Abstract
During intraerythrocytic development, Plasmodium falciparum exports proteins that interact with the host cell plasma membrane and subplasma membrane-associated spectrin network. Parasite-exported proteins modify mechanical properties of host RBCs, resulting in altered cell circulation. In this work, optical tweezers experiments of cell mechanical properties at normal physiological and febrile temperatures are coupled, for the first time, with targeted gene disruption techniques to measure the effect of a single parasite-exported protein on host RBC deformability. We investigate Pf155/Ring-infected erythrocyte surface antigen (RESA), a parasite protein transported to the host spectrin network, on deformability of ring-stage parasite-harboring human RBCs. Using a set of parental, gene-disrupted, and revertant isogenic clones, we found that RESA plays a major role in reducing deformability of host cells at the early ring stage of parasite development, but not at more advanced stage. We also show that the effect of RESA on deformability is more pronounced at febrile temperature, which ring-stage parasite-harboring RBCs can be exposed to during a malaria attack, than at normal body temperature.
Keywords: malaria, erythrocyte, membrane shear modulus, spectrin, cytoskeleton
After Plasmodium falciparum merozoite invasion of a human RBC, the parasite differentiates and multiplies for 48 h, leading to rupture of the parasitized RBC (Pf-RBCs) and release of new merozoites in the blood circulation. Throughout this 48-h period, several parasite proteins are introduced into the RBC plasma membrane and submembranous protein skeleton, thereby modifying a range of structural and functional properties of the Pf-RBCs (1–4). The best documented changes occur as P. falciparum matures to the trophozoite (24–36 h) and schizont (36–48 h) stages, when Pf-RBCs display decreased membrane deformability (2–6), become spherical, and develop cytoadherence properties responsible for parasite sequestration in the postcapillary venules of different organs (7, 8). In contrast, during early parasite development, ring-stage (0–24 h after invasion) Pf-RBCs preserve their biconcave shape, can circulate in peripheral blood (9), and thus are exposed to the spleen red pulp. Ring-stage Pf-RBCs may pass through this spleen compartment, be expelled from circulation, or return to the circulation once the parasite has been removed (10). Although the relative importance of these different processes and their underlying mechanisms are not fully understood, it is likely that altered deformability of ring-stage Pf-RBCs (11) plays a crucial role in determining Pf-RBCs spleen processing.
Introduction of parasite components within the Pf-RBC membrane and cortical cytoskeleton begins soon after RBC invasion, as demonstrated for the well characterized parasite protein Pf155/Ring-infected erythrocyte surface antigen (RESA) (12). This protein is discharged by the invading merozoite and exported to the Pf-RBC membrane where, once phosphorylated, it interacts with the spectrin network (13). Because spectrin plays a critical role in the ability of RBCs to deform (14, 15), it has been proposed that RESA could play an important role in the modulation of mechanical properties of ring-stage Pf-RBCs (1, 6). A connection between RESA and altered Pf-RBC mechanical properties has been demonstrated in a previous study on Pf-RBC membrane stability. Pf-RBCs resa1 knockout (KO) parasites obtained by gene disruption were prone to heat-induced vesiculation when exposed at 50°C, whereas resa1+ Pf-RBCs were not (16). This observation confirmed previous conclusions about the stabilization effect of RESA on the RBC submembranous cytoskeleton against heat-induced structural changes (17). However, analysis of RBC membrane deformability using a micropipette approach did not show any significant modification of membrane mechanical properties (16).
Here we demonstrate a new biophysical experimental technique for measuring the effect of a single parasite-exported protein, in this case RESA, on the overall deformability of pathologic RBCs harboring P. falciparum. Previously, optical tweezers were used to characterize mechanical property changes induced by P. falciparum over all intraerythrocytic developmental stages (4, 18). To study the effect of ultrastructural changes induced by parasite-exported proteins on parasitized RBC deformability, we coupled optical tweezers experiments with targeted gene disruption, a cell biology technique to prevent production of a specific protein. Through a combination of these methods adapted from different fields, we assessed, to a force resolution of a pN, the question of the role of RESA on RBC deformability. To ascertain the role of RESA, we constructed a set of three isogenic cloned parasite lines, a resa1 wild type, a resa1-KO targeted gene disruptant clone, and the resa1+ revertant of this clone. Tests were conducted at room temperature to comply with standard deformability assays, as well as at 37°C and 41°C to investigate possible effects on deformability resulting from febrile temperature to which ring-stage Pf-RBCs are exposed during a malaria attack.
RBC deformability is significantly dependent on RBC membrane stiffness. Our optical tweezers experiments led to a quantitative measure of membrane stiffness by determining the shear modulus, μ, of the RBC membrane. An increase in membrane stiffness corresponds to an overall decrease in RBC deformability.
Results
Effect of RESA Protein on Ring-Stage Pf-RBC Deformability.
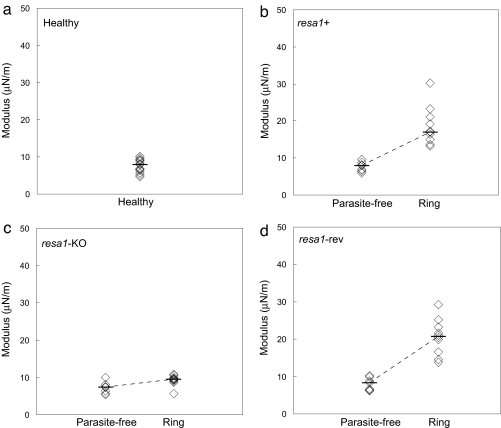
Measurement of RBC membrane stiffness (hereafter referred to as stiffness) for Pf-RBCs was performed on late ring-stage Pf-RBCs (14–20 h after invasion) harboring wild-type resa1+, resa1-KO, and resa1-rev parasites. The resa1-KO parasites were generated by insertion into the resa1 locus on chromosome 1 of a plasmid containing a positive and a negative selection marker. The resa1-rev were derived from the resa1-KO clone by negative selection using ganciclovir (see Materials and Methods). Control tests were performed on healthy RBCs maintained under standard Pf-RBC culture conditions and parasite-free RBCs. (Parasite-free RBCs were present in Pf-RBC cultures but did not harbor any parasites.) Tests were first performed at room temperature (25°C) (Fig. 1).
Fig. 1.
Stiffness of healthy, parasite-free, and late ring-stage (14–20 h after invasion) Pf-RBCs at room temperature (25°C). Tests were performed on cultures of healthy RBCs (a), wild-type resa1+ Pf-RBCs (b), resa1-KO Pf-RBCs (c), and resa1-rev Pf-RBCs (d).
Consistent with earlier work based on micropipette aspiration (19) and optical tweezers (20), the measured stiffness of healthy RBCs was 5–10 μN/m (median μ = 6.8 μN/m) (Fig. 1a). The stiffness of late ring-stage resa1+ Pf-RBCs (μ = 17.7 μN/m) (Fig. 1b) increased significantly compared with healthy RBCs (P < 0.0001). The 2-fold increase in membrane shear modulus for resa1+ Pf-RBCs is consistent with previous work using 3D7, a resa1+ P. falciparum clone (4, 11).
In contrast, late ring-stage resa1-KO Pf-RBCs (μ = 9.4 μN/m) (Fig. 1c) displayed a stiffness similar to healthy RBCs. The observed stiffness of resa1-KO Pf-RBCs was significantly different compared with wild-type resa1+ Pf-RBCs (P < 0.0001).
To ensure that targeted gene disruption of resa1 (and hence the absence of RESA) was responsible for the results observed in resa1-KO Pf-RBCs, we tested resa1-rev Pf-RBCs (Fig. 1d). The measured stiffness of resa1-rev Pf-RBCs (μ = 20.8 μN/m) was similar to that of the resa1+ Pf-RBCs, with a significantly higher shear modulus compared with healthy RBCs (P < 0.0001) and resa1-KO Pf-RBCs (P < 0.001).
Parasite-free RBCs from wild-type resa1+, resa1-KO, and resa1-rev parasite cultures (Fig. 1 b–d, respectively) displayed similar stiffness compared with healthy RBCs (Fig. 1a).
Effect of RESA Protein on Trophozoite-Stage Pf-RBC Deformability.
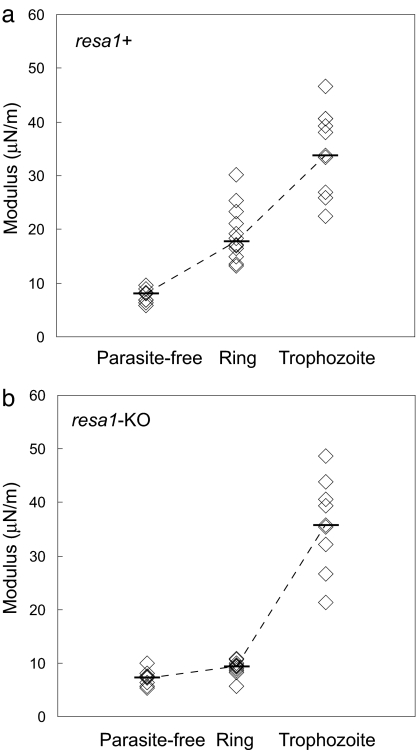
To determine whether the presence of RESA protein at the ring stage is needed for implementing the changes of the mechanical properties displayed by subsequent mature stages of Pf-RBCs, we measured the stiffness of resa1+ and resa1-KO trophozoite-stage Pf-RBCs (24–36 h after invasion). The stiffness of trophozoite-stage resa1+ (μ = 35.9 μN/m) and resa1-KO (μ = 35.7 μN/m) Pf-RBCs (Fig. 2) was significantly higher than ring-stage resa1+ Pf-RBCs (P < 0.001) and resa1-KO (P < 0.001), respectively. The 4-fold increase in shear modulus measured for both resa1+ and resa1-KO trophozoite-stage Pf-RBCs is consistent with previous studies (4, 11). No difference in stiffness between resa1+ and resa1-KO Pf-RBCs was observed at the trophozoite stage.
Fig. 2.
Stiffness over progressive parasite maturation from the ring stage (12–24 h after invasion) to the trophozoite stage (24–36 h after invasion) at room temperature (25°C). Tests were performed on cultures of wild-type resa1+ Pf-RBCs (a) and resa1-KO Pf-RBCs (b).
Impact of Physiological (Steady-State and Febrile) Temperatures on Ring-Stage Pf-RBC Deformability.
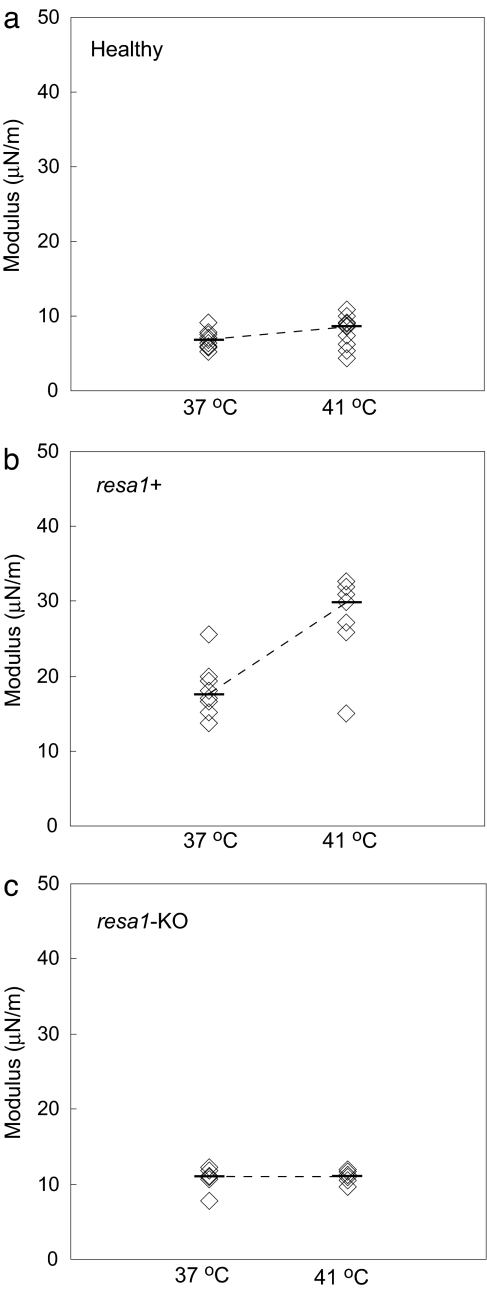
To study Pf-RBC deformation at physiological temperature and the consequences of elevated body temperature during febrile malaria episodes, tests on resa1+ and resa1-KO ring-stage Pf-RBCs were carried out at normal body temperature (37°C) and febrile temperature (41°C) (Fig. 3a).
Fig. 3.
Stiffness of RBCs and Pf-RBCs at normal body (37°C) and febrile (41°C) temperatures. Tests were performed on cultures of healthy RBCs (a), wild-type resa1+ Pf-RBCs (b), and resa1-KO Pf-RBCs (c).
The stiffness of healthy RBCs at 37°C (μ = 6.6 μN/m) and 41°C (μ = 8.6 μN/m) was similar to healthy RBCs at room temperature (5–10 μN/m) (Fig. 1a). The observation that stiffness of healthy RBCs remains approximately constant over this temperature range is consistent with similar measurements using other techniques (19, 21).
Ring-stage resa1+ Pf-RBCs displayed a marked increase in stiffness both at 37°C (μ = 17.6 μN/m) and 41°C (μ = 29.9 μN/m) (Fig. 3b) compared with healthy RBCs. The stiffness increase for resa1+ Pf-RBCs was significantly greater at 41°C than at 37°C (P < 0.05). In contrast, the stiffness of resa1-KO Pf-RBCs (Fig. 3c) at 37°C (μ = 11 μN/m) and 41°C (μ = 11.2 μN/m) remained similar to measurements at room temperature (μ = 9.4 μN/m) (Fig. 1c).
Discussion
During intraerythrocytic development of P. falciparum, parasite-exported proteins contribute to modifications of the deformability of Pf-RBCs. The contribution of specific parasite-exported proteins to decreased deformability has been demonstrated for KAHRP and PfEMP3 (5), which are present in the erythrocyte membrane in the trophozoite and schizont stages when Pf-RBCs are sequestered. Our study explores the effect of RESA, a ring-stage parasite-exported protein, on altered deformability of Pf-RBCs that are not sequestered and, therefore, must circulate. We document here a significantly reduced deformability by measuring the stiffness of ring-stage Pf-RBCs, and we show that RESA is largely responsible for this mechanical property change.
Whereas measured healthy RBC stiffness remained constant over all test temperatures, increased stiffness of ring-stage Pf-RBCs was greater at febrile temperature than at normal body temperature. Because resa1-KO Pf-RBCs exhibited stiffness similar to healthy RBCs at all temperatures, we conclude that the temperature-related change in membrane deformability is attributable, at least in part, to the presence of RESA in the ring-stage Pf-RBC membrane.
At the more advanced trophozoite stage, increased stiffness of Pf-RBCs was independent of a parasite's ability to express RESA. It has been shown that RESA can no longer be detected at the trophozoite stage (22), where parasite proteins other than RESA play a predominant role in decreased deformability, among which are KAHRP and PfEMP3 (5). Furthermore, our results indicate that absence of RESA in the ring stage does not impair subsequent remodeling in the late parasite stages.
Altered deformability during the ring stage is most probably a consequence of the attachment of RESA to spectrin (23). Binding of RESA to β1R16 spectrin structural repeat close to the interaction region between α1 and β1 spectrins significantly increases the stability of spectrin tetramers (24). In healthy RBCs, the spectrin network exists in a dynamic state of spectrin tetramers dissociating transiently into dimers, and shear deformation shifts the equilibrium of tetramer formation toward the dissociated dimer state (25). Thus, RESA stabilization of tetramers could modify spectrin network dynamic properties, thereby impairing membrane deformability.
This model is consistent with a proposed role for RESA on temperature-dependent membrane stability. Increased membrane resistance to thermal shift-induced structural change has been related to the stabilization of spectrin tetramers induced by RESA binding (24). Although the effect of temperature on the interaction between RESA and spectrin is unknown, we speculate that this specific binding alters temperature-dependent stabilization of spectrin tetramers and, as a consequence, contributes to the observed changes in ring-stage Pf-RBC deformability at febrile temperatures.
Deformability is critical for in vivo parasite survival during the ring stage when Pf-RBCs have not yet cytoadhered/sequestered and circulate in postcapillary microvessels and through the spleen. We have shown that ring-stage Pf-RBC decreased deformability is mediated/modulated by RESA, which binds to the RBC cytoskeleton. We find that the effect of RESA on deformability is greater at febrile temperature. It has been shown previously that exposure of ring-stage Pf-RBCs to febrile temperature negatively impacts parasite growth in the absence of RESA (16). RESA may thus play a role in the delicate balance between parasite and its host during the ring stage, on the one hand through contributing to parasite resistance to febrile temperatures and on the other hand through membrane deformability changes that may affect spleen processing of the Pf-RBC.
Materials and Methods
Plasmid Constructs and Gene Disruption.
PCR-amplified full-length P. falciparum resa1 gene was cloned in between SacII/XbaI sites of a modified pdTg23 vector (26) carrying the Toxoplasma gondii dihydrofolate reductase/thymidine synthase gene dhfr/ts (conferring resistance to pyrimethamine) fused in frame with the herpes virus thymidine kinase 1 (27, 28). Ring-stage FUP/CB (resa1+) clone parasites (FCR3-resa1+-like genotype) were transfected with 100 μg of Qiagen-purified targeting plasmid using the electroporation conditions described in ref. 29. Homologous recombination in the endogenous resa1 gene was obtained after a two-step pyrimethamine pressure selection (26). Pyrimethamine-resistant parasites were cloned by limiting dilution. Ganciclovir (10 μM) drug pressure was applied on genetically confirmed resa1-KO parasites in the absence of pyrimethamine to induce the resa1 gene reversion (resa1-rev parasites) (28).
Parasite Culture.
A clone derived from P. falciparum (FUP/CB) parasites, named here wild-type resa1+ P. falciparum, was maintained in leukocyte-free human O+ erythrocytes (Research Blood Components, Brighton, MA) and stored at 4°C for no longer than 2 weeks under an atmosphere of 3% O2, 5% CO2, and 92% N2 in RPMI medium 1640 (Gibco Life Technologies, Rockville, MD) supplemented with 25 mM Hepes (Sigma, St. Louis, MO), 200 mM hypoxanthine (Sigma), 0.209% NaHCO3 (Sigma), and 0.25% albumax I (Gibco Life Technologies). Cultures were synchronized successively by concentration of mature schizonts using plasmagel flotation (30) and sorbitol lysis 2 h after the merozoite invasion to remove residual schizonts (31). For wild-type resa1+, resa1-KO, and resa1-rev Pf-RBCs tests, optical tweezers tests were performed within and 14–20 h (ring stage) or 24–36 h (trophozoite stage) after merozoite invasion.
Bead Preparation.
Two-micrometer-diameter, streptavidin-coated polystyrene beads (Polysciences, Warrington, PA) were centrifuged three times at 16,000 × g in 0.1 mg/ml BSA-PBS. The beads were incubated for 30 min at 4°C in 1 mg/ml Con A (Sigma). The beads were then centrifuged three more times at 16,000 × g in 0.1 mg/ml BSA-PBS and stored in 0.1 mg/ml BSA-PBS.
Sample Preparation.
RBCs and Pf-RBCs were centrifuged at 125 × g three times in RPMI medium 1640 before suspension in 0.1 mg/ml BSA-PBS. Con A-coated polystyrene beads were added to the RBC suspension.
RBC Membrane Stiffness Measurement.
An optical tweezers technique was used to measure single RBC force-displacement response, from which shear modulus values can be determined based on computational modeling (32, 33). Uniaxial tension tests on single RBCs required attaching a small portion of an RBC to the coverslip and, on the diametrically opposite side, to an optically trapped Con A-coated polystyrene bead (Fig. 4e). Movement of the coverslip via a piezo-stage (Physik Instrumente) applied deformations to the RBC with resolution <1 nm. Applied forces up to 300 pN, with resolution <5 pN, were continuously measured by detecting the position of the optically trapped bead with a laser-based position detection system. A standard optical tweezers calibration of trapping force exerted on a bead was performed before tests by subjecting the trapped bead to known forces via fluid flow (Stokes flow technique). During a stretch experiment, deformations and forces were continuously acquired to determine the force-displacement response of a single RBC. Shear modulus values were computed immediately after each experiment by fitting the experimental data to an analytical expression derived from computational modeling using a finite element method (33). This method using continuous acquisition of optical tweezers test data and concurrent data analysis based on finite element modeling provides a substantial improvement to existing techniques for single RBC mechanical property evaluation with optical tweezers (18, 20, 34).
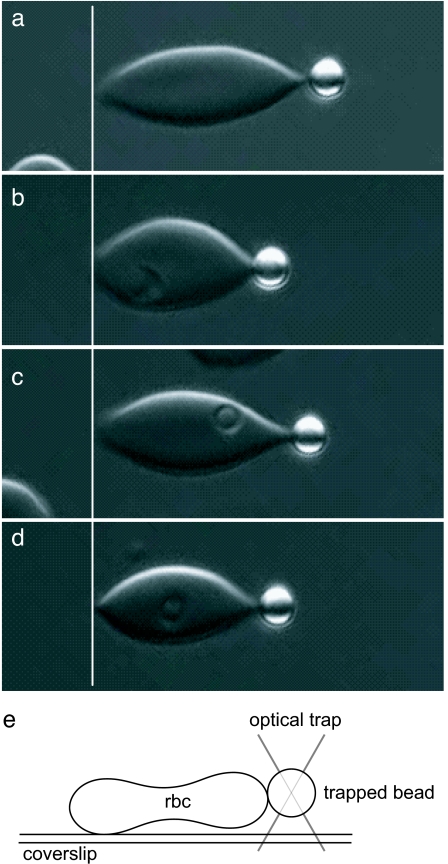
Fig. 4.
Optical microscopy images of RBCs stretched by using optical tweezers. (a–d) Representative RBCs stretched uniaxially with a 100-pN force. The resa1-KO Pf-RBC (c) shows deformability similar to parasite-free RBCs (a), whereas wild-type resa1+ (b) and resa1-rev Pf-RBCs (d) display similar decreased deformability. Stiffness values are determined from the entire force versus displacement response of each cell. (e) A schematic representation of a side view of an optical tweezers stretch test where a RBC is stretched by holding it at the coverslip surface and at the trapped bead.
Temperatures of 37°C and 41°C were achieved by locally heating the test sample with resistive heaters. Optical tweezers tests were conducted at these temperatures after maintaining the test temperature for 10 min. Two resistive heating pads attached to thin copper blocks were kept in thermal contact with the microslide to control the temperature at the sample. A thermocouple placed within the sample chamber was used to measure the local temperature, which was kept to ± 1°C.
Statistical Analysis.
Reported shear modulus values in the text are median values from multiple measurements shown in the figures. P values are calculated by two-tailed Mann–Whitney rank sum tests comparing shear modulus values between various test conditions.
Acknowledgments
This work was supported by an interinstitution research grant made available through the National University of Singapore as part of the Global Enterprise for MicroMechanics and Molecular Medicine, Pasteur Transversal Research Program Grant PTR#59 (to S.B.), and a research grant from the Agence Nationale de Recherches sur le SIDA (to G.M.). S.S. further acknowledges support from the Computational Systems Biology Program of the Singapore–MIT Alliance.
Abbreviations
- RESA
Ring-infected erythrocyte surface antigen
- KO
knockout.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cooke BM, Mohandas N, Coppel RL. Adv Parasitol. 2001;50:1–86. doi: 10.1016/S0065-308X(01)50029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cranston HA, Boylan CW, Carroll GL, Sutera SP, Williamson JR, Gluzman IY, Krogstad DJ. Science. 1984;223:400–403. doi: 10.1126/science.6362007. [DOI] [PubMed] [Google Scholar]
- 3.Paulitschke M, Nash GB. J Lab Clin Med. 1993;122:581–589. [PubMed] [Google Scholar]
- 4.Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Seufferlein T. Acta Biomater. 2005;1:15–30. doi: 10.1016/j.actbio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM. Blood. 2002;99:1060–1063. doi: 10.1182/blood.v99.3.1060. [DOI] [PubMed] [Google Scholar]
- 6.Nash GB, O'Brien E, Gordon-Smith EC, Dormandy JA. Blood. 1989;74:855–861. [PubMed] [Google Scholar]
- 7.Dondorp AM, Pongponratn E, White NJ. Acta Trop. 2004;89:309–317. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Miller LH, Baruch DI, Marsh K, Doumbo OK. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 9.Sherman IW, Eda S, Winograd E. Microbes Infect. 2003;5:897–909. doi: 10.1016/s1286-4579(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 10.Angus BJ, Chotivanich K, Udomsangpetch R, White NJ. Blood. 1997;90:2037–2040. [PubMed] [Google Scholar]
- 11.Mills JP, Qie L, Dao M, Tan KSW, Lim CT, Suresh S. Mater Res Soc Symp Proc. 2005;844:Y7.8.1–Y7.8.6. [Google Scholar]
- 12.Perlmann H, Berzins K, Wahlgren M, Carlsson J, Björkman A, Patarroyo ME, Perlmann P. J Exp Med. 1984;159:1686–1704. doi: 10.1084/jem.159.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley M, Tilley L, Sawyer WH, Anders RF. Mol Biochem Parasitol. 1991;46:137–147. doi: 10.1016/0166-6851(91)90207-m. [DOI] [PubMed] [Google Scholar]
- 14.Bennet V, Lambert S. J Clin Invest. 1991;87:1483–1489. doi: 10.1172/JCI115157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohandas N, Evans EA. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- 16.Silva MD, Cooke BM, Guillotte M, Buckingham DW, Sauzet J-P, Scanf CL, Contamin H, David P, Mercereau-Puijalon O, Bonnefoy S. Mol Microbiol. 2005;56:990–1003. doi: 10.1111/j.1365-2958.2005.04603.x. [DOI] [PubMed] [Google Scholar]
- 17.Da Silva E, Foley M, Dluzewski AR, Murray LJ, Anders RF, Tilley L. Mol Biochem Parasitol. 1994;66:59–69. doi: 10.1016/0166-6851(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 18.Sleep J, Wilson D, Simmons R, Gratzer W. Biophys J. 1999;77:3085–3095. doi: 10.1016/S0006-3495(99)77139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waugh R, Evans EA. Biophys J. 1979;26:115–131. doi: 10.1016/S0006-3495(79)85239-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills JP, Qie L, Dao M, Lim CT, Suresh S. Mech Chem Biosyst. 2004;1:169–180. [PubMed] [Google Scholar]
- 21.Li J, Huang YX, Ji T, Tu M, Mao X, Chen WX, Chen GW. Acta Biochim Biophys Sin. 2005;37:391–395. doi: 10.1111/j.1745-7270.2005.00056.x. [DOI] [PubMed] [Google Scholar]
- 22.Coppel RL, Lustigman S, Murray L, Anders RF. Mol Biochem Parasitol. 1988;31:223–231. doi: 10.1016/0166-6851(88)90152-1. [DOI] [PubMed] [Google Scholar]
- 23.Foley M, Corcoran L, Tilley L, Anders R. Exp Parasitol. 1994;79:340–350. doi: 10.1006/expr.1994.1096. [DOI] [PubMed] [Google Scholar]
- 24.Pei X, Guo X, Coppel R, Haldar K, Gratzer W, Mohandas N, An X. Blood. 2007 doi: 10.1182/blood-2007-02-076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An X, Guo X, Zhang X, Baines AJ, Debnath G, Moyo D, Salomao M, Bhasin N, Johnson C, Discher D, et al. J Biol Chem. 2006;281:10527–10532. doi: 10.1074/jbc.M513725200. [DOI] [PubMed] [Google Scholar]
- 26.Wu Y, Kirkman LA, Wellems TE. Proc Natl Acad Sci USA. 1996;93:1130–1134. doi: 10.1073/pnas.93.3.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garapin AC, Colbere-Garapin F, Cohen-Solal M, Horodniceanu F, Kourilsky P. Proc Natl Acad Sci USA. 1981;94:815–819. doi: 10.1073/pnas.78.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diez Silva MA. Paris: Université Pierre et Marie Curie; 2005. Ph.D thesis. [Google Scholar]
- 29.Fidock DA, Wellems TE. Proc Natl Acad Sci USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasvol G, Wilson RJ, Smalley ME, Brown J. Ann Trop Med Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 31.Lambros C, Vanderberg JP. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 32.Li J, Dao M, Lim CT, Suresh S. Biophys J. 2005;88:3707–3719. doi: 10.1529/biophysj.104.047332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dao M, Li J, Suresh S. Mater Sci Eng C. 2006;26:1232–1244. [Google Scholar]
- 34.Henon S, Lenormand G, Richert A, Gallet F. Biophys J. 1999;76:1145–1151. doi: 10.1016/S0006-3495(99)77279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]