Abstract
The calcein-AM (calcein-acetoxymethyl ester) method is a widely used technique that is supposed to assay the intracellular ‘labile iron pool’ (LIP). When cells in culture are exposed to this ester, it passes the plasma membrane and reacts with cytosolic unspecific esterases. One of the reaction products, calcein, is a fluorochrome and a hydrophilic alcohol to which membranes are non-permeable and which, consequently, is retained within the cytosol of cells. Calcein fluorescence is quenched following chelation of low-mass labile iron, and the degree of quenching gives an estimate of the amounts of chelatable iron. However, a requirement for the assay to be able to demonstrate cellular LIP in total is that such iron be localized in the cytosol and not in a membrane-limited compartment. For some time it has been known that a major part of cellular, redox-active, labile, low-mass iron is temporarily localized in the lysosomal compartment as a result of the autophagic degradation of ferruginous materials, such as mitochondrial complexes and ferritin. Even if some calcein-AM may escape cytosolic esterases and enter lysosomes to be cleaved by lysosomal acidic esterases, the resulting calcein does not significantly chelate iron at <pH 5. In the present study we show that the calcein-AM method does not capture lysosomal low-mass iron and, therefore, that the method seriously underestimates total cellular labile iron.
Keywords: autophagy, calcein, iron, labile iron pool, lysosomes, oxidative stress
Abbreviations: calcein-AM, calcein-acetoxymethyl ester; DMEM, Dulbecco's modified Eagle's medium; LIP, labile iron pool; SIH, salicylaldehyde isonicotinoyl hydrazone; SSM, sulfide-silver method
INTRODUCTION
The cytochemical calcein-AM (calcein-acetoxymethyl ester) method [1–4] is an established technique for the assay of the cellular ‘labile iron pool’ (LIP). There are however, as we shall demonstrate, reasons to doubt its efficacy.
Calcein-AM is a non-fluorescent lipophilic ester that easily penetrates cellular membranes. It is then rapidly cleaved by unspecific cytosolic esterases [compare with the FDA (fluorescein-diacetate) cellular viability test]. One of the reaction products is calcein, a fluorochromic alcohol that chelates labile iron under quenching of the green calcein fluorescence. Accordingly, following addition of calcein-AM to cells in culture, it diffuses into the cells and is quickly converted into calcein, which does not penetrate membranes; because of this (in the same way as fluorescein), calcein is retained within the cells. Therefore calcein-AM cannot enter cellular compartments, themselves confined by membranes, unless it is administered extracellularly at such high concentrations that it is not fully split by cytosolic esterases before some part of it has had the time to diffuse into these compartments. This point applies, for example, to lysosomes, endoplasmic reticulum and to mitochondria. It follows that for only as long as essentially all the intracellular LIP is in the cytosol is it possible to rely on the calcein-AM method for the evaluation of intracellular low-mas iron: the assay would not reliably detect iron that exists inside membrane-surrounded compartments. Moreover, since the binding of iron by calcein is pH-dependent (see the present study), calcein is not useful for an assessment of low-mass iron in compartments with a pH considerably lower than that of the cytosol.
It has been shown that lysosomes (which together with late endosomes constitute the acidic vacuolar compartment) are rich in low-mass iron [5–12]. This is a result of autophagic degradation of ferruginous material, such as ferritin and mitochondrial complexes. Accordingly, and especially if calcein does not readily chelate iron at lysosomal pH (pH 4–5), the calcein-AM method will not demonstrate all intracellular low-mass iron, but only a fraction of it.
In summary, a major part of intracellular low-mass iron is present in the lysosomal compartment and, therefore, cannot be assayed by the calcein-AM method. First, because calcein does not penetrate lysosomal membranes and, secondly, because it does not chelate iron at the low lysosomal pH.
EXPERIMENTAL
Materials
DMEM (Dulbecco's modified Eagle's medium) and L-glutamine were from Biochrom AG. Fetal calf serum, Nunc tissue culture plastics and penicillin/streptomycin were from Gibco BRL. Calcein-AM, calcein and Lysotracker Red were from Molecular Probes. Microscope slides and glass cover-slips were from the Menzel-Glaset Company. The reducing mounting medium Canada balsam [for the SSM (sulfide-silver method)] was from BDH. The specific iron chelator SIH (salicylaldehyde isonicotinoyl hydrazone) was a gift from Professor Prem Ponka (Department of Physiology and Medicine, McGill University, Montreal, Canada). All other chemicals were from Sigma.
Estimation of calcein fluorescence and iron binding at pH 4–7
Calcein was added (final concentration 60 nM) to 0.1 M acetate (pH 4.0, 5.0 and 6.0) and phosphate (pH 6.0 and 7.0) buffers. Ferrous ammonium sulfate was then repeatedly added to provide a 20 nM stepwise increase in the final iron concentration. The procedure was carried out in stirred cuvettes and the resulting fluorescence monitored (λex 488 nm; λem 518 nm) using an F-2500 Hitachi spectrofluorometer.
Cell culture, exposure to calcein or calcein-AM and ensuing confocal microscopy
HeLa cells were grown in DMEM containing 10% heat-inactivated fetal calf serum, 2 mM glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin in standard culture conditions (37 °C, humidified air, 5% CO2). Cells were propagated in plastic flasks and dishes using routine methods. In order to study the capacity of calcein to pass plasma and lysosomal membranes, cells growing on glass cover-slips were exposed for 2 h to calcein at a final concentration of 5 μM in otherwise standard culture conditions. The calcein-containing medium was then exchanged for normal complete medium, and the cells were subjected to confocal microscopy (Leica Microsystems AG) at different intervals during the next 24 h. To obtain a distinct lysosomal labelling, and to enable a judgement of the endocytic uptake of calcein and its ensuing transport to the lysosomal compartment, cells were exposed for 60 min to Lysotracker Red (50 nM) before microscopy.
In order to assay the efficiency by which the calcein-AM ester is cleaved by unspecific cellular esterases, and how that would influence the diffusion of calcein-AM into membrane-limited intracellular compartments, cells were exposed for 10 min to calcein-AM at concentrations between 10 pM and 12.5 nM before confocal microscopy was carried out following various periods of time under standard culture conditions.
Measurement of intracellular calcein-chelatable iron
The amounts of calcein-chelatable iron within both control cells and cells initially exposed to a bolus dose of hydrogen peroxide (an established way to rupture lysosomes [8–12]) was assayed along the lines described by Cabantchik et al. [1]. Briefly, control HeLa cells were incubated with 0.15 μM calcein-AM for 10 min at 37 °C in PBS containing 1 mg/ml BSA and 20 mM Hepes, pH 7.3. After calcein loading, cells were trypsinized, washed and re-suspended in 2.2 ml of the above buffer without calcein-AM, and placed in a stirred, thermostatically controlled (37 °C) fluorescence spectrophotometer (F-2500 Hitachi) cuvette and the fluorescence was monitored (λex 488 nm; λem 518 nm). Calcein-loaded cells show a fluorescence component (ΔF) that is quenched by intracellular iron. This iron-induced quenching was minimized by the addition of 10 μM SIH, a lipophilic, highly specific and membrane-permeable iron chelator. Cell viability (assayed as Trypan Blue dye exclusion) was >95% and unchanged during the assay.
Since hydrogen peroxide oxidizes calcein, and thus interferes with the assay, the estimation of cellular iron following lysosomal rupture had to be performed somewhat differently: cells were exposed to a bolus dose of hydrogen peroxide (40–200 μM) in culture medium for 2–60 min (hydrogen peroxide is degraded by the cells and, thus the concentration declines with time), rinsed in PBS, exposed to calcein-AM, trypsinized and assayed as described above.
Cytochemical detection of low-mass iron using the SSM
Cells grown on cover slips were subjected to the sensitive SSM for the cytochemical demonstration of cellular, low-mass, labile iron [5,8,12]. This technique is a modification (high pH, high S2−) of the original method by Timm [13]. As iron is the only cellular heavy metal that normally can be demonstrated in significant amounts (the main exceptions are some zinc-rich neurons and prostatic epithelial and endocrine cells), the method can be considered as specific for iron. Following rinsing in PBS, cells were fixed with 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) with 0.1 M sucrose for 2 h at 22 °C. Cells were then rinsed (×5) in glass-distilled water at 22 °C and sulfidated at approx. pH 9 with 1% (w/v) ammonium sulfide in 70% (v/v) ethanol for 15 min. After washing in glass-distilled water for 10 min at 22 °C, development was performed for 20–60 min at 26 °C in the dark using a physical colloid-protected (gum arabic) developer containing silver lactate and hydrochinone (the reaction is autocatalytic and the precipitation of metallic silver on the FeS cores depends on time and the amount of initiating FeS). Following dehydration in a graded series of diluted ethanol and mounting in reducing Canada balsam (to prevent oxidation of black silver to white silver oxide), the cells were examined and photographed under transmitted light using a Leitz DMR microscope connected to a DFC 480 digital camera (Leica) and Leica DFC Twain Software for image acquisition.
Statistical analysis
The Student's paired t test was used in order to determine significant differences between groups.
RESULTS
The hydrophilic alcohol calcein does not pass cellular membranes and has iron-binding and fluorochromic properties that are pH-dependent
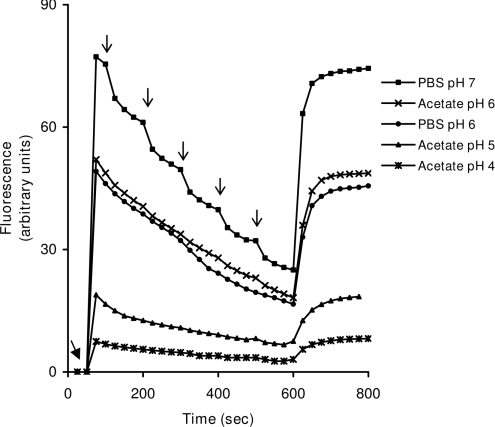
First we wanted to study the fluorescence intensity and iron-binding capacity of calcein at pH 4.0–7.0. As shown in Figure 1, not only is calcein fluorescence heavily influenced by pH, so is its iron-binding capacity, as reflected by the iron-induced quenching of calcein fluorescence. At pH 7.0, the fluorescence intensity of calcein was found to be about 10 times higher than at pH 4.0 and was dramatically quenched by added iron, while at pH 4.0–5.0 iron had only a minor quenching effect on the already low calcein fluorescence.
Figure 1. Fluorescence and iron-binding capacity of calcein is pH-dependent.
Calcein at a final concentration of 60 nM was added (filled arrow) to 2 ml buffers in stirred cuvettes [0.1 M acetate (pH 4.0, 5.0 and 6.0) and phosphate (pH 6.0 and 7.0)]. A freshly prepared solution of ferrous ammonium sulfate in distilled water was then added in aliquots to bring the final iron-concentration up in steps of 20 nM (arrows). Calcein fluorescence, depressed by iron-binding, was finally de-quenched by the addition of a strong iron chelator, SIH, at a final concentration of 10 μM (added at 600 s). Note that both the calcein fluorescence (λex 488 nm; λem 518 nm) and its iron-binding capacity are pH-dependent and much depressed at pH 4.0–5.0 as compared with pH 7.0.
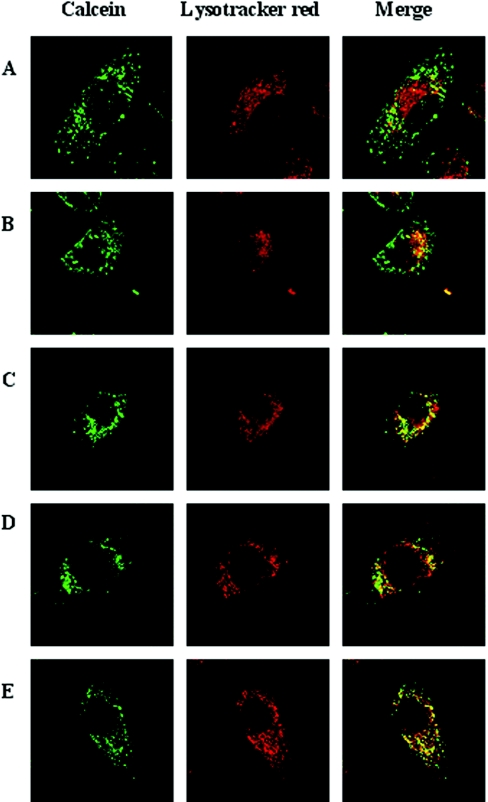
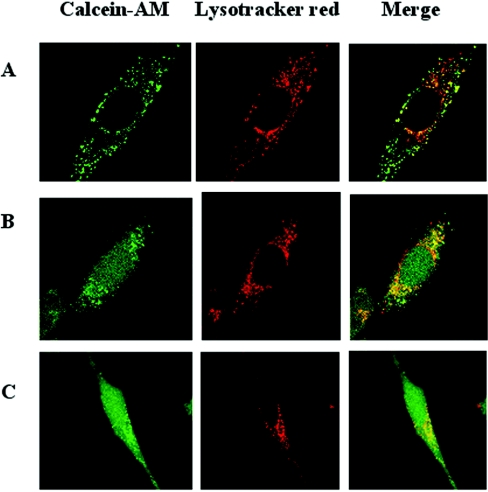
We then wanted to see whether calcein was able to pass cellular membranes, especially plasma and lysosomal membranes. As demonstrated by a series of confocal micrographs (Figures 2A–2E), calcein was taken up by fluid-phase endocytosis (the exposure time to calcein was 2 h) and transported to the lysosomal compartment, where it remained during the period studied (24 h) when cells were kept under standard culture conditions without calcein in the medium. Even at the end of the observation period there was no sign of cytosolic fluorescence, indicating that calcein, indeed, does not pass lysosomal membranes.
Figure 2. Calcein does not penetrate cellular membranes but is taken up by fluid-phase endocytosis.
HeLa cells (2.5×104/cm2), grown on glass cover-slips for 24 h, were exposed for 2 h to 5 μM calcein under otherwise standard culture conditions and then transferred to fresh culture medium without calcein and followed by confocal laser scanning microscopy for the next 24 h. Before each observation, lysosomes were labelled for 60 min with Lysotracker Red (final concentration 50 nM). (A) Calcein is mainly localized in early and late endosomes in the periphery of the cells, while some has reached a few centrally placed lysosomes (see yellow lysosome-sized granules in the merged picture). When the calcein-containing medium was replaced with normal medium for (B) 1 h, (C) 6 h, (D) 12 h or (E) 24 h, calcein entered lysosomes in a time-dependent way. However, even after 24 h there was no cytosolic staining. The results clearly demonstrate that the hydrophilic alcohol calcein does not pass plasma or lysosomal membranes. Note that even after 24 h there are several, mainly centrally located lysosomes that show only red fluorescence in the merged picture, indicating that the formation rate of new lysosomes (autophagolysosomes; compare with Figure 6) is substantial, taking into consideration the active fusion/fission processes within the lysosomal compartment that will introduce calcein into newly formed lysosomes.
Lysosomes contain a major part of cellular labile iron
We then wanted to confirm that lysosomal low-mass iron is a major part of the cell's LIP [5,8,9,12]. Our high pH, high S2− SSM [5] (modified from Timm [13]) is a catalytic cytochemical technique able to demonstrate even minute amounts of labile iron.
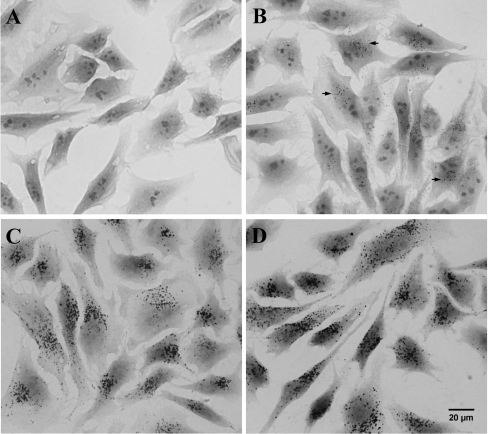
As demonstrated in Figures 3(A)–3(D), normal HeLa cell lysosomes contain substantial amounts of iron, although there are large individual variations between lysosomes within the same cell. Some lysosomes are rich in iron and are distinctly silver-impregnated already after a short period of development (see Figure 3B) while others require longer development to appear (see Figure 3D). Most probably this difference reflects varying participation in recent autophagic degradation of ferruginous materials. The staining of the cytoplasm is much weaker than that of lysosomes, indicating that the concentration of cytosolic low-mass iron is substantially lower than that of lysosomes.
Figure 3. Cellular iron is predominantly located inside lysosomes.
Cells were subjected to the sensitive (high pH, high S2−) SSM for the demonstration of low-mass, labile iron. This method is an autocatalytic one and the precipitation of atomic silver (indicating the presence of FeS) is dependent on the development time (for details see the Experimental section). Lysosomes with a higher concentration of low-mass iron thus appear silver-impregnated earlier than those with less iron. By increasing the development time [20, 25, 30 and 40 min, (A)–(D), respectively] a growing number of lysosomes appear, indicating a pronounced heterogeneity with respect to iron content between different lysosomes, most probably reflecting varying participation in recent autophagic degradation of ferruginous materials. In (A) no SSM-positive lysosomes are apparent, whereas in (B) about 50% of the cells (some are indicated by arrows) show at least a few SSM-positive lysosomes. In (C) and (D), all cells show SSM-positive lysosomes, although those in (D) are more abundant.
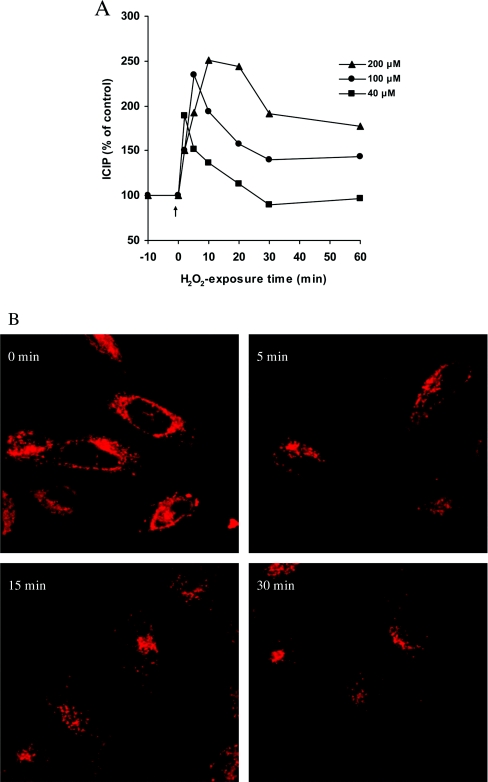
To further demonstrate the high amounts of iron within lysosomes, we exposed cells to a bolus dose of hydrogen peroxide, a well-known way to rupture lysosomes as a result of peroxidative damage to lysosomal membranes [8–12]. As demonstrated in Figures 4(A) and 4(B), the induced lysosomal rupture dramatically and quickly increased the cytosolic amount of labile iron.
Figure 4. The ‘cellular’ calcein-chelatable LIP increases following oxidative stress-induced lysosomal rupture.
(A) HeLa cells were plated (2.5×105/cm2) and, 24 h later, exposed for 2–60 min to a bolus dose of 40–200 μM hydrogen peroxide in culture medium. They were then exposed for 10 min to 0.15 μM calcein-AM in PBS containing 20 mM Hepes and 1 mg/ml BSA (and thereby loaded with calcein), trypsinized and rinsed in PBS containing 20 mM Hepes and 1 mg/ml BSA. The intracellular calcein-induced fluorescence was subsequently assayed (λex 488 nm; λem 518 nm) in a fluorescence spectrophotometer (F-2500 Hitachi). LIP was calculated by the increase in fluorescence following addition of the membrane-permeable, strong iron chelator SIH as described in the Experimental section. The initial cytosolic LIP value for HeLa cells was estimated to be 2.4±0.4 μM, a value that rapidly and significantly increased following exposure to hydrogen peroxide. The Figure is representative of two separate and mainly identical measurements. (B) In order to show the lysosome-rupturing effect of the oxidative stress event, Lysotracker Red-exposed cells (50 nM), growing on glass cover-slips, were exposed to 100 μM hydrogen peroxide in culture medium for 5, 15 and 30 min. The cells were then photographed under a confocal microscope. Note the time-dependent decrease in the number of intact lysosomes with distinct red fluorescence and the appearance of areas with diffuse cytosolic red fluorescence, indicating leakage of Lysotracker Red from the lysosomes.
Calcein-AM is split by cytosolic unspecific esterases, but there is time for some of it to enter lysosomes unless the concentration of calcein-AM is kept very low
Following unspecific cytosolic esterase-induced splitting of calcein-AM, calcein should be present in the cytosol only. However, we noted that within 10 min of the addition of calcein-AM at the ordinary concentration (12.5 nM) to the culture medium, some lysosomes clearly contained calcein. To check this phenomenon further, we labelled the lysosomes with Lysotracker Red for 60 min in medium without calcein-AM and then added calcein-AM for 10 min at concentrations between 10 pM and 12.5 nM and studied the cells by laser confocal scanning microscopy. As shown (Figures 5A–5C), when calcein-AM was used at 10 pM, tiny patches of green fluorescence were found along the plasma membrane but there was no lysosomal green fluorescence. The patches most probably represent areas with unspecific esterases where all of the calcein-AM is split directly after entering the cells. Following exposure to increasingly higher concentrations of calcein-AM, yellow lysosomes started to show up in the merged pictures, indicating the passage of calcein-AM first through the plasma membrane and then into lysosomes, where it is cleaved by lysosomal esterases. Thus some calcein-AM is able to diffuse into lysosomes if a high enough amount of calcein-AM penetrates the plasma membrane. However, since calcein has little or no capacity to chelate labile iron at lysosomal pH (pH 4–5) (see Figure 1), it is clear that the calcein-AM assay, nevertheless, does not evaluate lysosomal iron. Interestingly, the calcein-induced lysosomal green fluorescence is brighter than that of the cytosol, indicating that the effect of iron-induced quenching of cytosolic calcein fluorescence in HeLa cells is far greater than the reduction of calcein fluorescence by the low lysosomal pH.
Figure 5. The concentration of added calcein-AM determines early lysosomal labelling.
HeLa cells (2.5×104/cm2), grown on glass cover-slips, were exposed for 10 min to (A) 10 pM, (B) 100 pM or (C) 12.5 nM calcein-AM under otherwise standard culture conditions. Following lysosomal labelling for 60 min with Lysotracker Red (50 nM), cells were observed by confocal laser scanning microscopy. Note that almost no calcein-AM diffuses into lysosomes when calcein-AM is applied at a low concentration (the peripheral green patches most probably represent plasma membrane-oriented areas of unspecific esterase activity), while a substantial number of lysosomes are labelled when higher concentrations are used and, presumably, the cytosolic unspecific esterases were not able to split all the calcein-AM ester directly after its passage through the plasma membrane, allowing some calcein-AM to continue into lysosomes.
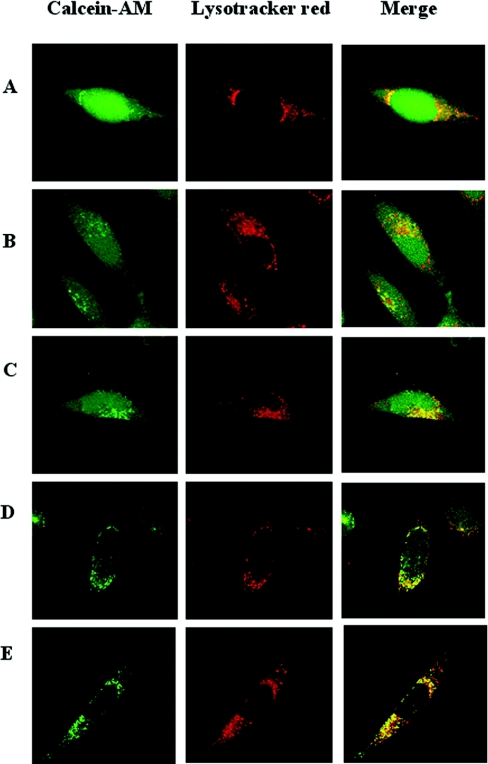
Calcein-AM can be used to assay normal autophagocytosis of the cytosol
Autophagy has recently been found to be a highly conserved turnover mechanism regulated by a family of genes (the ATG family) and of much greater significance to normal life and pathological conditions than generally anticipated [14–16]. Most long-lived proteins and all organelles are turned over by autophagy: that includes macro-, micro- and chaperone-mediated autophagy [16,17]. Since it might be possible to assay the autophagy of cytosolic components by following the lysosomal uptake of calcein-labelled cytosol, we wanted to follow cells exposed to calcein-AM for longer periods of time. Therefore cells were exposed to calcein-AM (12.5 nM) for 10 min and subsequently returned to standard culture conditions. As expected (Figure 6A), initially only a moderate number of calcein-labelled lysosomes could be seen after the return to standard conditions (see above about lysosomal calcein loading when cells are exposed to high concentrations of calcein-AM). However, in time an increasing number of lysosome-sized granules started to appear (Figures 6B–6D); and 24 h after the return to standard conditions the lysosomal pattern was impressive, showing that, as expected [16], normal autophagy does indeed effectively bring cytosol into the lysosomal compartment as part of the normal turnover of cellular material. As seen (Figure 6E), the cytosol only contains little calcein when cells have been kept for 24 h in calcein-AM-free medium following an initial exposure for 10 min to this compound. This finding indicates that the turnover of the cytosol in HeLa cells is rapid, and confirms that calcein does not pass lysosomal membranes.
Figure 6. The calcein-AM method can be used to follow the kinetics of cytosolic autophagocytosis.
(A) Cells growing on glass cover-slips were exposed for 10 min to a bolus dose of 12.5 nM calcein-AM at otherwise standard culture conditions, rinsed and analysed by confocal microscopy. Note cytosolic and some lysosomal (due to the high calcein-AM concentration, compare with Figure 5) calcein-mediated fluorescence. Cells were then kept under standard culture conditions (without calcein-AM) for periods up to 24 h. After 3, 6, 12 and 24 h (B, C, D and E, respectively) an increasing number of calcein-containing lysosomes started to appear, while the cytoplasm became less and less fluorescent. The result indicates that the calcein-labelled cytosol is autophagocytosed into the lysosomal compartment in a time-dependent way and suggests that in HeLa cells a major part of the cytosol is turned over within 24 h. Although lysosomes are known to be rich in iron (compare with Figure 3), quenching of the calcein-mediated fluorescence does not occur in lysosomes because calcein does not bind iron at lysosomal pH (compare with Figure 1), implying that the calcein-AM technique does not assay all intracellular, low-mass, labile iron, but only the cytosolic fraction of it. Further, the retention of calcein inside lysosomes for a long period of time following autophagy of the calcein-labelled cytosol confirms that calcein does not pass lysosomal membranes.
DISCUSSION
By exposing cultured human HeLa cells to the fluorochromic and iron-binding hydrophilic alcohol calcein, we showed that this compound is taken up by fluid-phase endocytosis and is transported to the lysosomal compartment despite being unable to pass either plasma or lysosomal membranes. This explains why calcein is retained in the cell sap following exposure of cells in culture to the lipophilic ester calcein-AM, which is cleaved by unspecific esterases with calcein being one of the reaction products. It follows that once inside the cytosol, calcein will neither diffuse back to the extracellular medium, nor into intracellular membrane-bound compartments.
The calcein-AM method is well-established as a technique for assaying the ‘intracellular’ LIP. Since we have previously shown that lysosomes are rich in iron and that most cellular low-mass, redox-active iron is probably localized inside this compartment [5,7–12,18], the demonstrable inability of calcein to diffuse from the cytosol to the interior of lysosomes strongly suggests that the calcein-AM technique does not measure the totality of ‘intracellular labile iron’, but only the cytosolic fraction of it.
Since it is clear that calcein does not pass lysosomal or plasma membranes, it would probably neither pass inner mitochondrial membranes. Because mitochondria are important sites of cellular iron metabolism, and another example of cellular labile iron existing in compartments, this would form another serious limit to the usefulness of the calcein-AM method for assaying cellular labile iron. In addition, the fact that calcein is a strong iron chelator may also induce problems with the estimation of cytosolic labile iron. By chelating such iron, feedback mechanisms may induce release of iron from ferritin, either directly or following its autophagic degradation [19,20], and rapidly cause an artificial elevation of cytosolic labile iron. If lysosomes are burst, e.g. by exposure of cells to oxidative stress or lysosomotropic detergents, such as MSDH (O-methylserine dodecylamide hydrochloride) or sphingosine, or lysosomotropic aldehydes, such as 3-AP (3-amino propanal), iron will be relocalized to the cytosol or to nuclear DNA and mitochondrial DNA with resulting site-specific iron-induced damage [10,21–27]. In the present study lysosomal rupture was induced by exposing cells to a bolus dose of hydrogen peroxide and the release of iron to the cytosol demonstrated by the quenching of calcein fluorescence. The high concentration of iron inside lysosomes, which is also obvious when the sensitive cytochemical SSM technique is applied (see Figure 3), must be sizeable since a rather incomplete rupture of lysosomes (see Figures 4A and 4B) caused by the exposure for 10 min to a bolus dose of 100–200 μM hydrogen peroxide more than doubled the calcein-chelatable cytosolic iron. In a liver cell the lysosomal compartment constitutes about 2% of the total cell volume but in HeLa cells it is significantly less. Therefore the doubling of the cytosolic iron concentration found following an incomplete lyosomal rupture means that the intralysosomal concentration of low-mass iron should be no less than 100 times that of the cytosol.
Because of the large quantity of labile iron within lysosomes, one would expect that the small amounts of calcein present within lysosomes following diffusion of calcein-AM (see Figure 5) should be subjected to severe fluorescence quenching. In reality this is not the case and is explained by the fact that calcein does not significantly bind low-mass iron at <pH 5 (see Figure 1).
The calcein fluorescence was also found to be pH-dependent (see Figure 1). Consequently, calcein in lysosomes, where the pH is in the range of 4–5, might be expected generally to show a lower fluorescence than in the cytosol (approx. pH 7). Nevertheless, as judged from the confocal pictures (see for example Figure 5C) lysosomal calcein gives a brighter fluorescence compared with cytosolic calcein. The explanation is, of course, the absence of iron-mediated quenching that occurs in the cytosol but not in lysosomes: the depression of calcein fluorescence due to low pH is less than that caused by iron binding.
Initially after the exposure of cells to a bolus dose of calcein-AM, a few lysosomes were labelled unless very low concentrations of calcein-AM were used, indicating that some calcein-AM is not cleaved by cytosolic esterases but is left to diffuse into lysosomes, where it is cleaved by lysosomal esterases. Later, however, when cells were kept at standard culture conditions, calcein-labelled lysosomes started to show up in large numbers reflecting the autophagy of calcein-containing cytosol. Autophagocytosis is a normal and continuously ongoing process that is responsible for the turnover of a major part of the cell's long-lived proteins and all organelles [14–17]. The exposure of cells to a bolus dose of calcein-AM followed by the study of calcein redistribution seems to be an easy way to assay the kinetics of this process. In the present study we found that within 24 h most of the cell sap was turned over by autophagy (Figure 6E). Moreover, the obviously rather rapid formation of autophagolysosomes explains why cells exposed to calcein for 2 h and then kept under standard culture conditions for another 24 h, show many lysosomes without calcein (red cytosolic granules in Figure 2E) in spite of the fusion/fission processes that are known to go on within the lysosomal compartment [28]. If new lysosomes were formed only slowly, it would be expected that almost all of them should contain some calcein and show up as yellow granules in the merged pictures.
In conclusion, a major part of cellular low-mass labile iron is concentrated in the lysosomal compartment and is not demonstrable by the calcein-AM technique for estimation of the ‘cellular’ LIP. First because calcein does not penetrate cellular membranes and, thus, never reaches lysosomes and, secondly, because even if it did, it would not indicate iron because at the pH observed in lysosomes calcein does not chelate it. Thus the calcein-AM technique shows ‘cytosolic’ rather than ‘cellular’ calcein-chelatable iron.
Acknowledgments
This work was supported by the European Union and the Hellenic Ministry of Education within the framework of program “Pythagoras I” (1694) and the Linköping University Hospital Foundations. We thank the confocal laser microscopy facility of the University of Ioannina for the use of their equipment. The expert linguistic advice by Stephen Hampson is gratefully recognized.
References
- 1.Cabantchik Z. I., Glickstein H., Milgram P., Breuer W. A fluorescence assay for assessing chelation of intracellular iron in a membrane model system and in mammalian cells. Anal. Biochem. 1996;233:221–227. doi: 10.1006/abio.1996.0032. [DOI] [PubMed] [Google Scholar]
- 2.Kakhlon O., Cabantchik Z. I. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Radical Biol. Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 3.Esposito B. P., Epsztejn S., Breuer W., Cabantchik Z. I. A review of fluorescence methods for assessing labile iron in cells and biological fluids. Anal. Biochem. 2002;304:1–18. doi: 10.1006/abio.2002.5611. [DOI] [PubMed] [Google Scholar]
- 4.Glickstein H., El R. B., Shvartsman M., Cabantchik Z. I. Intracellular labile iron pools as direct targets of iron chelators: a fluorescence study of chelator action in living cells. Blood. 2005;106:3242–3250. doi: 10.1182/blood-2005-02-0460. [DOI] [PubMed] [Google Scholar]
- 5.Zdolsek J. M., Roberg K., Brunk U. T. Visualization of iron in cultured macrophages: a cytochemical light and electron microscopic study using autometallography. Free Radical Biol. Med. 1993;15:1–11. doi: 10.1016/0891-5849(93)90120-j. [DOI] [PubMed] [Google Scholar]
- 6.Brunk U. T., Neuzil J., Eaton J. W. Lysosomal involvement in apoptosis. Redox Rep. 2001;6:91–97. doi: 10.1179/135100001101536094. [DOI] [PubMed] [Google Scholar]
- 7.Petrat F., de Groot H., Rauen U. Subcellular distribution of chelatable iron: a laser scanning microscopic study in isolated hepatocytes and liver endothelial cells. Biochem. J. 2001;356:61–69. doi: 10.1042/0264-6021:3560061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Z., Persson H. L., Eaton J. W., Brunk U. T. Intralysosomal iron: a major determinant of oxidant-induced cell death. Free Radical Biol. Med. 2003;34:1243–1252. doi: 10.1016/s0891-5849(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 9.Persson H. L., Yu Z., Tirosh O., Eaton J. W., Brunk U. T. Prevention of oxidant-induced cell death by lysosomotropic iron chelators. Free Radical Biol. Med. 2003;34:1295–1305. doi: 10.1016/s0891-5849(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 10.Kurz T., Leake A., von Zglinicki T., Brunk U. T. Relocalized redox-active lysosomal iron is an important mediator of oxidative-stress-induced DNA damage. Biochem. J. 2004;378:1039–1045. doi: 10.1042/BJ20031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baird S. K., Kurz T., Brunk U. T. Metallothionein protects against oxidative stress-induced lysosomal destabilization. Biochem. J. 2006;394:275–283. doi: 10.1042/BJ20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurz T., Gustafsson B., Brunk U. T. Intralysosomal iron chelation protects against oxidative stress-induced cellular damage. FEBS J. 2006;273:3106–3117. doi: 10.1111/j.1742-4658.2006.05321.x. [DOI] [PubMed] [Google Scholar]
- 13.Timm F. Histochemistry of heavy metals: the sulfide-silver procedure. Dtsch. Z. Gesamte Gerichtl. Med. 1958;46:706–711. [PubMed] [Google Scholar]
- 14.Cuervo A. M., Bergamini E., Brunk U. T., Dröge W., Ffrench M., Terman A. Autophagy and aging: the importance of maintaining ‘clean’ cells. Autophagy. 2005;1:131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 15.Baehrecke E. H. Autophagy: dual roles in life and death? Nat. Rev. Mol. Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- 16.Terman A., Brunk U. T. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc. Res. 2005;68:355–365. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Cuervo A. M. Autophagy: many paths to the same end. Mol. Cell. Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- 18.Zdolsek J., Zhang H., Roberg K., Brunk U. H2O2-mediated damage to lysosomal membranes of J-774 cells. Free Radical Res. Commun. 1993;18:71–85. doi: 10.3109/10715769309147344. [DOI] [PubMed] [Google Scholar]
- 19.De Domenico I., Vaughn M. B., Li L., Bagley D., Musci G., Ward D. M., Kaplan J. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006;25:5396–5404. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terman A., Gustafsson B., Brunk U. Autophagy, organelles and ageing. J. Pathol. 2007;211:134–143. doi: 10.1002/path.2094. [DOI] [PubMed] [Google Scholar]
- 21.Li W., Yuan X., Nordgren G., Dalen H., Dubowchik G. M., Firestone R. A., Brunk U. T. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 2000;470:35–39. doi: 10.1016/s0014-5793(00)01286-2. [DOI] [PubMed] [Google Scholar]
- 22.Kagedal K., Zhao M., Svensson I., Brunk U. T. Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem. J. 2001;359:335–343. doi: 10.1042/0264-6021:3590335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Z., Li W., Brunk U. T. 3-Aminopropanal is a lysosomotropic aldehyde that causes oxidative stress and apoptosis by rupturing lysosomes. APMIS. 2003;111:643–652. doi: 10.1034/j.1600-0463.2003.1110607.x. [DOI] [PubMed] [Google Scholar]
- 24.Yu Z., Li W., Hillman J., Brunk U. T. Human neuroblastoma (SH-SY5Y) cells are highly sensitive to the lysosomotropic aldehyde 3-aminopropanal. Brain Res. 2004;1016:163–169. doi: 10.1016/j.brainres.2004.04.075. [DOI] [PubMed] [Google Scholar]
- 25.Doulias P. T., Christoforidis S., Brunk U. T., Galaris D. Endosomal and lysosomal effects of desferrioxamine: protection of HeLa cells from hydrogen peroxide-induced DNA damage and induction of cell-cycle arrest. Free Radical Biol. Med. 2003;35:719–728. doi: 10.1016/s0891-5849(03)00396-4. [DOI] [PubMed] [Google Scholar]
- 26.Tenopoulou M., Doulias P. T., Barbouti A., Brunk U., Galaris D. Role of compartmentalized redox-active iron in hydrogen peroxide-induced DNA damage and apoptosis. Biochem. J. 2005;387:703–710. doi: 10.1042/BJ20041650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simunek T., Boer C., Bouwman R. A., Vlasblom R., Versteilen A. M., Sterba M., Gersl V., Hrdina R., Ponka P., de Lange J. J., Paulus W. J., Musters R. J. SIH-a novel lipophilic iron chelator-protects H9c2 cardiomyoblasts from oxidative stress-induced mitochondrial injury and cell death. J. Mol. Cell. Cardiol. 2005;39:345–354. doi: 10.1016/j.yjmcc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Bright N. A., Gratian M. J., Luzio J. P. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr. Biol. 2005;15:360–365. doi: 10.1016/j.cub.2005.01.049. [DOI] [PubMed] [Google Scholar]