PubMed] [Google Scholar]
- 44.Rahemtulla, A., T. M. Kundig, A. Narendran, M. F. Bachmann, M. Julius, C. J. Paige, P. S. Ohashi, R. M. Zinkernagel, and T. W. Mak. 1994. Class II major histocompatibility complex-restricted T cell function in CD4-deficient mice. Eur. J. Immunol. 24:2213-2218. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez, J. C., G. M. Gerhardi, and M. Esteban. 2000. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J. Virol. 74:923-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riviere, M., J. Tartaglia, M. E. Perkus, E. K. Norton, C. M. Bongermino, F. Lacoste, C. Duret, P. Desmettre, and E. Paoletti. 1992. Protection of mice and swine from pseudorabies virus conferred by vaccinia virus-based recombinants. J. Virol. 66:3424-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson, A. J. and D. J. Lyttle. 1992. Parapoxviruses: their biology and potential as recombinant vaccines, p. 285-327. In M. M. Binns and G. L. Smith (ed.), Recombinant poxviruses. CRC Press, Inc., Boca Raton, Fla.
- 48.Rooney, J. F., C. Wohlenberg, K. J. Cremer, B. Moss, and A. L. Notkins. 1988. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J. Virol. 62:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rziha, H.-J., B. Bauer, K.-H. Adam, M. Röttgen, R. Cottone, M. Henkel, C. Dehio, and M. Büttner. 2003. Relatedness and heterogeneity at the near-terminal end of the genome of parapoxvirus bovis 1 strain (B177) compared with parapoxvirus ovis (Orf virus). J. Gen. Virol. 84:1111-1116. [DOI] [PubMed] [Google Scholar]
- 50.Rziha, H. J., M. Henkel, R. Cottone, B. Bauer, U. Auge, F. Götz, E. Pfaff, M. Röttgen, C. Dehio, and M. Büttner. 2000. Generation of recombinant parapoxviruses: non-essential genes suitable for insertion and expression of foreign genes. J. Biotechnol. 83:137-145. [DOI] [PubMed] [Google Scholar]
- 51.Savory, L. J., S. A. Stacker, S. B. Fleming, B. E. Niven, and A. A. Mercer. 2000. Viral vascular endothelial growth factor plays a critical role in Orf virus infection. J. Virol. 74:10699-10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schijns, V. E., B. L. Haagmans, and M. C. Horzinek. 1995. IL-12 stimulates an antiviral type 1 cytokine response but lacks adjuvant activity in IFN-gamma-receptor-deficient mice. J. Immunol. 155:2525-2532. [PubMed] [Google Scholar]
- 53.Schijns, V. E., B. L. Haagmans, E. O. Rijke, S. Huang, M. Aguet, and M. C. Horzinek. 1994. IFN-gamma receptor-deficient mice generate antiviral Th1-characteristic cytokine profiles but altered antibody responses. J. Immunol. 153:2029-2037. [PubMed] [Google Scholar]
- 54.Sha, Z., and R. W. Compans. 2000. Induction of CD4+ T-cell-independent immunoglobulin responses by inactivated influenza virus. J. Virol. 74:4999-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharpe, S., N. Polyanskaya, M. Dennis, G. Sutter, T. Hanke, V. Erfle, V. Hirsch, and M. Cranage. 2001. Induction of simian immunodeficiency virus (SIV)-specific CTL in rhesus macaques by vaccination with modified vaccinia virus Ankara expressing SIV transgenes: influence of pre-existing anti-vector immunity. J. Gen. Virol. 82:2215-2223. [DOI] [PubMed] [Google Scholar]
- 56.Sin, J. I., J. J. Kim, D. Zhang, and D. B. Weiner. 2001. Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum. Gene Ther. 12:1091-1102. [DOI] [PubMed] [Google Scholar]
- 57.Snapper, C. M., and W. E. Paul. 1987. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236:944-947. [DOI] [PubMed] [Google Scholar]
- 58.Wittmann, G., and H.-J. Rziha. 1989. Aujeszky's disease (pseudorabies) in pigs, p. 230-325. In G. Wittmann (ed.), Herpesvirus diseases of cattle, horses, and pigs. Kluwer Academic, Boston, Mass.
- 59.Zarnke, R. L., and R. A. Dieterich. 1985. Attempted reactivation of contagious ecthyma in Dall sheep. Am. J. Vet. Res. 46:1775-1776. [PubMed] [Google Scholar]
- 60.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271:173-178. [DOI] [PubMed] [Google Scholar]
- 61.Zuckermann, F. A., L. Zsak, T. C. Mettenleiter, and T. Ben-Porat. 1990. Pseudorabies virus glycoprotein gIII is a major target antigen for murine and swine virus-specific cytotoxic T lymphocytes. J. Virol. 64:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]