Abstract
The poliovirus (PV) eradication campaign is conducted on the premise that this virus, because of the lack of a zoonotic reservoir, will not reemerge once eradicated. This report examines the origin of PV using theoretical and experimental approaches. Our rooted phylogenetic analysis suggests a speciation of PV from a C-cluster coxsackie A virus (C-CAV) ancestor through mutation of the capsid that caused a receptor switch from intercellular adhesion molecule-1 to CD155, leading to a change of pathogenicity. This hypothesis is supported experimentally with chimeras generated from three different pairs of PV and C-CAV. Those carrying the PV capsid and the replication proteins of C-CAVs replicated well, whereas their reciprocal counterparts were either debilitated or dead. This phenomenon of asymmetry is observed also in recombinants between PV1 and C-CAV20, selected in tissue culture cells using a previously undescribed protocol. The recombinants are generated at frequencies of 10−6 typical for PV interserotype recombination. Strikingly, they resemble genetically and phenotypically, including neurovirulence in CD155 transgenic mice, the large majority of circulating vaccine-derived PVs that have caused poliomyelitis outbreaks in different parts of the world. These data provide experimental evidence for C-CAVs being partners to PVs in generating diverse PV progeny by homologous recombination. They support speciation of a novel human pathogen (PV) from a pool of different human pathogens (C-CAVs). In a PV-free world without PV neutralizing antibodies, contemporary C-CAV, like their ancestor(s), could be fertile ground for a PV-like agent to emerge by mutation.
Keywords: circulating vaccine-derived poliovirus, genetic recombination, rooted phylogenetic analysis, transgenic mouse neurovirulence, emerging infections
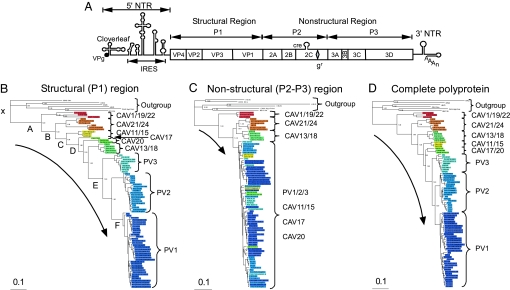
Poliovirus (PV), a member of the genus Enterovirus of the Picornaviridae, is a human pathogen feared for its ability to cause a neurological disease called poliomyelitis that can lead to irreversible paralysis and death (1). Its genome is a plus-stranded ≈7-kb RNA encoding a single polyprotein that is cleaved by virus-encoded proteinases into polypeptides forming the capsid (P1, structural region) and the replication proteins (P2–P3, nonstructural region) (2) (Fig. 1A). Cap-independent translation of the genome is directed by an internal ribosomal entry site (IRES) (2), whereas its replication is controlled by at least three RNA structures mapping to the 5′ and 3′ nontranslated regions (NTRs) and to the 2CATPase-coding region (Fig. 1A) (3). The capsid defines cellular receptor specificity and virus antigenicity (4).
Fig. 1.
Phylogenetic analysis of HEV-C viruses based on polyprotein sequences. (A) Genome organization of PV, which is representative for all C-cluster HEVs. The single-stranded genomic RNA is covalently linked to the viral-encoded protein VPg at the 5′ end of the NTR (5′NTR). The 5′NTR consists of two cis-acting domains, the cloverleaf, and the IRES. The coding region (open box) depicts the structural (P1) and nonstructural (P2 and P3) regions. Within the 2CATPase coding region, the cis replication element (cre) and the locus of the Gdn·HCl-resistant marker (gr) are indicated. The 3′NTR contains a heteropolymeric region and is polyadenylated. (B–D) Phylogenetic analyses of HEV-C. Six sequences of picornaviruses that are distant from the HEV-C were designated as outgroup. Branch points in the trees have a posterior probability >0.50. The bar at the bottom left indicates the number of changes per site. See SI Fig. 5 for more detailed versions of these trees. Sequence names are listed in SI Table 3. (B) Tree for the structural (P1) region of the polyprotein. The pseudoroot and six major nodes that were affected in five trees, generated by reshuffling (SI Materials and Methods), are labeled in alphabetic order from the root (indicated by “x”). The original and derived trees were used to verify the PV origin from C-CAVs using likelihood tests that supported, besides the original tree, only one with the least perturbed topology (SI Table 4). (C) Tree for the nonstructural (P2+P3) region of the polyprotein. (D) Tree for the complete polyprotein.
Based on their genotypic relationships, three serotypes of PV and 11 coxsackie A viruses (CAV) form the C cluster, which is considered a single species (HEV-C) within the human enteroviruses (HEVs); other HEVs form clusters (species) A, B, and D (4). Although PVs and C-cluster CAVs (C-CAVs) both use the gastrointestinal tract as their primary site of replication (where they may recombine), they cause remarkably different diseases in humans (paralysis and upper respiratory disease, respectively) (1, 5). (Hereafter and unless otherwise stated, all CAVs mentioned belong to HEV-C). The distinct tissue tropism is responsible for the different pathogenicity of these enteroviruses and is largely determined by the use of different cellular receptors for entry: intercellular adhesion molecule-1 (ICAM-1) for CAVs (6) and CD155 (PVR) for PVs (7, 8).
PV is the subject of an intense eradication campaign conducted by the World Health Organization (WHO), using predominantly the live, oral PV vaccine (OPV) developed by A. Sabin (1). The global eradication effort has been a success, considering the greatly reduced incidence of poliomyelitis. Expectations are high that all PVs will be eradicated in the near future (9), an optimism not shared by everyone (10). Moreover, it is commonly believed that PV may not reemerge because of the lack of a zoonotic reservoir.
In this report, we provide theoretical and experimental evidence suggesting that C-CAVs are the source of the ancestral PV by mutation as well as contemporary partners to (vaccine) PVs giving rise to novel PV variants in the field. The latter include highly neurovirulent, circulating vaccine-derived PVs, cVDPVs (11), that have been largely generated by homologous recombination between PV and CAVs. cVDPVs have caused numerous outbreaks of poliomyelitis globally and present a serious health threat (12). In our studies of genetic exchanges between PV and CAV, we observed that genetically engineered chimeras and in vivo selected recombinants with the genetic structure resembling that of cVDPVs with CD155 receptor specificity consistently survived much better than did their reciprocal counterparts with ancestral ICAM-1 receptor specificity. This asymmetry correlates with the direction of the ancestral PV speciation from CAV, as determined by phylogenetic analysis. These data exemplify how a significant pathogen can emerge from a benign ancestor in humans.
Results and Discussion
PVs May Have Originated from C-CAVs That Remain a Reservoir for PV Evolution.
We have proposed that ancestral PV may have originated from C-CAVs (13). To analyze the relationships among these viruses in greater detail, we have performed a phylogenetic analysis of the HEV-Cs using Bayesian posterior probability trees (14) (Fig. 1 B–D). For the initial rooting of these trees, we relied on analysis of paralogous proteins, in which the direction of evolution is naturally determined by a duplication event (15). The Picornaviridae encode a pair of paralogous cysteine proteases, 3Cpro conserved in the entire family, and 2Apro specific for a subset including entero/rhinoviruses. Because of the highly restricted phyletic distribution of 2Apro, it is likely that this protease originated by a duplication of a common 3Cpro in an ancestor of entero/rhinoviruses. Phylogenetic analysis of proteases of entero/rhinoviruses confirmed that 3Cpro and 2Apro form separate branches, which are similarly organized and rooted [supporting information (SI) Fig. 4]. Both branches contain a cluster of HEV-Cs next to clusters of other entero/rhinoviruses, implying that PV and C-CAV have originated from an immediate common ancestor.
The trees shown in Fig. 1 B–D were rooted according to the 2Apro + 3Cpro tree, using six sequences representing HEV-A (CAV4), HEV-B (CBV2), HEV-D (HEVD), human rhinovirus A (HRV2), human rhinovirus B (HRVB), and bovine enterovirus (BEV2) species that were designated as outgroup. The tree for the P1 region, where recombination is not common (16, 17), revealed a fully resolved clustered organization: nine groups were identified in the HEV-C, starting with CAV1/19/22, followed by CAV21/24, CAV11/15, CAV17, CAV20, CAV13/18, and finally PV3, PV2, and PV1 serotypes (in sequential order from the root; Fig. 1B; SI Fig. 5A). The capsid controls receptor specificity, and C-CAV capsid evolution has been accompanied by a diversification of ICAM use (although CAV1/19/22 and CAV17 are yet to be characterized in this respect) (6). As our rooted tree suggests, this continuous evolution must have eventually led to the replacement of the ICAM specificity to that of CD155 in the PV ancestor. The three PVs that have evolved from the ancestral PV refined this specificity. According to a maximum-likelihood estimate, contemporary PVs may be separated from the most recent common ancestors of PVs and HEV-C at, respectively, the 0.086 and 0.364 distances in the PI region. Hence, only a small fraction of mutations accumulated in the PV capsid since the HEV-C origin has been used to evolve the CD155 specificity.
In contrast to the P1 region, PVs and some C-CAVs (except the CAV1/19/22 subcluster) interleave in a poorly resolved cluster in phylogenetic trees generated for the P2+P3 region and its individual proteins (Fig. 1C; SI Fig. 5 B, D–L), in agreement with the results of others (16, 17). This topology was confirmed by using a more complicated model (amino acid GTR, using autocorrelated gamma rate variation) in a MrBayes-mediated analysis and a maximum-likelihood tree inferred using the TREE-PUZZLE package (18) under default parameters (data not shown). The P2+P3 region compared with the P1 region has accepted fewer substitutions (compare Fig. 1 B and C), indicating that its evolution has been constrained much stronger. Two polar effects, linked to intervirus distances and relative polyprotein positions, are evident in this region. First, the closer the C-CAVs are to PVs in the P1 area, the more readily these viruses intertwine in trees generated for proteins downstream of P1. Second, the closer a protein is located to the C terminus of the P2+P3 regions, the larger is the number of C-CAVs that intertwine with PVs. As a result of these trends, only the most distant CAV1/19/22 subcluster remains separated from all other C-CAVs/PVs in a phylogenetic tree for the most C-terminal 3Dpol (SI Fig. 5 D–L).
The tree topology for the complete polyprotein (Fig. 1D; SI Fig. 5C) resembles that obtained for the capsid (Fig. 1B; SI Fig. 5B). Thus, the phylogenetic signal generated by the nonsynonymous mutations accumulated during HEV-C evolution in the structural region dominates that inferred for the nonstructural region. Deviations among the trees for the complete polyprotein and capsid are of a limited scale and include relocation of the CAV13/18 subcluster closer to the root and merging of CAV17 and CAV20 into a single subcluster closer to the PVs.
Intertwining between PVs and CAVs in the P2+P3 trees, confirmed by using nucleotide sequences (J.A.J.F. and A.E.G., unpublished data), is restricted to proteins separated by rather small distances. It may be due to homologous recombination and, indeed, some cVDPVs seem to be field recombinants of OPV and CAVs that also accepted mutations (19). These cVDPVs and their reciprocal counterparts (with CAV capsid and PV nonstructural proteins) that have never been identified in the field may be viewed as recombinants generated, respectively, along and counter to the direction of PV speciation from the C-CAV base. The viability of recombinants with PV capsid and C-CAV replicase may be expected if contemporary C-CAVs resemble the C-CAV/PV ancestors that gave rise to PVs. Thus experimental analyses of recombination between PV and C-CAV, and the products thereof, could verify both the origin of some cVDPVs and the direction of PV evolution in the field.
Parent-Specific Asymmetry of Genetic Compatibility Between PV1(M) and CAV20 Revealed by Reciprocal Exchanges in Vitro.
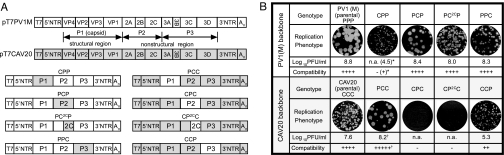
To test implications of phylogenetic analysis and to study the molecular genetics of cVDPV in view of PV evolution, we analyzed different gene combinations (chimeras) between a PV and a CAV. For the initial set of experiments, the two serotypes CAV20 and PV1 (Mahoney) [PV1(M)] were chosen because of their relatively close genetic kinship in the capsid and nonstructural regions (Fig. 1 B and C). (The nonvaccine PV strain was used to ensure broad applicability of our analysis.) To facilitate the genetic manipulation of the CAV20 genome, an infectious cDNA clone of CAV20, pT7CAV20 carrying the T7 RNA polymerase promoter (20), was constructed from a progenitor strain obtained from American Type Culture Collection (Bethesda, MD). CAV20 derived from transfection of in vitro RNA transcript of pT7CAV20 was comparable to that of the prototype strain in replication kinetics and progeny yield and indistinguishable in sequence (after four passages) from the progenitor strain (data not shown).
Gene swapping among different picornavirus species by genetic engineering has been frequently used to study gene function or to assess pathogenesis (21, 22). We used a similar approach to evaluate genetic compatibility between PV and CAV. Infectious cDNA clones of PV1(M) (pT7PVM) and CAV20 (pT7CAV20) were used as backbone, and the P1, P2, and P3 coding regions were exchanged in vitro, resulting in three reciprocal pairs of chimeric genomes (Fig. 2A). In addition, we exchanged the coding region of 2CATPase, a protein with multiple distinct functions in HEV replication (3). 2CATPase would be expected to serve as a highly sensitive indicator of compatibility within the chimeric HEV-C. The percentage of amino acid and nucleotide sequence identities of the different genetic regions between CAV20 and PV1(M) is shown in SI Table 5. Transcript RNAs of the chimeric plasmids were transfected into HeLa H1 cells, and the progeny viruses, if any, were analyzed. Compatibility was estimated by calculating the titer differences (TD) between the parental backbone virus and the chimeric virus (Fig. 2B).
Fig. 2.
In vitro genetic recombination between PV1(M) and CAV20. (A) Schematic diagram of the infectious cDNA of pT7PVM (open box), pT7CAV20 (shaded box), and the four reciprocal pairs of chimeric genomes. Chimeras are identified by using the following nomenclature: the first letter is for the P1 region, the second is for the P region, and the third is for the P3 region [P, PV1(M); C, CAV20], whereas the 2C chimeras were named by putting 2C in superscript. (B) Comparison of replication of chimeric viruses by virus titers and plaque phenotypes (23). The compatibility of donor and recipient (backbone) viruses in different genetic regions is assessed by the TD between the parental backbone strain and the chimera. +++++, TD < 0; ++++, 0 ≤ TD < 1; +++, 1 ≤ TD < 2; ++, 2 ≤ TD < 3; +, 3 ≤ TD; −, chimera is not viable. ∗, CPP yielded viable virus only after the fourth passage. †, the higher-than-expected compatibility score of the PCC chimera, exceeding that of its backbone parent, indicates that the donor P1 region is likely to have partially conferred the higher intrinsic proliferating ability of PV to this chimera on HeLa H1 cells. This is reflected by the 1.2 log difference in titer (8.8 vs. 7.6) between the parental PV1(M) and CAV20.
Interestingly, analysis of reciprocal chimeras revealed parent-specific asymmetry in compatibility between PV1(M) and CAV20 (Fig. 2B). All PV-based chimeras with a replacement in the nonstructural region (PCP, PC2CP, and PPC) yielded progeny replicating with nearly wild-type efficiency, whereas a chimera with the CAV20 capsid (CPP) was quasi-infectious and yielded virus only after four blind passages (Fig. 2B). In contrast, the CAV20-based chimera with the PV1(M) capsid (PCC) displayed a wild-type-like proliferation phenotype, whereas replacements in the nonstructural region were either compromised (CCP) or dead (CPC and CP2CC). Transcript RNAs of the latter two chimeras never produced progeny even after six blind passages. Patterns of in vitro translation and polyprotein processing in HeLa cell-free extracts (23) are indistinguishable between CAV20, CPC, and CP2CC RNAs (SI Fig. 6A), an observation suggesting that these processes are not impaired in the chimeras. Instead, the dead phenotype resulted from a complete inhibition of RNA synthesis (SI Fig. 6B), which cannot be linked to the cre in the coding region of 2CATPase, because this replication element is identical in PV1(M) and CAV20. Overall, in vitro genetic recombination between PV1(M) and CAV20 showed a parent-specific asymmetry in compatibility.
Parent-Specific Asymmetry in Genetic Compatibility Is Reproduced with Other Pairs of PV and CAV.
To determine whether the parent-specific asymmetry is a general phenomenon for CAV and PV, we have extended our analysis to two other virus pairs, CAV20+PV3(Leon) and CAV21(Kuykendall)+PV1(M), that are separated by either smaller or larger distances, respectively (Fig. 1 B–D). Indeed, the same asymmetry was observed and, additionally, its scale was found to be modulated by the intervirus genetic distance of parents (Table 1). Specifically, a P3C20C20 chimera resembled PV3(Leon) in replication phenotype and progeny yield, whereas its reciprocal C20P3P3 counterpart, although viable, was impaired in replication (data not shown; subscript numbers indicate viral serotype) (Table 1). The P1C21C21 chimera generated viable virus upon transfection (data not shown). In contrast, the reciprocal C21P1P1 chimera proved to be dead, never yielding a replicating variant even after numerous blind passages. Interestingly, the mechanism of incompatibility between CAV capsid and PV nonstructural proteins in PV-based CAV capsid chimeras (C20P1P1, C21P1P1) is related to a defect in encapsidation rather than RNA replication (P.J., S. Mueller, J. Yin, E. Rieder, H. Shimizu, A.P., and E.W., unpublished results). Collectively, all chimeras with the PV capsid (P1C20C20, P3C20C20, and P1C21C21) are viable, whereas their reciprocal counterparts (C20P1P1, C20P3P3, and C21P1P1) display compromised or even dead phenotypes. These data strongly confirm and support the phylogenetic analysis of C-CAVs and PVs (Fig. 1 B–D), including the origin of PV capsid from a C-CAV ancestor by mutation.
Table 1.
Comparison of genetic compatibility of in vitro chimeras generated between PVs and CAVs at different intervirus genetic distances
| Intervirus genetic distance | Chimeric PVs (PV capsid, CAV backbone) |
Chimeric CAVs (CAV capsid, PV backbone) |
||
|---|---|---|---|---|
| Genotype | Compatibility | Genotype | Compatibility | |
| smaller | P3C20C20 | ++++ | C20P3P3 | +++ |
 |
P1C20C20 | ++++ | C20P1P1 | +/− |
| larger | P1C21C21 | +++ | C21P1P1 | − |
The P1 coding regions were exchanged between two pairs of viruses CAV20+PV3 (shorter genetic distance than between CAV20+PV1) and CAV21+PV1 (longer genetic distance than between CAV20+PV1), respectively. Together with that of P1C20C20 and C20P1P1, the genetic compatibility of chimeric PVs (P3C20C20 and P1C21C21) and of their chimeric CAV counterparts (C20P3P3 and C21P1P1) is listed. Compatibility is rated as described in Fig. 2.
Parent-Specific Asymmetry of Genetic Compatibility Is Evident in Viable Recombinants Between PV1(M) and CAV20 Selected in Vivo.
To validate the asymmetry phenomenon further, we studied PV/CAV recombinants obtained through coinfection and selection in vivo. This allowed us to check naturally chosen cross-over sites between partners in reactions mediated by cognate replication complexes (2). To proceed, we developed a previously undescribed protocol, because genetic recombination in vivo among CAVs and PVs, in contrast to that among PVs (2, 24–26), has not yet been experimentally demonstrated. (In fact, recombination in vivo has not been experimentally demonstrated between any different nonpolio members of HEV). HeLa H1 cells, which express both CD155 and ICAM-1, were coinfected with PV1(M) and CAV20. Subsequent selection of viable recombinants was based on host range and drug resistance. We used transformed mouse L cell lines L20B and Hei expressing CD155 and ICAM-1, respectively, which were treated with 2 mM guanidine hydrochloride (Gdn·HCl). PV is exceptionally sensitive (gs) to this drug that blocks RNA replication (2), but the virus readily acquires resistance (gr) by an Asn179Gly exchange in 2CATPase (Fig. 1A; ref. 27). The same mutation introduced into CAV20 also rendered this virus Gdn·HCl resistant (Fig. 3A).
Fig. 3.
In vivo genetic recombination between PV1(M) and CAV20. (A) Plaque phenotype of four parental strains used for in vivo genetic recombination experiments. Cross 1 was carried out between PV1(M)gs and CAV20gr, and cross 2 was carried out between PV1(M)gr and CAV20gs. (B) The cross-over regions of recombinant polioviruses were mapped throughout the P2 coding region with the selected gr marker being maintained. The cross-over regions of recombinants from coinfection of cross 1 at different temperatures are listed, and the plaque phenotypes of the recombinants are shown. (C) In vivo recombination occurs at sites in the vicinity of homologous sequences. The nucleotide sequences of in vivo recombinants with cross-over regions in 2Apro, 2B, and 2CATPase, designated as 2A[111Gly(gr)], 2B[41Leu(gr)], and 2C[140Ala(gr)], were determined and aligned with those of the parental PV1(M) and CAV20 sequences. The nucleotide sequence of a recombinant that was inherited from either PV1(M) or CAV20 is indicated by the black bar above or underneath a respective parental sequence. The part of the recombinant sequence whose origin was not resolved, and where recombination has likely occurred, is depicted with the dotted bar either above or underneath a parent. The parental nucleotides that are different from those of the recombinant are in bold. The codon(s) where recombination occurs are italic and underlined, whereas their corresponding amino acid(s) (bold, italic) are numbered according to their position in the respective protein of the P2 region. The homologous nucleotide sequences among the recombinant and parental viruses, located downstream of the cross-over points, are shaded. (D) In vivo recombination frequency in a coinfection of PV1(M)gs and CAV20gr at 34°C, 37°C, and 39°C.
Four different viruses were chosen to make two reciprocal crosses for in vivo recombination experiments: PV1(M)gs, CAV20gs, PV1(M)gr, and CAV20gr. Their replication phenotypes are shown in Fig. 3A. In cross 1, HeLa cells were coinfected with PV1(M)gs and CAV20gr and in cross 2, with PV1(M)gr and CAV20gs (SI Fig. 7). In the presence of 2 mM Gdn·HCl, progeny of cross 1 was subsequently incubated with L20B cells and progeny of cross 2 with Hei cells. Infection of permissive cells combined with drug treatment allowed selection of gr recombinants recognizing cells expressing either ICAM-1 or CD155. Cross 2 progeny, unlike its reciprocal counterpart, never produced detectable recombinants. This indicates that the impaired replication of these recombinants, initially described for the in vitro generated CAV-based chimeras (Fig. 2B), may be a genuine phenomenon. Because CAV-based chimeras (in vitro constructed) and recombinants (in vivo selected) differ in their 5′- and 3′-NTRs (compare Figs. 2A and 3B), these regions may not contribute significantly to the observed asymmetry phenomenon.
Sequence analyses of in vivo recombinants from cross 1 revealed that the cross-overs mapped to the coding regions of 2Apro, 2B, or 2CATPase. These data are compatible with the proposed cross-over regions in cVDPVs from the field (28, 29). Also, the preferred site(s) of cross-overs depended on the temperature during the coinfection (Fig. 3B), a phenomenon observed earlier but not fully understood (30). Titers of recombinants with crossover regions in 2Apro, 2B, and 2CATPase in HeLa H1 cells in the absence of 2 mM Gdn·HCl were similar to that of the parental strain PV1(M)(gs) (data not shown). Alignment of the nucleotide sequences of three in vivo recombinants (Fig. 3C), designated as 2A[111Gly(gr)], 2B[41Leu(gr)], and 2C[140Ala(gr)], with those of the parental strains revealed cross-overs adjacent to conserved regions, a hallmark of homologous recombination (2). Gdn·HCl resistant variants of PV1(M)gs are also likely to emerge spontaneously during selection in the presence of Gdn·HCl (2) (SI Fig. 8). Taking this into account, the recombination frequency between PV1(M)gs and CAV20gr was calculated to be 10−6 (Fig. 3D). This value falls into the range of recombination frequencies observed between heterotypic PVs (26, 31). Recombination experiments in vivo with the Sabin vaccine strain of poliovirus type 1 (OPV1) instead of PV1(M) as a partner of CAV20 have yielded similar results (data not shown).
In Vivo PV1(M)/CAV20 Recombinants Are Highly Neurovirulent in Transgenic Mice.
Central to this study is the analysis of neurovirulence phenotypes of the PV1/CAV20 recombinants, which we have determined in CD155 transgenic mice (strain ICR.PVR.Tg I) (32). To avoid possible artifacts, the gr mutation (Asn179Gly) in recombinants 2A[111Gly(gr)], 2B[41Leu(gr)], and 2C[140Ala(gr)] (Fig. 3C) was reverted by site-directed mutagenesis to the wild-type gs genotype, yielding 2A[111Gly(gs)], 2B[41Leu(gs)], and 2C[140Ala(gs)]. As shown in Table 2, the replication phenotypes of the three modified recombinants are comparable to that of parental PV1(M). Intracerebral inoculation with the three in vivo recombinants revealed neurovirulence nearly identical to that of PV1(M). This observation demonstrated that, in this important aspect, these chimeras are also similar to cVDPVs.
Table 2.
Neurovirulence test of in vivo PV1(M)/CAV20 recombinants in CD155 transgenic mice
| Virus | PLD50 | Virus titer (pfu/ml) | Replication phenotype |
|---|---|---|---|
| PV1(M) | 101.8 | 109 |  |
| 2A[111Gly(gs)] | 102.3 | 4 × 108 |  |
| 2B[41Leu(gs)] | 101.7 | 4 × 108 |  |
| 2C[140Ala(gs)] | 101.8 | 5 × 108 |  |
| Sabin1 | >106 | 109 |  |
After the mutation N179G in 2CATPase was reverted back to the wild-type sequence (179N), the modified recombinants 2A[111Gly(gs)], 2B[41Leu(gs)], and 2C[140Ala(gs)] were compared with PV1(M) in their growth phenotypes by measuring the viral titer and plaque size. Neurovirulence of the in vivo recombinants was tested after intracerebral inoculation in experimental animals. PV1(M) and the PV Sabin1 vaccine strain (OPV1) were tested as controls. The PLD50 value indicates the virus titer that induced paralysis or death in 50% of the mice (38).
Concluding Remarks.
In the current study, we provide evidence suggesting a dual role played by nonpolio enteroviruses, the C-CAVs, in PV origin and evolution: as the source of the ancestral PV that originated by mutation and as contemporary partners to PVs in the generation of novel PV variants by recombination. These two scenarios describe the emergence and continuous evolution of a significant pathogen from benign ancestors within humans. Our rooted phylogenetic analysis suggests speciation of PV from a C-CAV ancestor accomplished by mutation of the capsid that caused a receptor switch from ICAM-1 to CD155. The most recent common ancestors of PVs+CAVs (having ICAM-1 specificity) and that of PVs (CD155) might have differed at ≈85 amino acid positions (9.5%) in the P1 region (J.A.J.F. and A.E.G., unpublished results). All or a fraction of these positions must have been mutated for the receptor switch to occur. The new receptor specificity could have subsequently been refined in three PV serotype lineages. It is interesting to note that PV excreted from an immune-compromised individual was reported to have 24 amino acid positions mutated in the capsid during 5.5 years (33), an observation providing a window on the time frame of mutation fixation. The closely related CAV and PV capsids both dock their respective Ig-like receptors into “canyons” near the 5-fold apex of their icosahedron, a process involving ≈12 residues (34). Receptor switching from ICAM to CD155, however, has not been reproduced experimentally.
Chimeras consisting of the PV capsid and CAV nonstructural proteins, whether generated in vitro or by recombination in vivo at high frequency (10−6), replicate with PV wild-type kinetics. Importantly, the in vivo recombinants described here resemble phenotypically (replication and neurovirulence) the cVDPVs isolated from the field. Thus, our data provide insight into the origin of some of cVDPVs. In contrast, proliferation of reciprocal recombinants, for example CPP, was compromised. These observations reveal that PVs and C-CAVs are engaged in asymmetric partnership in recombination. The asymmetry phenomenon, which has not been observed among recombinants among the three PV serotypes (2, 24–26), can be correlated with the direction of the ancestral PV speciation. In this framework, viruses with the newly acquired receptor specificity (CD155) appear to be favored compared with those that use the ancestral specificity (I-CAM). Mechanistically, the replication apparatus of contemporary CAVs seems to have retained compatibility with the refined PV capsid. This is not observed in recombinants generated counter to the PV speciation direction, e.g., CPP, in which the PV replication proteins are “forced” to cooperate with the CAV capsid that inherited ancestral ICAM-1 specificity. The underlying molecular mechanism of this incompatibility phenomenon, whose magnitude seems to be proportional to the intervirus distance between PV and CAV, is currently being studied. Collectively, our data support a description of PV as “species in the making” that is already distinguished by unique receptor specificity but has been separated only partially from the parental C-CAV lineage.
The global campaign of PV eradication was initiated and has been conducted on the premise that PV has no natural host other than humans. However, the PV reservoir is not restricted to wild-type viruses, because it also includes the live OPV and C-CAVs, the latter being viruses that cause a benign disease. Overall, the global control of poliomyelitis has been successful for more than four decades, predominantly through the use of OPV (9, 35). Although OPV is an excellent vaccine, our results reinforce concerns about the risk associated with its use. Due to recombination of OPV with CAVs, highly virulent cVDPVs may essentially replace wild-type PVs in regions of low vaccine coverage. In case of global PV eradication, worldwide cessation of OPV vaccination has been proposed (9) as a radical measure to target OPV as a new genetic base of PV evolution. However, if the vaccination is terminated, the world is destined to become free of anti-PV antibodies, and contemporary C-CAVs may provide a fertile ground for PV (re)emergence by mutation (13).
Materials and Methods
Bioinformatics Analysis of HEV-Cs.
Virus sequence data set collection, multiple sequence alignment generation, and evolutionary analyses are described in detail in SI materials and methods. Several software packages (14, 18, 36, 37) and the Viralis platform (A.E.G., unpublished data) were used in this analysis.
Genetic Analysis of in Vitro and in Vivo Recombination Between PVs and C-CAVs.
The materials and methods used for in vitro and in vivo genetic recombination between PVs and C-CAVs are described in detail in SI Materials and Methods.
Neurovirulence Assay.
The gs revertants of in vivo recombinant polioviruses 2A[111Gly(gr)], 2B[41Leu(gr)], and 2C[140Ala(gr)] were generated and tested in a neurovirulence assay as detailed in SI Materials and Methods. PLD50 was calculated by the method of Reed and Muench (38). All procedures involving experimental mice were conducted according to protocols approved by the institutional committees on animal welfare.
Supplementary Material
Acknowledgments
We thank E. Rieder (Plum Island Animal Disease Center, Greenport, NY) for sharing initial results of C-cluster enteroviruses; M. Gromeier (Duke University, Durham, NC) for providing the Hei cell line; and A. Wimmer for editing the manuscript. A.E.G. and J.A.J.F. thank W. Spaan for encouragement, D. Samborskiy and A. Kravchenko for helping with the Viralis platform, B. Brandt for helpful suggestions, and K. Miaskiewicz for access to National Cancer Institute NR protein database. This study was partially supported by National Institutes of Health Grants AI 15122 and GM 650300 (to E.W.), by European Union Grant FP6 IP Vizier LSHG-CT-2004-511960, and by Netherlands Bioinformatics Centre Grant SP 3.2.2 (to A.E.G.). H.T. was the recipient of a Fellowship from the Pediatrics Oncology Research Foundation (Japan).
Abbreviations
- PV
poliovirus
- IRES
internal ribosomal entry site
- NTR
nontranslated region
- HEV
human enterovirus
- CAV
coxsackie A virus
- C-CAV
C-cluster CAV
- WHO
World Health Organization
- OPV
oral PV vaccine
- cVDPV
circulating vaccine-derived PVs
- ICAM-1
intercellular adhesion molecule-1
- TD
titer difference
- Gdn·HCl
guanidine hydrochloride.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700451104/DC1.
References
- 1.Mueller S, Wimmer E, Cello J. Virus Res. 2005;111:175–193. doi: 10.1016/j.virusres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Wimmer E, Hellen CUT, Cao X. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 3.Paul A. In: Molecular Biology of Picornaviruses. Semler BL, Wimmer E, editors. Washington, DC: Am Soc Microbiol Press; 2002. pp. 227–246. [Google Scholar]
- 4.Stanway G, Hovi T, Knowles NJ, Hyypia T. In: Molecular Biology of Picornaviruses. Semler BL, Wimmer E, editors. Washington, DC: Am Soc Microbiol Press; 2002. pp. 17–24. [Google Scholar]
- 5.Pallansch MA, Roos RP. In: Fields Virology. Knipes DM, Howley PM, Griffin DE, editors. Philadelphia: Lippincott, Williams & Wilkins; 2001. pp. 730–739. [Google Scholar]
- 6.Newcombe NG, Andersson P, Johansson ES, Au GG, Lindberg AM, Barry RD, Shafren DR. J Gen Virol. 2003;84:3041–3050. doi: 10.1099/vir.0.19329-0. [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn CL, Wimmer E, Racaniello VR. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 8.Koike S, Horie H, Ise I, Okitsu A, Yoshida M, Iizuka N, Takeuchi K, Takegami T, Nomoto A. EMBO J. 1990;9:3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heymann DL, Sutter RW, Aylward RB. Nature. 2005;434:699–700. doi: 10.1038/434699a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arita I, Nakane M, Fenner F. Science. 2006;312:852–854. doi: 10.1126/science.1124959. [DOI] [PubMed] [Google Scholar]
- 11.Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA. Annu Rev Microbiol. 2005;59:587–635. doi: 10.1146/annurev.micro.58.030603.123625. [DOI] [PubMed] [Google Scholar]
- 12.Agol VI. Biologicals. 2006;34:103–108. doi: 10.1016/j.biologicals.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Gromeier M, Wimmer E, Gorbalenya AE. In: Origin and Evolution of Viruses. Domingo E, Webster RG, Holland JF, editors. San Diego: Academic; 1999. pp. 287–316. [Google Scholar]
- 14.Ronquist F, Huelsenbeck JP. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 15.Iwabe N, Kuma K, Hasegawa M, Osawa S, Miyata T. Proc Natl Acad Sci USA. 1989;86:9355–9359. doi: 10.1073/pnas.86.23.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown B, Oberste MS, Maher K, Pallansch MA. J Virol. 2003;77:8973–8984. doi: 10.1128/JVI.77.16.8973-8984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmonds P, Welch J. J Virol. 2006;80:483–493. doi: 10.1128/JVI.80.1.483-493.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt HA, Strimmer K, Vingron M, von Haeseler A. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- 19.Arita M, Zhu SL, Yoshida H, Yoneyama T, Miyamura T, Shimizu H. J Virol. 2005;79:12650–12657. doi: 10.1128/JVI.79.20.12650-12657.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Werf S, Bradley J, Wimmer E, Studier FW, Dunn JJ. Proc Natl Acad Sci USA. 1986;78:2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Proc Natl Acad Sci USA. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvala H, Kalimo H, Bergelson J, Stanway G, Hyypia T. J Gen Virol. 2005;86:1897–1907. doi: 10.1099/vir.0.80603-0. [DOI] [PubMed] [Google Scholar]
- 23.Molla A, Paul AV, Wimmer E. Science. 1991;254:1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- 24.Hirst GK. Cold Spring Harbor Symp Quant Biol. 1962;27:303–309. doi: 10.1101/sqb.1962.027.001.028. [DOI] [PubMed] [Google Scholar]
- 25.Kew OM, Nottay BK, Hatch MH, Nakano JH, Obijeski JF. J Gen Virol. 1981;56:337–347. doi: 10.1099/0022-1317-56-2-337. [DOI] [PubMed] [Google Scholar]
- 26.Tolskaya EA, Romanova LA, Kolesnikova MS, Agol VI. Virology. 1983;124:121–132. doi: 10.1016/0042-6822(83)90295-7. [DOI] [PubMed] [Google Scholar]
- 27.Pincus SE, Wimmer E. J Virol. 1986;60:793–796. doi: 10.1128/jvi.60.2.793-796.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shaw J, Garib Z, Andre J, Blackman E, Freeman CJ, Jorba J, et al. Science. 2002;296:356–359. doi: 10.1126/science.1068284. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu H, Thorley B, Paladin FJ, Brussen KA, Stambos V, Yuen L, Utama A, Tano Y, Arita M, Yoshida H, et al. J Virol. 2004;78:13512–13521. doi: 10.1128/JVI.78.24.13512-13521.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duggal R, Wimmer E. Virology. 1999;258:30–41. doi: 10.1006/viro.1999.9703. [DOI] [PubMed] [Google Scholar]
- 31.Kirkegaard K, Baltimore D. Cell. 1986;47:433–443. doi: 10.1016/0092-8674(86)90600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koike S, Taya C, Kurata T, Abe S, Ise I, Yonekawa H, Nomoto A. Proc Natl Acad Sci USA. 1991;88:951–955. doi: 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellmunt A, May G, Zell R, Pring-Akerblom P, Verhagen W, Heim A. Virology. 1999;265:178–184. doi: 10.1006/viro.1999.0003. [DOI] [PubMed] [Google Scholar]
- 34.Xiao C, Bator-Kelly CM, Rieder E, Chipman PR, Craig A, Kuhn RJ, Wimmer E, Rossmann MG. Structure (London) 2005;13:1019–1133. doi: 10.1016/j.str.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Wkly Epidemiol Rec. 2006;81:417–424. [PubMed] [Google Scholar]
- 36.Edgar RC. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drummond AJ, Rambaut A. BEAST. 2003 http://evolve.zoo.ox.ac.uk/beast.
- 38.Reed LJ, Muench M. Am J Hyg. 1938;27:493–497. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.