Abstract
Five pentacyclic triterpenoids isolated from Campsis grandiflora were tested for insulin-mimetic and insulin-sensitizing activity. The compounds enhanced the activity of insulin on tyrosine phosphorylation of the IR (insulin receptor) β-subunit in CHO/IR (Chinese-hamster ovary cells expressing human IR). Among the compounds tested, CG7 (ursolic acid) showed the greatest enhancement and CG11 (myrianthic acid) the least. We characterized the effect of CG7 further, and showed that it acted as an effective insulin-mimetic agent at doses above 50 μg/ml and as an insulin-sensitizer at doses as low as 1 μg/ml. Additional experiments showed that CG7 increased the number of IRs that were activated by insulin. This indicates that a major mechanism by which CG7 enhances total IR auto-phosphorylation is by promoting the tyrosine phosphorylation of additional IRs. CG7 not only potentiated insulin-mediated signalling (tyrosine phosphorylation of the IR β-subunit, phosphorylation of Akt and glycogen synthase kinase-3β), but also enhanced the effect of insulin on translocation of glucose transporter 4 in a classical insulin-sensitive cell line, 3T3-L1 adipocytes. The results of the present study demonstrate that a specific pentacyclic triterpenoid, CG7, exerts an insulin-sensitizing effect as an IR activator in CHO/IR cells and adipocytes. The enhancement of insulin activity by CG7 may be useful for developing a new class of specific IR activators for treatment of Type 1 and Type 2 diabetes.
Keywords: adipocyte, glucose transporter 4 (GLUT4), insulin signal transduction, pentacyclic triterpenoids, receptor tyrosine phosphorylation, ursolic acid
Abbreviations: Ab, antibody; CHO/IR, Chinese-hamster ovary cells expressing human insulin receptor; CG6, oleanolic acid; CG7, ursolic acid; CG9, hederagenin acid; CG10, tormentic acid; CG11, myrianthic acid; DMEM, Dulbecco's modified Eagle's medium; ERK, extracellular-signal-regulated kinase; FBS, foetal bovine serum; GLUT4, glucose transporter 4; GSK3β, glycogen synthase kinase 3β; HRP, horseradish peroxidase; IBMX, isobutylmethylxanthine; IP, immunoprecipitation; IR, insulin receptor; IRβ, IR β-subunit; IRS, IR substrate; αMEM, α-minimal Eagle's medium; PI3K, phosphoinositide 3-kinase; pTyr, phosphotyrosine
INTRODUCTION
The IR (insulin receptor) is a tetrameric protein consisting of two identical extracellular α-subunits and two identical transmembrane β-subunits that have intracellular tyrosine kinase activity [1,2]. Binding of insulin to the α-subunits leads to a conformational change and stimulation of the receptor kinase activity via auto-phosphorylation of tyrosine residues in the β-subunits [1–3]. The activated IR kinase then phosphorylates substrate proteins, including the family of IRS (IR substrate) proteins, on specific tyrosine residues that serve as docking sites for downstream effector molecules [4–6]. This triggers two major kinase signalling cascades: the mitogen-activated protein kinase and PI3K (phosphoinositide 3-kinase) pathways. Activation of PI3K is one of the earliest steps in the insulin-signalling pathway and plays a major role in many insulin-regulated responses [7]. Several kinases, including the serine/threonine kinase, Akt, appear to relay the signal initiated by PI3K activation. The involvement of Akt in the translocation of GLUT4 (glucose transporter 4) and glucose uptake has been reported in various cell types [8–11]. PI3K and Akt are thus two important signalling molecules regulating glucose homoeostasis in target cells.
One of the major roles of insulin is to maintain whole-body glucose homoeostasis by stimulating the transport of glucose into peripheral tissues via GLUT4, which is mainly expressed in skeletal and cardiac muscle, and in adipose tissue [12,13]. In normal individuals, the response to an increased plasma glucose level is to increase secretion of insulin from the β-cells of the pancreatic islets. In response to acute insulin stimulation, intracellular vesicles that store GLUT4 are translocated to the plasma membrane and this results in redistribution of GLUT4 to the plasma membrane, where it facilitates glucose uptake [14,15]. In Type 2 diabetes, the decreased ability of insulin to stimulate glucose disposal, and the reduced glucose uptake into muscle or adipose tissues in response to insulin, results in a condition called insulin resistance [3]. Although the molecular basis of Type 2 diabetes is poorly understood, it is well established that insulin signalling, including activation of IR tyrosine kinase activity, is impaired in most patients with Type 2 diabetes [16,17].
Previously, small non-peptide molecules known as IR activators have been developed that restore IR auto-phosphorylation in insulin-resistant cells [18–22]. Such pharmacological agents could be useful for treating Type 2 diabetes [23,24]. Since traditional Chinese herbs and herbal formulae have been used to treat diabetes mellitus, we have investigated whether such medicinal herbs contain IR activators with insulin-mimetic and/or insulin-sensitizing activity.
Campsis grandiflora (Thunb.) K. Schum. (Bignoniaceae), also known by its synonym, Campsis chinensis (Lam.) Voss. is a creeping plant with large, deep orange to red flowers. Its flowers, leaves and roots have long been used as herbal remedies in traditional Chinese medicine to promote blood circulation and remove blood stasis in diseases caused by blood stagnation. For these reasons, C. grandiflora is a traditional Chinese anti-diabetic medicine. The isolation of iridoids, phenyl propanoid glycosides and triterpenoids from the leaves and flowers of this plant has been reported previously [26–33]. Solvent extracts and active compounds isolated from extracts from the flowers and leaves of C. grandiflora have several pharmacological actions [30–33]. However, the mechanisms underlying the anti-diabetic action of C. grandiflora, as well as the identities of its anti-diabetic components are not clear.
In the present study, we have used CHO/IR cells (Chinese-hamster ovary cells expressing modest amounts of human IR) [34], as well as 3T3-L1 primary adipocytes, to identify IR activators with insulin-mimetic and/or insulin-sensitizing activity. We evaluated five pentacyclic triterpenoid compounds isolated from C. grandiflora. Of the five, CG7 (ursolic acid) greatly enhanced the effects of insulin on IR signalling. It induced the substantial increase of insulin-mediated tyrosine auto-phosphorylation of the IR β-subunit, as well as GLUT4 translocation. We found that CG7 increased the number of IRs that were tyrosine-phosphorylated in response to insulin. We also found that it was an effective insulin-mimetic agent at doses over 50 μg/ml and an insulin-sensitizer at doses as low as 1 μg/ml in a cellular model of insulin-sensitive adipose tissue.
EXPERIMENTAL
Materials
DMEM (Dulbecco's modified Eagle's medium), αMEM (α-minimal Eagle's medium), FBS (foetal bovine serum), bovine calf serum, L-glutamine, penicillin and streptomycin were purchased from Gibco® BRL (Invitrogen). Trypsin/EDTA, insulin, dexamethasone, IBMX (isobutylmethylxanthine), o-phenylenediamine dihydrochloride, and GammaBind Sepharose beads were obtained from Sigma Chemical Co. Abs (antibodies) against the IRβ (IR β-subunit; C-19), GLUT4 (H-61), phospho-(Tyr989-IRS-1) (pIRS-1), and ERK (extracellular-signal-regulated kinase) were from Santa Cruz Biotechnology. Abs against protein kinase B (PKB/Akt), phospho-Ser473-Akt (pAkt), phospho-Ser9-GSK3β (glycogen synthase kinase 3β pGSK3β), and phospho-Thr202/Tyr204-ERK1/2 (pERK) were from Cell Signaling Technology. Abs against phosphotyrosine (pTyr; clone 4G10) and biotin-conjugated Ab 4G10 were from Upstate Biotechnology. Monoclonal anti-human IRβ Ab for coating, and streptavidin conjugated to HRP (horseradish peroxidase), were obtained from Biosource International. HRP-conjugated secondary Abs, affinity-purified mouse anti-rabbit IgG and rabbit anti-mouse IgG were purchased from Bio-Rad Laboratories, and the ECL® kit was from Amersham Biosciences.
Plant compounds
Five pentacyclic triterpenoid compounds: CG6 (oleanolic acid), CG7, CG9 (hederagenin acid), CG10 (tormentic acid) and CG11 (myrianthic acid) were isolated from the dried leaves of C. grandiflora. The dried leaves (3.8 kg) were extracted with 90% (v/v) methanol three times for 6 h each. The methanol extract concentrated in vacuo (400 g) was partitioned with chloroform and H2O, and the chloroform layer (120 g), after concentration, was partitioned with hexane and 90% (v/v) methanol to obtain hexane (40 g) and methanol (80 g) fractions. The methanol fraction was subjected to chromatography on a silica-gel column (chloroform/methanol, 95:5–70:30, v/v) to yield ten subfractions (II-1–10). Further chromatography of the subfractions with silica gel, followed by a purification step, gave five pure compounds: CG6 (30 mg) and CG7 (50 mg) from II-3, and CG9 (15 mg), CG10 (10 mg) and CG11 (15 mg) from II-7. The structure of each compound was obtained by infra-red, electron impact MS, one- and two-dimensional NMR spectroscopic methods. The spectroscopy data have been reported previously [30]. The compounds were dissolved in DMSO and diluted with cell culture medium (final DMSO concentration ≤0.01%).
Cell culture
3T3-L1 adipocytes (American Type Culture Collection, Manassas, VA, U.S.A.) were grown and differentiated as described previously [35]. They were cultured in fibroblast medium [DMEM with high (4.5 mg/ml) glucose content] containing 10% (v/v) bovine calf serum, 1% (w/v) glutamine and 1% (w/v) penicillin/streptomycin at 37 °C in a 5% CO2/95% air humidified incubator. After the 3T3-L1 cells had grown to confluence, the pre-adipocytes were induced to differentiate into adipocytes in adipocyte medium [DMEM with high glucose, 10% (v/v) FBS, 1% (w/v) L-glutamine and 1% (v/v) penicillin/streptomycin] supplemented with 0.25 μM dexamethasone, 0.5 mM IBMX and 100 nM insulin. After 4 days in this medium, the adipocytes were transferred to the same medium containing 10 μg/ml insulin for 4 days; thereafter the cells were grown in fresh adipocyte medium without insulin, with fresh medium added every 4 days. The cells were normally used at 12 days post-differentiation, as the full adipocyte phenotype appears approx. 5–8 days after transfer to differentiation medium. CHO/IR cells [23] were kindly provided by Dr Jeffrey Pessin (University of Iowa, Iowa City, U.S.A.). They were maintained in αMEM supplemented with 10% (v/v) FBS, 1% (w/v) L-glutamine and 1% (w/v) penicillin/streptomycin at 37 °C in a humidified 5% CO2/95% air incubator. When the cells reached 90% confluence they were removed from the plate by incubation with trypsin and transferred to a new dish for the next passage.
ELISA for whole-cell IR auto-phosphorylation
The ELISA for tyrosine phosphorylation of the IRβ was performed as described previously [36], with some modifications. After incubation, cells were washed three times with ice-cold PBS and solubilized in Triton X-100 lysis buffer containing 150 mM NaCl, 10 mM Hepes (pH 7.4), 1% (v/v) Triton X-100, protease inhibitors (50 μg/ml aprotinin, 10 μg/ml leupeptin, 40 μg/ml pepstatin A and 1 mM PMSF) and phosphatase inhibitors (400 μM sodium vanadate, 10 mM sodium fluoride and 10 mM sodium pyrophosphate) for 30 min at 4 °C. Cell debris was removed by centrifugation at 800 g for 30 min at 4 °C. Equal amounts of cell lysates (40 μg of protein) were applied to 96-well Immulon-1 flat-bottomed-plates coated with monoclonal anti-human IRβ Ab in carbonate/bicarbonate buffer (15 mM Na2CO3 and 35 mM NaHCO3, pH 9.6) at 4 °C overnight. IRβ was allowed to bind overnight at 4 °C. Next, the plates were washed with PBS containing 0.05% Tween 20, and biotin-conjugated anti-pTyr (4G10) Ab was added to the wells for 1 h at room temperature (25 °C). The wells were again washed and streptavidin–HRP was added. Following the addition of the peroxidase substrate, o-phenylenediamine dihydrochloride, tyrosine phosphorylation was quantified by measuring absorbance at 490 nm with a Spectra MAX 190 microplate reader (Molecular Devices).
Subcellular fractionation
After washing in ice-cold PBS, 3T3-L1 cells were incubated in 300 μl of ice-cold hypotonic buffer S1 (10 mM Hepes/KOH, pH 7.4, and 38 mM NaCl, with protease and phosphatase inhibitors described for the Triton X-100 lysis buffer) and subjected to four freeze–thaw cycles. The resulting suspension was centrifuged at 800 g for 10 min to remove nuclei and intact cells, and the supernatant was centrifuged at 48000 rev./min for 1 h at 4 °C in a TLA 120.2 rotor (Beckman Coulter). The supernatant (S100) was collected, and the membrane pellet (M) was resuspended in 50 μl of 6 M urea before the addition of SDS sample buffer [350 mM Tris/HCl, pH 6.8, 10% (w/v) SDS, 30% (v/v) glycerol, 0.6 M dithiothreitol and 0.1% Bromophenol Blue].
Western blotting
Total cell lysates, and membrane (M) and soluble (S100) fractions, were subjected to SDS/PAGE [8% (v/v) gels]. The proteins were transferred on to nitrocellulose filters and incubated with anti-pTyr Ab to detect phosphorylated proteins (IRS-1, Akt, GSK3β or ERK) and Glut4. Anti-IRβ, anti-Akt and anti-ERK polyclonal Abs were used to assess total protein levels. After incubation with HRP-conjugated secondary Ab, signals were detected by ECL®.
IP (immunoprecipitation)
Cell lysates (30–100 μg of protein) were diluted to 1 ml with Triton X-100 lysis buffer and incubated overnight at 4 °C with 0.5–1 μg of anti-pTyr (4G10) Ab or anti-IRβ Ab conjugated to GammaBind Sepharose beads at 4 °C overnight. The immune complexes were pelleted by centrifugation, washed three times with lysis buffer, and boiled with 2× Laemmli reducing sample buffer [120 mM Tris/HCl, pH 6.8, 20% (v/v) glycerol, 4% (w/v) SDS, 2% (v/v) 2-mercaptoethanol and 0.05% Bromophenol Blue] for Western blotting.
Other methods
Protein concentrations were determined with the Bradford reagent (Bio-Rad Laboratories). The effects of compounds in combination with insulin or on multiple cell lines were examined by two-factor analysis of variance. Comparisons of multiple treatment conditions were made by one factor analysis of variance. Post hoc analysis was by paired t tests when a significant interaction was obtained. Values of P<0.05 were considered significant.
RESULTS
Stimulation of IRβ auto-phosphorylation by pentacyclic triterpenoids in CHO/IR cells
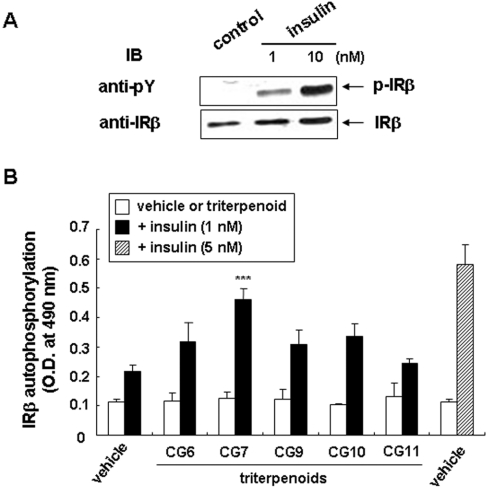
Tyrosine phosphorylation of IRβ increased in response to insulin in a dose-dependent manner in CHO/IR cells, as determined by Western blotting of cell lysates with anti-pTyr Ab (Figure 1A). The effects of insulin on IRβ auto-phosphorylation were also measured using ELISA, as shown in Figure 1(B). Insulin at 5 nM had an approx. 3-fold greater effect than at 1 nM. As also shown in Figure 1(B), none of the triterpenoid compounds at a concentration of 1 μg/ml induced phosphorylation of IRβ. On the other hand, these compounds tended to enhance low dose (1 nM) insulin-mediated tyrosine phosphorylation of IRβ. The effect of CG7 on insulin-mediated IR activation was the most significant (P<0.001), and, therefore, all the subsequent experiments were performed with CG7.
Figure 1. Insulin-stimulated IRβ auto-phosphorylation in CHO/IR cells and effects of triterpenoids on IR.
(A) Cells were treated with vehicle alone, and 1 nM or 10 nM insulin for 15 min. Auto-phosphorylation of IRβ (p-IRβ) was analysed in total cell lysates by Western blotting with anti-pTyr Ab, 4G10 (anti-pY). The level of total IRβ as an index of equal loading of the lysates was analysed with anti-IRβ. The blots shown are one representative of three independent experiments. (B) Cells were incubated in the absence (vehicle) or presence of each triterpenoid at 1 μg/ml for 15 min at 37 °C with 1 nM insulin (closed bars) or without (open bars). Cells treated with 5 nM insulin alone (hatched bar) were included as a positive control. IRβ auto-phosphorylation was measured by ELISA as described in the Experimental section. Values are means±S.D. of the D490 for three experiments. ***, P<0.001 compared with 1 nM insulin alone.
Effect of CG7 on IRβ auto-phosphorylation
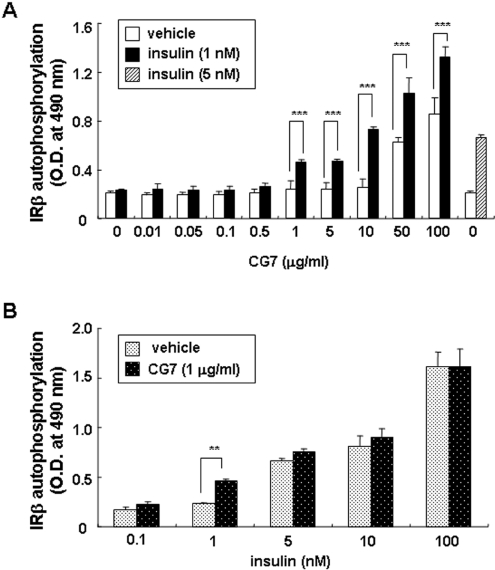
We investigated further the effects of CG7 on IRβ auto-phosphorylation in CHO/IR cells (Figure 2A). In the absence of insulin CG7 had no effect on IRβ auto-phosphorylation at concentrations up to 10 μg/ml, whereas at 50 μg/ml we detected a 2- to 3-fold effect, with the maximal effect observed at 100 μg/ml. The significant IR-sensitizing effects of the compound at low doses of insulin (1 nM) were observed with 1 μg/ml CG7 (P<0.001) and greatly increased at 10 μg/ml (P<0.001). In subsequent IR-sensitizing action experiments, CG7 was used at 10 μg/ml.
Figure 2. Effect of CG7 on the IR in CHO/IR cells.
(A) Cells were incubated with CG7 at the indicated concentrations with (closed bars) or without 1 nM insulin (open bars) for 15 min at 37 °C, and direct effects of CG7 on IRβ auto-phosphorylation were measured by ELISA. Values are means±S.D. for three experiments. ***, P<0.001 indicates values significantly greater than with CG7 alone. (B) Cells were incubated in the presence of the indicated concentrations of insulin (black-dotted bars) or insulin plus 1 μg/ml CG7 (white-dotted bars) for 15 min at 37 °C, and IR β-subunit auto-phosphorylation was measured by ELISA. Values are means±S.D. for three experiments. **, P<0.005 indicates values significantly greater than insulin alone.
In the absence of CG7, a detectable effect of insulin on IR auto-phosphorylation in CHO/IR cells was observed at 1 nM, with half-maximal effects at approx. 5–10 nM and maximal effects at 100 nM (Figure 2B). IR auto-phosphorylation by 1 nM insulin was greatly enhanced in the presence of 1 μg/ml CG7 (P<0.005).
Time course of CG7 action
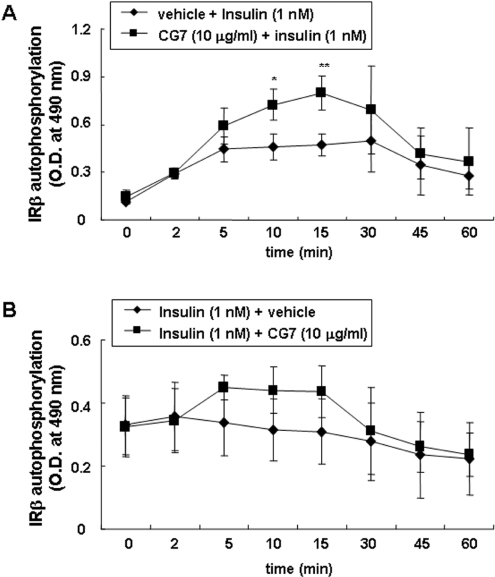
We investigated the time course of the sensitizing effect of CG7 on insulin-mediated IRβ auto-phosphorylation (Figure 3A). With insulin alone, a maximal effect was observed at 5 min; phosphorylation levels were stable up to 30 min and then began to decay, reaching approximately half-maximal values at 60 min. In response to insulin plus CG7, IRβ phosphorylation levels increased continuously for 15 min (P<0.005) and then decayed at a rate similar to that with insulin alone. However, IRβ phosphorylation levels were higher within 5 min in response to insulin plus CG7, and continuously increased and remained higher after 30 min. These IR-sensitizing effects of CG7 were similar when the pre-treatment with CG7 lasted for between 10 min and 6 h (results not shown).
Figure 3. Time course of CG7 action.
(A) CHO/IR cells pre-incubated for 10 min with CG7 or vehicle were stimulated with 1 nM insulin. At various times the cells were solubilized, and ELISAs for IRβ auto-phosphorylation were performed as described in the Experimental section. Values are means±S.D. for three experiments. *, P<0.05; **, P<0.005. (B) CHO/IR cells were incubated with 1 nM insulin, and 10 μg/ml CG7 was added after 10 min. Cells were lysed after various incubation times and ELISAs for IRβ auto-phosphorylation were performed. Values are means±S.D. for three experiments.
We next asked whether CG7 had a sensitizing effect if it was added after insulin had activated IRβ auto-phosphorylation (Figure 3B). We incubated cells with insulin for 10 min and then added CG7. Within 5 min CG7 had an effect, which was constant for 10 min and then decayed to the level with insulin alone.
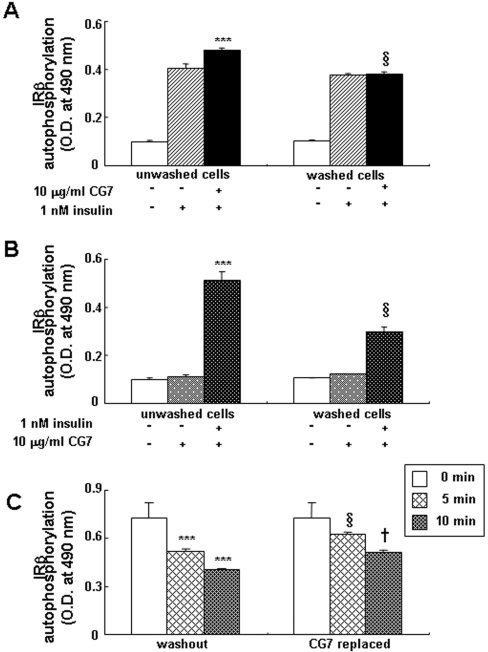
We also determined whether the compound had to be present together with insulin to exert its effect (Figure 4A). Cells were pre-incubated for 10 min with or without CG7, washed and then insulin was added. The removal of the CG7 resulted in complete loss of potentiation of the insulin effect (P<0.001). In the reverse experiments, with insulin pre-incubation for 10 min, followed by washing and addition of CG7, no sensitizing effect was detected (P<0.001; Figure 4B). We then assessed the effect of CG7 on decay of the insulin effect. When both insulin and CG7 were removed after incubation with cells, there was a rapid decay of IRβ auto-phosphorylation to approx. 50% of the level observed for the unwashed cells after a 10 min washout (Figure 4C). However, re-addition of CG7 alone to the incubation medium following this washout slowed the decay markedly (P<0.05).
Figure 4. Effect of CG7 and/or insulin washout on IRβ auto-phosphorylation in CHO/IR cells.
(A) In parallel samples, cells were pre-treated with vehicle or CG7 (10 μg/ml) for 10 min. In one sample the medium containing CG7 was washed out. Insulin (1 nM) was then added to both samples and the cells were incubated for 5 min. IRβ auto-phosphorylation was assayed by ELISA. Values are means±S.D. for triplicate samples per condition. ***, P<0.001 indicates values significantly greater than insulin alone. §, P<0.001indicates values significantly reduced compared with unwashed cells. Similar results were obtained in three separate experiments. (B) In parallel samples, cells were pre-treated with vehicle or 1 nM insulin for 10 min. In one sample, the medium containing insulin was washed out and 10 μg/ml CG7 was then added to both samples. After 5 min of incubation IRβ auto-phosphorylation was quantified by ELISA. Values are means±S.D. of the ODs for three experiments per condition. ***, P<0.001 indicates values significantly greater than CG7 alone. §, P<0.001 indicates values significantly reduced compared with unwashed cells. (C) Effect of CG7 on IRβ auto-phosphorylation after insulin wash-out in CHO/IR cells. In parallel samples CHO/IR cells were pre-treated with CG7 (10 μg/ml) for 10 min, followed by incubation with 1 nM insulin for 10 min. In one set of samples, the medium containing insulin and CG7 was removed and replaced with serum-free medium. In the other sample, the medium was removed and replaced with serum-free medium containing 10 μg/ml CG7. After subsequent incubations for 5 and 10 min, cells were lysed and IRβ auto-phosphorylation was quantified by ELISA. Values are means±S.D. for three experiments per condition. ***, P<0.001 indicates values significantly reduced compared with unwashed (0 min) cells. §, P<0.05 indicates values significantly increased compared with washed cells at 5 min. †, P<0.05 indicates values significantly increased compared with washed cells at 10 min.
Activation of IR signal transduction in CHO/IR cells
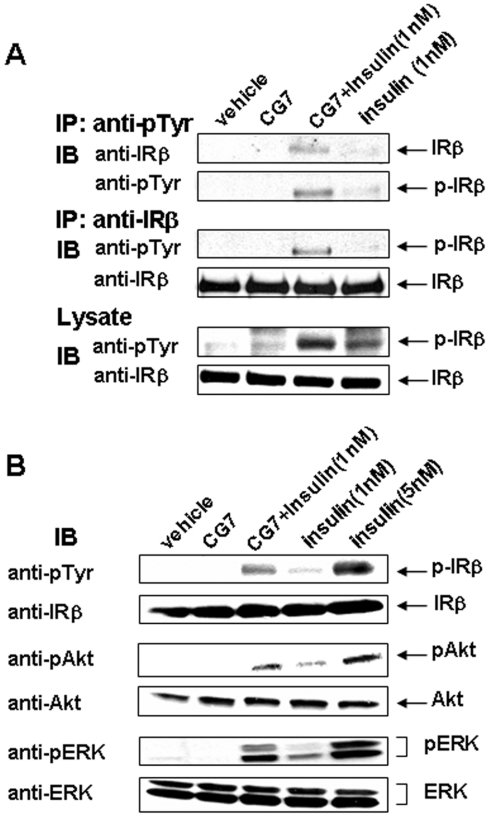
We next investigated if CG7 increased IRβ auto-phosphorylation by increasing the number of IRs that underwent auto-phosphorylation or the number of phosphorylated tyrosine residues per IR molecule. To assess the content of tyrosine-phosphorylated IR, cells were stimulated with insulin plus CG7, lysed, immunoprecipitated with the anti-pTyr Ab and Western blotted with anti-IR Ab. The anti-pTyr Ab immunoprecipitated a significantly greater number of IR molecules from cells incubated with insulin plus CG7 compared with cells incubated with insulin alone (Figure 5A). This result indicates that the increase in the IR pTyr signal from cells incubated with CG7 plus insulin, as detected by ELISA, is due mainly to an increase in the number of phosphorylated IR molecules.
Figure 5. CG7 increases the number of IR undergoing IRβ auto-phosphorylation in CHO/IR cells.
(A) CHO/IR cells were incubated in the presence or absence of 1 μg/ml CG7 for 15 min at 37 °C as described in the Experimental section. Cells were also incubated simultaneously with 1 nM insulin in the presence or absence of CG7. Cell lysates were immunoprecipitated (IP) using Ab against pTyr or IRβ, followed by Western blotting with anti-IRβ or anti-pTyr Ab, as described in the Experimental section. The levels of IRβ and its auto-phosphorylation were also analysed in total cell lysates by Western blotting with anti-IRβ and anti-pTyr Abs. The data shown are one representative for three independent experiments. (B) Lysates were prepared from CHO/IR cells incubated with 1 nM insulin for 15 min at 37 °C in the presence or absence of 1 μg/ml CG7. They were also prepared from cells incubated with vehicle, CG7, or 5 nM insulin alone as controls. Equal amounts of soluble proteins were used for Western blotting with Abs specific for the phosphorylated forms of IRβ, Akt and ERK. Blots were stripped and re-probed with Abs directed against total cellular forms of IRβ, Akt and ERK. The data shown are one representative for three independent experiments. IB, immunoblot.
We also tested whether activation of IR by CG7 results in increased phosphorylation of a number of other proteins, namely IR, Akt and ERK. CHO/IR cells were treated in various ways and lysates were Western blotted with phospho-specific Abs. As shown in Figure 5(B), phosphorylation of these proteins was higher in cells incubated with CG7 plus insulin compared with cells incubated with insulin alone.
Potentiation of insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes
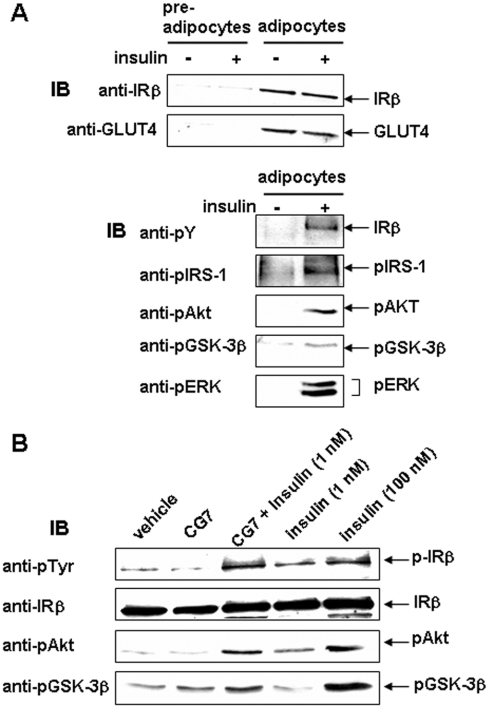
We next enquired whether the action of CG7 on the IR affected the classical insulin effect on glucose transport. Because CHO/IR cells do not have an insulin-sensitive glucose transport system, we used 3T3-L1 adipocytes, which do. We first examined the characteristics of 3T3-L1 adipocytes formed from pre-adipocytes. As shown in Figure 6(A), levels of IR and GLUT4 were greatly enhanced in the differentiated cells, and insulin signalling, including activation of IR tyrosine kinase activity, followed by phosphorylation of endogenous substrates, such as IRS-1, Akt, GSK3β and ERK, was induced in these cells. We then examined the effect of CG7 on insulin signalling. As shown in Figure 6(B), CG7 stimulated tyrosine phosphorylation of the IRβ-subunit as well as phosphorylation of Akt and GSK3β.
Figure 6. Activation of IR signalling in 3T3-L1 adipocytes.
(A) Top panel: 3T3-L1 pre-adipocytes before and after differentiation into adipocytes were incubated with vehicle or 100 nM insulin at 37 °C for 15 min as described in the Experimental section. Adipocyte differentiation was determined by Western blotting of equal amounts of soluble protein with Abs directed against IRβ or GLUT4. Bottom panel: equal amounts of soluble protein from adipocytes were used for Western blotting with Abs specific for the phosphorylated forms of IRβ, IRS-1, Akt, GSK3β and ERK. The blots are representative of three independent experiments. Anti-pY, anti-pTyr. (B) Differentiated 3T3-L1 adipocytes were incubated in the presence or absence of 1 μg/ml CG7 for 15 min at 37 °C. Cells were also incubated simultaneously with 1 nM insulin in the presence or absence of CG7. Cells incubated with 100 nM insulin served as controls. Equal amounts of soluble proteins from each preparation were Western blotted with Abs specific for the phosphorylated forms of IRβ, Akt and GSK3β. Blots were stripped and re-probed with Ab directed against IRβ. The data are one representative for three separate experiments. IB, immunoblot.
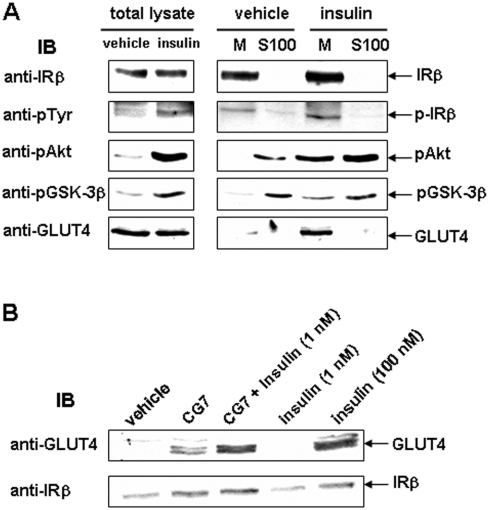
To examine insulin-stimulated translocation of GLUT4 to the plasma membrane, the membrane pellet (M) was separated from the supernatant (S100). Insulin stimulation of 3T3-L1 adipocytes caused translocation of the phosphorylated forms of IRβ, Akt and GSK3β, as well as of GLUT4 to the membrane fraction (Figure 7A). Incubation with 1 nM insulin plus CG7 led to a similar extent of GLUT4 translocation to the plasma membrane as observed with 100 nM insulin alone (Figure 7B).
Figure 7. Effects of CG7 on insulin-independent and insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes.
(A) 3T3-L1 adipocytes were serum-starved and incubated with vehicle or 100 nM insulin at 37 °C for 20 min. Subcellular fractionation was performed as described in the Experimental section. Total cell lysate, cytosol fraction (S100) and membrane pellet (M), 30 μg each, were used for Western blotting with Abs specific for the phosphorylated forms of IRβ, Akt and GSK3β. Blots were stripped and reprobed with Abs directed against IRβ or GLUT4. The data are one representative for at least three independent experiments. (B) Serum-starved 3T3-L1 adipocytes were incubated in the presence or absence of CG7 at 1 μg/ml for 20 min at 37 °C. Cells were also incubated simultaneously with 1 nM insulin in the presence or absence of CG7. Cells incubated with 100 nM insulin served as controls. Membrane pellet (M) fraction (30 μg) was used for Western blotting to determine GLUT4 translocation using an Ab directed against GLUT4. Blots were stripped and reprobed with Ab directed against IRβ. The data shown are one representative for at least three independent experiments. IB, immunoblot.
DISCUSSION
We have investigated whether medicinal herbs contain IR activators with insulin-mimetic and/or insulin-sensitizing activity by analysing their effects on IR signalling, and have identified several components of medicinal plants that greatly stimulate the effect of insulin on IR signalling. In the present study, we tested the effects of five pentacyclic triterpenoids isolated from C. grandiflora on insulin signalling. The extent of stimulation of insulin-mediated tyrosine phosphorylation of the IRβ depended on details of the structures of the pentacyclic triterpenoids. CG7 gave the greatest enhancement, whereas CG11 gave the least. Our findings show that the extent of stimulation of insulin action may depend on the positions and numbers of hydroxyl groups in these compounds: hydroxyl groups at the C3 position have a greater effect on stimulation and increased numbers of hydroxyl groups have an inhibitory effect.
We characterized the effect of CG7 further and showed that it is also an IR activator: it acted as an effective insulin-mimetic agent at levels above 50 μg/ml and an insulin-sensitizer at doses over 1 μg/ml. Pre-incubation with CG7 for approx. 5 min significantly enhanced the activity of a relatively low dose of insulin (1 nM). Kinetic analysis of the action of CG7 demonstrated that it was effective up to 5 min after a maximal response of IR auto-phosphorylation to insulin. CG7 enhancement of insulin activity required the continuous presence of CG7 along with the insulin. However, when cells were stimulated with CG7 plus insulin and only the insulin was removed, the presence of CG7 led to a higher level of IR auto-phosphorylation. These observations suggest that IRβ is the site of action of CG7. Moreover, the results of the wash-out experiments, after exposure of the cells to CG7 for 10 min, also suggested that CG7 could act outside the cells, although both insulin and CG7 are small lipid-soluble molecules that can easily cross the plasma membrane.
Additional experiments showed that CG7 increased the number of IR molecules that were activated by insulin. This indicates that a major mechanism by which CG7 enhances total IR auto-phosphorylation is by promoting the tyrosine phosphorylation of additional IR molecules. Although it is possible that the effects of CG7 are due to inhibition of cellular protein tyrosine phosphatase activity, as reported recently [37], our results support the idea that it acts directly on the IR to expose it to auto-phosphorylation and to maintain its phosphorylation. After stimulation with insulin alone or insulin plus CG7, there was a parallel rapid increase in IR auto-phosphorylation, followed by a parallel decrease, which would not be expected if CG7 were acting solely by inhibiting tyrosine phosphatase activity. Although CG7 did not directly activate the IR at low concentrations, at higher concentrations it had a direct effect on both IR auto-phosphorylation and glucose transport. These results suggest that CG7 does not act on the IR-binding site, but rather on the IRβ and that it specifically enhances IRβ auto-phosphorylation and subsequent downstream signalling.
Defects in the IR and its signal transduction pathway have been found in insulin-resistant patients, including decreased IR- and IRS-1-phosphorylation and decreased PI3K activity [38,39]. Impaired insulin signalling leads to hyperglycaemia and other metabolic abnormalities [40]. Therefore pharmacological agents that enhance IRβ tyrosine kinase activity could be useful in the treatment of Type 2 diabetes, which is characterized by abnormal insulin secretion due to impaired β-cell function and insulin resistance in target tissues [41]. Previously, two small non-peptide molecules that enhance insulin action in cultured cells were reported: one is a direct IR agonist [18] and the other an IR sensitizer [20]. The results of the present study demonstrate that a specific pentacyclic triterpenoid, CG7, also exerts an insulin-sensitizing effect as an IR activator in CHO/IR cells and adipocytes.
Pentacyclic triterpenoids, such as oleanolic acid and CG7, are ubiquitous in the plant kingdom and in medicinal herbs, and are integral components of the human diet. There have been other reports that the naturally occurring pentacyclic triterpenoids act as anti-diabetic agents [42–44]. The present study confirms these findings and provides a possible explanation for the effects of triterpenoids. Although the detailed mechanisms of CG7 action need to be investigated further, our results suggest a new class of compounds as possible IR activators for the treatment of diabetes. Compared with the compounds reported as IR activators previously, CG7 at 1 μg/ml had a greater sensitizing effect on the activity of insulin at concentrations as low as 1 nM. In addition, CG7 is derived from medicinal plants, mixtures of which are often used as traditional remedies for the treatment of diabetes. The identification of a significant IR activator in these medicinal plants may provide the opportunity to develop a novel class of anti-diabetic agent that is more effective and less toxic than those currently available.
Acknowledgments
This study was supported by a grant from the Korea Health 21 R&D Project (01-PJ2-PG6-01NA01-0002) of the Ministry of Health and Welfare, a grant from the Basic Research Program of the Korea Research Foundation (KRF-2002-015-EP0083), and another from the National Core Research Center Program (R15-2006-020) of the Ministry of Science and Technology, and the Korea Science and Engineering Foundation through the Center for Cell Signaling and Drug Discovery Research at Ewha Womans University. S.H.J., Y.J.H. and E.K.S. were supported in part by the Brain Korea 21 programme of the Korea Ministry of Education.
References
- 1.Goldfine I. D. The insulin receptor: molecular biology and transmembrane signaling. Endocrin. Rev. 1987;8:235–255. doi: 10.1210/edrv-8-3-235. [DOI] [PubMed] [Google Scholar]
- 2.Moller D. E., Flier J. S. Insulin resistance: mechanisms, syndromes, and implications. New Eng. J. Med. 1991;325:938–948. doi: 10.1056/NEJM199109263251307. [DOI] [PubMed] [Google Scholar]
- 3.Kahn C. R. Banting Lecture. Insulin action, diabetogenes, and the cause of Type 2 diabetes. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 4.White M. F. The IRS-signaling system: a network of docking proteins that mediate insulin and cytokine action. Rec. Prog. Hor. Res. 1998;53:119–138. [PubMed] [Google Scholar]
- 5.Virkamaki A., Ueki K., Kahn C. R. Protein–protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn C. R., White M. F. The insulin receptor and the molecular mechanism of insulin action. J. Clin. Invest. 1988;82:1151–1156. doi: 10.1172/JCI113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd P. R., Withers D. J., Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem. J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong L. N., Chen H., Li Y., Zhon L., McGibbon M. A., Taylor S. I., Quon M. J. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol. Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 9.Hajduch E., Alessi D. R., Hemmings B. A., Hundal H. S. Constitutive activation of protein kinase Bα by membrane targeting promotes glucose and system A amino acid transport, protein synthesis and inactivation of glycogen synthase kinase-3 in L6 muscle cells. Diabetes. 1998;47:1006–1019. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 10.Wang Q., Somwar R., Bilan P. J., Liu Z., Jin J., Woodgett J. R., Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol. Cell. Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith U., Carvalho E., Mosialou E., Beguinot F., Formisano P., Rondinone C. PKB inhibition prevents the stimulatory effect of insulin on glucose transport and protein translocation but not the antilipolytic effect in rat adipocytes. Biochem. Biophys. Res. Commun. 2000;268:315–320. doi: 10.1006/bbrc.2000.2145. [DOI] [PubMed] [Google Scholar]
- 12.Haruta T., Morris A. J., Rose D. W., Nelson J. G., Mueckler M., Olefsky J. M. Insulin-stimulated GLUT4 translocation is mediated by a divergent intracellular signaling pathway. J. Biol. Chem. 1995;270:27991–27994. doi: 10.1074/jbc.270.47.27991. [DOI] [PubMed] [Google Scholar]
- 13.Charron M. J., Katz E. B., Olson A. L. GLUT4 gene regulation and manipulation. J. Biol. Chem. 1999;274:3253–3256. doi: 10.1074/jbc.274.6.3253. [DOI] [PubMed] [Google Scholar]
- 14.Pessin J. E., Thurmond D. C., Elmendorf J. S., Coker K. J., Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J. Biol. Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 15.Bryant N. J., Govers R., James D. E. Regulated transport of the glucose transporter GLUT4. Nature Rev. Mol. Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 16.Thies R. S., Molina J. M., Ciaraldi T. P., Freidenberg G. R., Olefsky J. M. Insulin-receptor autophosphorylation and endogenous substrate phosphorylation in human adipocytes from control, obese, and NIDDM subjects. Diabetes. 1990;39:250–259. doi: 10.2337/diab.39.2.250. [DOI] [PubMed] [Google Scholar]
- 17.Goldfine I. D. Unraveling the riddle of insulin resistance. J. Lab. Clin. Med. 1999;134:100–102. doi: 10.1016/s0022-2143(99)90112-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B. B., Salituro G., Szalkowski D., Li Z. H., Zhang Y., Royo I., Vilella D., Diez M. T., Pelaez F., Ruby C., et al. Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science. 1999;284:974–977. doi: 10.1126/science.284.5416.974. [DOI] [PubMed] [Google Scholar]
- 19.Quresh S. A., Ding V., Li Z., Szalkowski D., Biazzo-Ashnault D. E., Xie D., Saperstein R., Brady E., Huskey S., Shen X., et al. Activation of insulin signal transduction pathway and anti-diabetic activity of small molecule insulin receptor activators. J. Biol. Chem. 2000;275:36590–36595. doi: 10.1074/jbc.M006287200. [DOI] [PubMed] [Google Scholar]
- 20.Manchem V. P., Goldfine I. D., Kohanski R. A., Cristobal C. P., Lum R. T., Schow S. R., Shi S., Spevak W. R., Laborde E., Toavs D. K., et al. A novel small molecule that directly sensitizes the insulin receptor in vitro and in vivo. Diabetes. 2001;50:824–830. doi: 10.2337/diabetes.50.4.824. [DOI] [PubMed] [Google Scholar]
- 21.Ding V. D. H., Qureshi S. A., Szalkowski D., Li Z., Biazzo-Ashnault D. E., Xie D., Liu K., Jones A. B., Moller D. E., Zhang B. B. Regulation of insulin signal transduction pathway by a small-molecule insulin receptor activator. Biochem. J. 2002;367:301–306. doi: 10.1042/BJ20020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pender C., Goldfine I. R., Manchem V. P., Evans J. L., Spevak W. R., Shi S., Rao S., Bajjalieh S., Maddux B. A., Youngren J. F. Regulation of insulin receptor function by a small molecule insulin receptor activator. J. Biol. Chem. 2002;277:43565–43571. doi: 10.1074/jbc.M202426200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B. B., Moller D. E. New approaches in the treatment of Type II diabetes. Curr. Opin. Chem. Biol. 2000;4:461–467. doi: 10.1016/s1367-5931(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 24.Salituro G. M., Pelaez F., Zhang B. B. Discovery of a small molecule insulin receptor activator. Rec. Prog. Horm. Res. 2001;56:107–126. doi: 10.1210/rp.56.1.107. [DOI] [PubMed] [Google Scholar]
- 25. Reference deleted.
- 26.Guiso M. Pondraneoside, a new iridoid glucoside from Pondranea ricasoliana. J. Nat. Prod. 1982;45:462–465. [Google Scholar]
- 27.Imakura Y., Kobayashi S., Kida K., Kido M. Iridoid glucosides from Campsis chinensis. Phytochemistry. 1984;23:2263–2269. [Google Scholar]
- 28.Imakura Y., Kobayashi S., Mima A. Bitter phenyl propanoid glycosides from Campsis chinensis. Phytochemistry. 1985;24:139–146. [Google Scholar]
- 29.Imakura Y., Kobayashi S., Yamahara Y., Kihara M., Tagawa M., Murai F. Studies on constituents of bignoniaceae plants. IV. Isolation and structure of a new iridoid glucoside, campsiside, from Campsis chinensis. Chem. Pharm. Bull. 1985;33:2220–2227. [Google Scholar]
- 30.Jin J. L., Lee Y. Y., Heo J. E., Lee S., Kim J. M., Yun-Choi H. S. Anti-platelet pentacyclic triterpenoids from leaves of Campsis grandiflora. Arch. Pharm. Res. 2004;27:376–380. doi: 10.1007/BF02980076. [DOI] [PubMed] [Google Scholar]
- 31.Kim D. H., Han K. M., Chung I. S., Kim D. K., Kim S. H., Kwon B. M., Jeong T. S., Park M. H., Ahn E. M., Baek N. I. Triterpenoids from the flower of Campsis grandiflora K. Schum. as human acyl-CoA: cholesterol acyltransferase inhibitors. Arch. Pharm. Res. 2005;28:550–556. doi: 10.1007/BF02977757. [DOI] [PubMed] [Google Scholar]
- 32.Jin J. L., Lee S., Lee Y. Y., Heo J. E., Kim J. M., Yun-Choi H. S. Two new non-glycosidic iridoids from the leaves of Campsis grandiflora. Planta Med. 2005;71:578–580. doi: 10.1055/s-2005-864165. [DOI] [PubMed] [Google Scholar]
- 33.Cui X.-Y., Kim J.-H., Zhao X. C. B.-Q., Lee B.-C., Pyo H.-B., Yun Y.-P., Zhang Y.-H. Antioxidative and acute anti-inflammatory effects of Campsis grandiflora flower. J. Ethnopharmacol. 2006;103:223–228. doi: 10.1016/j.jep.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Frattali A. L., Treadway J. L., Pessin J. E. Evidence supporting a passive role for the insulin receptor transmembrane domain in insulin-dependent signal transduction. J. Biol. Chem. 1991;266:9829–9834. [PubMed] [Google Scholar]
- 35.Olson A. L., Knight J. B., Pessin J. E. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 1997;17:2425–2435. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Youngren J. F., Goldfine I. D., Pratley R. E. Insulin receptor autophosphorylation in cultured myoblasts correlates to glucose disposal in Pima Indians. Amer. J. Physiol. 1999;39:E990–E994. doi: 10.1152/ajpendo.1999.276.5.E990. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W., Hong D., Zhou Y., Zhang Y., Shen Q., Li J., Hu L., Li J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta. 2006;1760:1505–1512. doi: 10.1016/j.bbagen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Dozio N., Micossi P., Galimberti G., Sartori S., Pozza G., Dosio F., Savi F., Gerundini P. G., Fazio F., Chiumello G., et al. In vivo demonstration of insulin-receptor defect with 123I-labeled insulin and scintigraphic scanning in severe insulin resistance. Diabetes Care. 1992;15:651–656. doi: 10.2337/diacare.15.5.651. [DOI] [PubMed] [Google Scholar]
- 39.Goodyear L. J., Giorgino F., Sherman L. A., Carey J., Smith R. J., Dohm G. L. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips for obese subjects. J. Clin. Inv. 1995;95:2195–2204. doi: 10.1172/JCI117909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor S. I. Deconstructing Type 2 diabetes. Cell. 1999;97:9–12. doi: 10.1016/s0092-8674(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 41.Pessin J. E., Saltiel A. R. Signaling pathways in insulin action: molecular target of insulin resistance. J. Clin. Inv. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 43.Huang T. H., Peng G., Kota B. P., Li G. Q., Yamahara J., Roufogalis B. D., Li Y. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-γ and identification of an active component. Toxicol. Appl. Pharmacol. 2005;207:160–169. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz-Andrade R. R., Garcia-Jimenez S., Castillo-Espana P., Ramirez-Avila G., Villalobos-Molina R., Estrada-Soto S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: an anti-hyperglycemic agent. J. Ethnopharmacol. 2007;109:48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]