Abstract
S-nitrosothiol compounds are important mediators of NO signalling and can give rise to various redox derivatives of NO: nitrosonium cation (NO+), nitroxyl anion (NO−) and NO• radical. Several enzymes and transporters have been implicated in the intracellular delivery of NO from S-nitrosothiols. In the present study we have investigated the role of GPx (glutathione peroxidase), the L-AT (L-amino acid transporter) system and PDI (protein disulfide-isomerase) in the delivery of NO redox derivatives into human platelets.
Washed human platelets were treated with inhibitors of GPx, L-AT and PDI prior to exposure to donors of NO redox derivatives (S-nitrosoglutathione, Angeli's salt and diethylamine NONOate). Rapid delivery of NO-related signalling into platelets was monitored by cGMP accumulation and DAF-FM (4-amino-5-methylamino-2′7′-difluorofluorescein) fluorescence.
All NO redox donors produced both a cGMP response and DAF-FM fluorescence in target platelets. NO delivery was blocked by inhibition of PDI in a dose-dependent manner. In contrast, inhibition of GPx and L-AT had only a minimal effect on NO-related signalling.
PDI activity is therefore required for the rapid delivery into platelets of NO-related signals from donors of all NO redox derivatives. GPx and the L-AT system appeared to be unimportant in rapid NO signalling by the compounds used in the present study. This does not, however, exclude a possible role during exposure of cells to other S-nitrosothiol compounds, such as S-nitrosocysteine. These results further highlight the importance of PDI in mediating the action of a wide range of NO-related signals.
Keywords: L-amino acid transporter (L-AT), Angeli's salt, diethylamine NONOate (DEANO), glutathione peroxidase (GPx), protein disulphide-isomerase (PDI), S-nitrosoglutathione (GSNO)
Abbreviations: L-AT, L-amino acid transporter; BCH, 2-aminobicyclo[2,2,1]heptane-2-carboxylic acid; CSNO, S-nitrosocysteine; DAF-FM, 4-amino-5-methylamino-2′7′-difluorofluorescein; DEANO, diethyl NONOate sodium salt; GPx, glutathione peroxidase; GSNO, S-nitrosoglutathione; HBS, Hepes-buffered saline; NOx, reactive nitrogen species; PAO, phenylarsine oxide; PDI, protein disulfide-isomerase; PRP, platelet-rich plasma; RSNO, S-nitrosothiol compound; YC-1, 1-benzyl-3-(5-hydroxymethylfur-2-yl)-indazole
INTRODUCTION
RSNOs (S-nitrosothiol compounds), produced by S-nitrosation of cysteine thiols, feature prominently in the biology of NO as mediators of both signalling events and nitrosative stress [1]. The easy synthesis, relative stability and NO-related biological activity of RSNOs make them potentially useful therapeutic tools with a wide range of clinical applications [2], in particular as platelet-selective anti-thrombotic agents [3]. Nevertheless, the NO-related signalling actions of RSNOs are independent of their rate of NO release [4,5] and the mechanism by which they deliver NO into cells is debated. There exists what has been described as a “gulf between our chemical understanding of S-nitrosothiols and the proposed biological activities of these compounds” [6]. However, a picture is emerging of RSNO action being mediated at the target cell surface via stereoselective recognition sites [7], L-ATs (L-amino acid transporters) [8,9] and metabolizing enzymes [10–12].
Several reports have highlighted the possibility that NO delivery may involve an extracellular transnitrosation step in which the RSNO acts as a nitrosonium (NO+) donor, transferring this either to a cell surface thiol-bearing protein such as PDI (protein disulfide-isomerase) [13] or to a low molecular mass thiol such as cysteine, prior to transmembrane transport of CSNO (S-nitrosocysteine) via the L-AT system [9]. A further interesting observation is that extracellular GPx (glutathione peroxidase) potentiates platelet inhibition by RSNOs, possibly by facilitating transnitrosation reactions [14]. It may be that RSNO action involves a variety of modes of cellular metabolism and transmembrane NO delivery, tailored to provide both signalling specificity and also, under conditions of RSNO excess, cellular defence against nitrosative stress. A further layer of complexity arises from the diverse chemistry of RSNO compounds, which under physiological conditions can give rise to various redox derivatives of NO. Thus metal-catalysed RSNO decomposition produces NO radical (NO•), transnitrosation involves transfer of nitrosonium cation (NO+) and S-thiolation reactions release nitroxyl anion (NO−) [15]. There has been recent interest in the fact that these various redox derivatives, in particular NO−, show distinct biological properties [16], including a differential ability to stimulate generation of the classic second messenger cGMP. In fact, some reports have suggested that NO• is the only redox form to stimulate soluble guanylate cyclase [17].
In the present study we have investigated the role of GPx, L-AT and PDI in the delivery of NO signalling into human platelets from the RSNO compound GSNO (S-nitrosoglutathione). In addition, we have used well-characterized donors of both NO• and NO− to determine whether L-AT, GPx and PDI also mediate intra-platelet delivery of these NO redox derivatives.
EXPERIMENTAL
In order to establish which, if any, of the candidate enzyme/transporter systems may be involved in the delivery of NO across the platelet membrane, recognized pharmacological inhibitors of GPx, the L-AT system and PDI were used and NO entry into platelets was measured after addition of NO donors. Three different donors of NO redox derivatives were used: GSNO (an NO+ donor), Angeli's salt (an NO− donor), and DEANO (diethylamine NONOate sodium salt; a donor of free NO, NO•).
Materials and reagents
Angeli's salt was purchased from Merck Biosciences, DAF-FM (4-amino-5-methylamino-2′7′-difluorofluorescein) diacetate was from Molecular Probes, and an antibody against PDI (RL90) was from Alexis Biochemicals. Reagents for measurement of cGMP using an enzyme immunoassay were obtained from Amersham Biosciences. All other chemicals were purchased from Sigma.
Preparation of washed platelets
Washed platelets were prepared from informed healthy volunteers who had not received any medication known to alter platelet function for at least 2 weeks before the study. Blood was anticoagulated with acid citrate dextrose and processed within 1 h of collection. PRP (platelet-rich plasma) was obtained by centrifuging the blood twice at 170 g for 10 min, keeping the upper layer each time. PRP was acidified with 0.5 M citric acid to pH 6.3, and prostaglandin E1 (1.5 μM) and apyrase (2 units/ml) were added prior to centrifugation at 1000 g for 12 min. The platelet pellet obtained was then resuspended in 1.5 ml HBS (Hepes-buffered saline), containing NaCl (140 mM), KCl (2.7 mM), glucose (5 mM), BSA (1 mg ml−1) and Hepes (10 mM; pH 7.3) and loaded on to a Sepharose 2B column (5–6 ml packed Sepharose in a 1.5 cm column equilibrated with HBS). The platelet fraction was eluted with HBS and the platelet count adjusted to 200×109 l−1. Finally, CaCl2 and MgCl2 were added to a final concentration of 1 mM.
Preparation of GSNO
GSNO was prepared as previously described [5] by nitrosation under acid conditions. Briefly, equal volumes of glutathione (20 mM) and sodium nitrite (20 mM) were incubated in the presence of 50 mM HCl on ice for 30 min. GSNO was stabilized by the addition of 1 mM EDTA. GSNO was freshly prepared each day and stored on ice in the dark until used. The concentration of GSNO was estimated by its absorbance at 334 nm using a molar absorption coefficient of 0.85 mM−1 cm−1.
Preparation of Angeli's salt and DEANO
Stock solutions (20 mM each) of Angeli's salt and DEANO were prepared in 10 mM NaOH and diluted in HBS immediately prior to use.
cGMP accumulation in response to an NO donor
NO donors were incubated with platelets at 37 °C for 2 min, after which an equal volume of 20% (v/v) perchloric acid was added and samples were stored at −20 °C. Immediately prior to the assay, the pH was corrected to 7.4 by addition of K3PO4 (0.54 M), after which samples were centrifuged (6500 g for 1 min) to remove the precipitate and cGMP was measured using an enzyme immunoassay.
Measurement of the entry of NOx (reactive nitrogen species) using DAF-FM fluorescence
DAF-FM diacetate is a membrane-permeant fluorescent probe used for detecting intracellular NO. Platelets suspended in HBS without BSA were treated with DAF-FM diacetate (1 μM) for 30 min at 37 °C and then loaded on to a Sepharose 2B column. The DAF-FM-loaded platelets were clearly separated from free DAF-FM and were collected and adjusted to 200×109 l−1, in HBS containing 1 mM CaCl2 and MgCl2 as before. Following addition of the NO donors, fluorescence intensity at 515 nm with excitation at 495 nm was measured using a FLUOstar OPTIMA plate reader (BMG Labtechnologies) as an indicator of NOx entry into platelets.
Investigation of the role of GPx in NO delivery
PRP was pre-incubated with 10–1000 μM β-mercaptosuccinic acid or 0.1–10 mM zinc chloride for 30 min at 37 °C to inhibit GPx, prior to the addition of the NO donor. cGMP levels were then measured as described above.
In separate experiments, washed platelet suspensions were supplemented with GPx (Sigma) to final concentrations of 0.1–20 units ml−1, prior to measurement of cGMP responses. A GPx cellular activity assay kit from Sigma was used to measure GPx in platelet-poor plasma. Concentrations of 0.0075 units ml−1 were found and this plasma GPx activity was completely inhibited by β-mercaptosuccinic acid and zinc chloride at concentrations of 1000 μM and 10 mM respectively (results not shown).
Investigation of the role of L-AT in NO delivery
L-Leucine, as a typical substrate of the L-AT system, and BCH (2-aminobicyclo[2,2,1]heptane-2-carboxylic acid), as a selective inhibitor of L-AT, were used at concentrations of 1–10 mM to competitively block the activity of this transport mechanism. Both have previously been shown to inhibit the entry of CSNO into human epithelial cells at this concentration range [8]. BCH, L-leucine and D-leucine (as a control), were pre-incubated with washed platelets for 30 min at 37 °C prior to the addition of NO donor when measuring cGMP levels, and with DAF-FM-loaded washed platelets when measuring fluorescence as an indicator of NO entry.
Investigation of the role of PDI in NO delivery
The ability of bacitracin, PAO (phenylarsine oxide) and the anti-PDI antibody, RL90, to inhibit the disulphide exchange activity of 0.16 μM PDI (Sigma) was monitored using an insulin turbidity assay, as described previously [18]. cGMP levels and DAF-FM fluorescence were measured after pre-incubation of washed platelets for 30 min at 37 °C with 0.05–5 mM bacitracin, 1–100 μM PAO and 0.2–20 μg ml−1 RL90.
RESULTS
A similar cGMP response was promoted in washed platelets with the addition of 10 μM GSNO, 10 μM Angeli's salt and 1 μM DEANO and therefore these concentrations were used in subsequent studies (Figure 1a). When cGMP was measured in PRP, 10-fold higher concentrations of each donor were required.
Figure 1. Measurement of cGMP accumulation and DAF-FM fluorescence intensity following exposure of washed platelets to NO redox derivatives.
Intracellular delivery of NOx from donors of NO redox derivatives GSNO, Angeli's salt (AS) and DEANO, was measured by (a) cGMP accumulation and (b) the change in DAF-FM fluorescence intensity (ΔFI).
For studies using DAF-FM fluorescence to monitor delivery of NOx into platelets, 10 μM Angeli's salt and 1 μM DEANO were found to give similar increases in fluorescence intensity and these concentrations were therefore used in experiments with DAF-FM-loaded platelets. Smaller increases in fluorescence intensity were obtained with GSNO, however treatment of platelets with 100 μM GSNO provided a usable response (Figure 1b).
GPx
Effect of inhibition of GPx on cGMP generation
cGMP generation stimulated by GSNO, Angeli's salt and DEANO in PRP was inhibited to a minor degree (maximum 30%) by both β-mercaptosuccinic acid and zinc chloride (Figure 2), however this inhibition failed to show a clear dose-response relationship.
Figure 2. Inhibition of cGMP accumulation by inhibitors of GPx, in response to donors of different NO redox derivatives.
GSNO, Angeli's salt (AS) and DEANO were added to platelets which resulted in an accumulation of cGMP. Inhibition of this accumulation by inhibitors of GPx, (a) β-mercaptosuccinate and (b) zinc chloride, was monitored. Results represent the means for six to eight experiments. Error bars are omitted for clarity.
Effect of GPx supplementation on GSNO-stimulated cGMP generation in washed platelets
Addition of GPx had the effect of increasing GSNO-stimulated cGMP production in platelets, however levels of GPx required to show this effect were approx. 1000 times higher than the concentration of GPx found in plasma (0.0075 units ml−1). GPx supplementation had no effect on Angeli's salt- or DEANO-stimulated cGMP production (Figure 3).
Figure 3. cGMP accumulation in response to donors of different NO redox derivatives, following addition of GPx to washed platelet suspensions.
GPx was added to washed platelets. cGMP accumulation was then measured following exposure of the platelets to GSNO, Angeli's salt and DEANO. Results represent means±S.E.M. from four experiments.
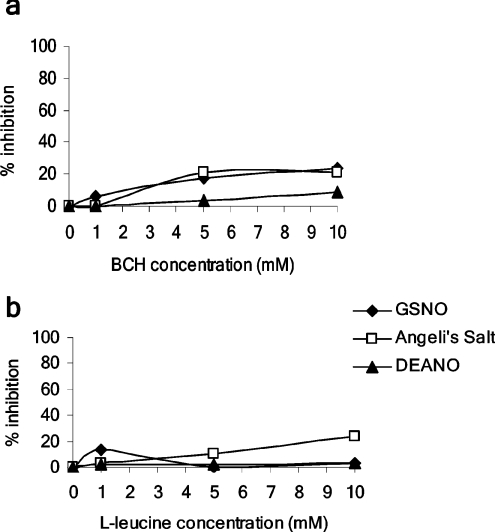
L-AT system
Neither BCH (1–10 mM) nor L-leucine (1–10 mM) significantly inhibited cGMP generation stimulated by any of the NO donors (Figures 4a and 4b). D-leucine was also shown to have no effect (results not shown).
Figure 4. Inhibition of cGMP accumulation by inhibitors of L-AT, in response to donors of different NO redox derivatives.
The effect of L-AT inhibitors: (a) BCH and (b) L-leucine, on cGMP accumulation in response to donors of different NO redox derivatives was monitored. Results represent the means of three to four experiments. Error bars are omitted for clarity.
In parallel experiments, NOx entry measured fluorometrically in DAF-FM-loaded platelets was unaffected by either BCH or L-leucine (results not shown).
PDI
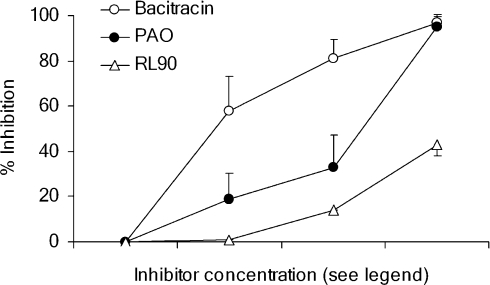
Inhibition of PDI by bacitracin, PAO and RL90
All three agents produced concentration-dependent inhibition of PDI activity however the degree of inhibition was smaller with RL90 than with bacitracin or PAO (Figure 5).
Figure 5. Inhibition of PDI enzyme activity by bacitracin, PAO and RL90.
Disulphide exchange activity of authentic PDI (0.16 μM) was measured by an insulin turbidity assay in the presence of bacitracin (0.05, 0.5 and 5 mM), PAO (1, 10 and 100 μM) and RL90 anti-PDI antibody (0.2, 2 and 20 μg ml−1) and the degree of enzyme inhibition calculated by comparison with the activity obtained using vehicle control alone. Values shown represent means±S.E.M. from three to four experiments.
Platelet cGMP responses are inhibited by bacitracin
NO donors were added to washed platelets in the presence of bacitracin (0–5 mM), RL90 (0–20 μg ml−1) and PAO (0–100 μM). cGMP accumulation resulting from stimulation by all NO donors was inhibited in a dose-dependent manner by bacitracin (Figure 6). The effect of RL90 and PAO, however, was less clear. RL90 had a minimal effect on cGMP accumulation in response to GSNO and DEANO, although it had some effect with Angeli's salt (results not shown). Conversely, PAO, at concentrations of 10 and 100 μM completely inhibited cGMP accumulation with all NO donors (results not shown).
Figure 6. Inhibition of cGMP accumulation by an inhibitor of PDI, in response to donors of different NO redox derivatives.
The PDI inhibitor, bacitracin, was pre-incubated with washed platelets. Inhibition of cGMP accumulation in response to donors of different NO redox derivatives [GSNO, Angeli's salt (AS) and DEANO], was measured. Results represent means±S.E.M. from six to eight experiments.
Experiments using YC-1 [1-benzyl-3-(5-hydroxymethylfur-2-yl)-indazole] suggest direct interference of RL90 and PAO with soluble guanylate cyclase
To investigate the inconsistent effects of the different PDI inhibitors on cGMP responses we investigated whether the inhibitors might exert a direct effect on the action of soluble guanylate cyclase, using the NO-independent activator YC-1 [19]. PDI inhibitors were pre-incubated with washed platelets after which 100 μM YC-1 was added. cGMP responses were unchanged with bacitracin (5 mM), increased approx. 3-fold with RL90 (20 μg ml−1), and almost completely abolished with PAO (100 μM) (results not shown). Since RL90 and PAO interfered directly with soluble guanylate cyclase, only experiments using bacitracin to inhibit PDI are regarded as reliable.
NO entry into DAF-FM-loaded platelets is inhibited by bacitracin, RL90 and PAO
Experiments using DAF-FM-loaded platelets indicated that PDI plays a role in delivery of all redox derivatives of NO. Pre-incubation of DAF-FM-loaded platelets with bacitracin, RL90 and PAO produced a dose-dependent inhibition of fluorescence intensity in response to all three NO donor compounds (Figure 7). Control experiments performed in the absence of NO donors showed that the PDI inhibitors themselves produced no change in DAF-FM fluorescence over the experimental period (results not shown).
Figure 7. Increases in DAF-FM fluorescence in response to donors of different NO redox derivatives are inhibited by inhibitors of PDI.
GSNO, Angeli's salt and DEANO were added to DAF-FM-loaded platelets which resulted in increases in DAF-FM fluorescence over a period of 10 min. Increasing concentrations of (a) bacitracin, (b) RL90 and (c) PAO pre-incubated with these platelets resulted in a dose-related inhibition of this increase in fluorescence intensity. Results represent means±S.E.M. from three to five experiments.
DISCUSSION
The principal finding of the present study is that PDI activity is required for the delivery into platelets of NO-related signals from donors of all NO redox derivatives. Under the experimental conditions used, GPx and the L-AT system appeared to be unimportant in NOx delivery.
Two systems, cGMP accumulation and DAF-FM fluorescence, were used to detect the arrival of NOx inside the target platelets and a positive response was obtained with both detection systems following treatment of platelets with donors of all three NO redox derivatives. Previous reports have suggested that only NO• is capable of activating soluble guanylate cyclase [17], however this result was obtained using the purified enzyme and there are numerous data showing that in whole platelets a cGMP response is obtained in response to GSNO [20,21], DEANO [5] and Angeli's salt [22]. This indicates either that the responsiveness of soluble guanylate cyclase is in fact wider than previously believed, or that NO− is at least partly converted to NO• once inside the cell [23]. Diamino fluorophores, including DAF-FM, are widely used as intracellular indicators of NO• production, however it is now recognized that fluorescence marks the reaction of the probe with NOx rather than the NO• radical itself [24,25]. Thus in the current study a positive signal is interpreted simply as evidence of NOx entry, and the exact identity of the delivered NOx remains uncertain.
We were interested in investigating the role of GPx in platelet regulation by NO because of its possible clinical significance. In the late 1990s a novel thrombotic tendency was reported, first in a single family [26] and later in a larger study of seven families [27], in which deficiency of plasma GPx was associated with both platelet resistance to GSNO and an increased incidence of childhood stroke. An earlier paper [14] had suggested that plasma GPx could potentiate the anti-platelet action of GSNO via two mechanisms: (i) reduction of lipid peroxides thereby preventing them from inactivating NO, and (ii) an ability of extracellular GPx to catalyse transnitrosation. Certain observations reported in these studies suggested to us that the second mechanism was more likely. For example, sodium nitroprusside-induced inhibition of platelet aggregation was not altered by GPx [14], suggesting its effects might be an RSNO-related phenomenon, and not more generally relevant to other NO donor compounds. Also, intraplatelet GPx (as opposed to extracellular GPx in the plasma) was found to be normal when measured in one affected family [26], suggesting that the GPx effect was exerted outside the platelet, for example via catalysis of transnitrosation from an RSNO to a target on the exofacial surface of the plasma membrane. Despite these considerations, we did not find evidence in the current study that plasma GPx mediates the transfer of NO-related signalling into platelets. β-Mercaptosuccinate and zinc chloride, at concentrations shown to completely inhibit plasma GPx, showed only a limited ability to inhibit cGMP generation following addition of NO donors, and this minor inhibition was not dose-dependent. It should be noted that previous authors have monitored platelet aggregation and P-selectin surface expression, whereas in the present study we focussed on cGMP accumulation as a marker of intracellular NO delivery. This technical difference may in part explain our different findings. Consistent with Freedman et al. [14], we found that addition of GPx to washed platelets increased cGMP accumulation in response to GSNO (though not to DEANO or Angeli's salt), however, the amounts of GPx required were approx. 1000-fold higher than the levels we found in plasma. Given this, it appears unlikely that the effect has physiological relevance.
There is ample evidence that CSNO is transported across the plasma membrane in a stereoselective manner via the L-AT system. This has been demonstrated in a variety of cell types including PC12 neuronal cells [28], RAW 264.7 macrophages [9] and the carcinoma cell lines A431 and T24 [8]. We found, however, no inhibition of intraplatelet NOx delivery by GSNO, DEANO or Angeli's salt, in the presence of the well-characterized inhibitors BCH and L-leucine, at concentrations shown in previous studies to block CSNO uptake on the L-AT system [8]. GSNO is not a direct substrate for this transporter, and for L-AT to mediate NO delivery from GSNO, extracellular cysteine must be available (either directly or via reduction of cystine) so that CSNO can be produced via transnitrosation prior to transport [9,29]. Neither cysteine nor cystine was supplied in our experiments and this may explain why we found no evidence for the involvement of L-AT. The fact remains, however, that all three NO donors were capable of rapidly and effectively bringing about both cGMP accumulation and DAF-FM fluorescence in platelets, and so some other entry mechanism must exist.
The present study produced clear evidence that inhibitors of PDI prevent the intracellular delivery of NO-related signals (cGMP accumulation and DAF-FM fluorescence) from GSNO in a dose-dependent manner. These results are consistent with those of Zai et al. [30] and Ramachandran et al. [13] who also found that PDI inhibition produced inhibition of NO delivery from RSNOs (measured as cGMP accumulation and quenching of N-dansylhomocysteine fluorescence respectively). PDI has been shown to denitrosate GSNO [18] and it is postulated that the NO• released by this reaction combines with oxygen in the hydrophobic environment of either the cell membrane or the PDI protein itself to form N2O3 [31], which then passes on the NO signal via nitrosation of intracellular thiols, completing the transmembrane delivery process [13]. We were surprised, however, to also find evidence of PDI involvement in the intracellular delivery of NOx from both DEANO and Angeli's salt. We had anticipated that free NO• released by DEANO would be able to diffuse into platelets unaided by virtue of its lipophilicity. If the role of platelet membrane PDI in RSNO action is to denitrosate prior to transmembrane transfer of NOx, then it is not clear why such a mechanism should be required following platelet exposure to DEANO. The ingress of NO−-derived species from Angeli's salt is reported to involve reaction with oxygen to form a long-lived diffusible intermediate which penetrates the cell via partitioning into the cell membrane [32]. Our own results showed that the degree of inhibition of intracellular NOx entry caused by bacitracin, PAO and RL90 varied between different donor compounds (GSNO, DEANO and Angeli's salt) and detection systems (cGMP accumulation and DAF-FM fluorescence), and did not always correlate closely with the degree of inhibition of authentic PDI caused by these agents, suggesting a variable degree of PDI dependence for the delivery of different NO redox derivatives. Although the detailed chemistry is not yet clear, overall our data suggest that PDI facilitates the transfer across the platelet membrane of both NO•-derived and NO−-derived species, possibly through the intermediate formation of ‘NO-charged PDI’ [31].
Recent research has revealed a significant role for both PDI [33] and other thiol isomerases [34,35] in platelet responses. NO is an important physiological regulator of platelet function and our data further highlight the importance of PDI in facilitating the delivery of a wide range of NO-related signals.
Acknowledgments
The present study was supported by a project grant from the British Heart Foundation.
References
- 1.Liu L., Yan Y., Zeng M., Zhang J., Hanes M. A., Ahearn G., McMahon T. J., Dickfeld T., Marshall H. E., Que L. G., Stamler J. S. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 2.Richardson G., Benjamin N. Potential therapeutic uses for S-nitrosothiols. Clin. Sci. 2002;102:99–105. [PubMed] [Google Scholar]
- 3.de Belder A. J., MacAllister R., Radomski M. W., Moncada S., Vallance P. J. Effects of S-nitroso-glutathione in the human forearm. Cardiovasc. Res. 1994;28:691–694. doi: 10.1093/cvr/28.5.691. [DOI] [PubMed] [Google Scholar]
- 4.Mathews W. R., Kerr S. W. Biological activity of S-nitrosothiols: the role of nitric oxide. J. Pharmacol. Exp. Ther. 1993;267:1529–1537. [PubMed] [Google Scholar]
- 5.Gordge M. P., Hothersall J. S., Noronha-Dutra A. A. Evidence for a cyclic GMP-independent mechanism in the anti-platelet activity of S-nitrosoglutathione. Br. J. Pharmacol. 1998;124:141–148. doi: 10.1038/sj.bjp.0701821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogg N. The biochemistry and physiology of S-nitrosothiols. Annu. Rev. Pharmacol. Toxicol. 2002;42:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- 7.Lewis S. J., Hoque A., Bates J. N. Differentiation of L- and D-S-nitrosothiol recognition sites in vivo. J. Cardiovasc. Pharmacol. 2005;46:660–671. doi: 10.1097/01.fjc.0000181714.94827.5d. [DOI] [PubMed] [Google Scholar]
- 8.Li S., Whorton A. R. Identification of stereoselective transporters for S-nitroso-L-cysteine: role of LAT1 and LAT2 in biological activity of S-nitrosothiols. J. Biol. Chem. 2005;280:20102–20110. doi: 10.1074/jbc.M413164200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Hogg N. S-nitrosothiols: cellular formation and transport. Free Radical Biol. Med. 2005;38:831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Liu L., Hausladen A., Zeng M., Que L., Heitman J., Stamler J. S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 12.Mani A. R., Ebrahimkhani M. R., Ippolito S., Ollosson R., Moore K. P. Metalloprotein-dependent decomposition of S-nitrosothiols: studies on the stabilization and measurement of S-nitrosothiols in tissues. Free Radical Biol. Med. 2006;40:1654–1663. doi: 10.1016/j.freeradbiomed.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran N., Root P., Jiang X. M., Hogg P. J., Mutus B. Mechanism of transfer of NO from extracellular S-nitrosothiols into the cytosol by cell-surface protein disulfide-isomerase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9539–9544. doi: 10.1073/pnas.171180998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman J. E., Frei B., Welch G. N., Loscalzo J. Glutathione peroxidase potentiates the inhibtion of platelet function by S-nitrosothiols. J. Clin. Invest. 1995;96:394–400. doi: 10.1172/JCI118047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg N. Biological chemistry and clinical potential of S-nitrosothiols. Free Radical Biol. Med. 2000;28:1478–1486. doi: 10.1016/s0891-5849(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 16.Miranda K. M., Paolocci N., Katori T., Thomas D. D., Ford E., Bartberger M. D., Espey M. G., Kass D. A., Feelisch M., Fukuto J. M., Wink D. A. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dierks E. A., Burstyn J. N. Nitric oxide (NO), the only nitrogen monoxide redox form capable of activating soluble guanylyl cyclase. Biochem. Pharmacol. 1996;51:1593–1600. doi: 10.1016/0006-2952(96)00078-0. [DOI] [PubMed] [Google Scholar]
- 18.Root P., Sliskovic I., Mutus B. Platelet surface protein disulfide-isomerase mediated S-nitrosoglutathione consumption. Biochem. J. 2004;382:575–580. doi: 10.1042/BJ20040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko F. N., Wu C. C., Kuo S. C., Lee F. Y., Teng C. M. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- 20.Radomski M. W., Rees D. D., Dutra A., Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br. J. Pharmacol. 1992;107:745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordge M. P., Meyer D. J., Hothersall J., Neild G. H., Payne N. N., Noronha-Dutra A. Copper chelation-induced reduction of the biological activity of S-nitrosothiols. Br. J. Pharmacol. 1995;114:1083–1089. doi: 10.1111/j.1476-5381.1995.tb13317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermejo E., Saenz D. A., Alberto F., Rosenstein R. E., Bari S. E., Lazzari M. A. Effect of nitroxyl on human platelet function. Thromb. Haemostasis. 2005;94:578–584. doi: 10.1160/TH05-01-0062. [DOI] [PubMed] [Google Scholar]
- 23.Vanuffelen B. E., Van Der Zee J., De Koster B. M., Vansteveninck J., Elferink J. G. Intracellular but not extracellular conversion of nitroxyl anion into nitric oxide leads to stimulation of human neutrophil migration. Biochem. J. 1998;330:719–722. doi: 10.1042/bj3300719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balcerczyk A., Soszynski M., Bartosz G. On the specificity of 4-amino-5-methylamino-2′, 7′ -difluorofluorescein as a probe for nitric oxide. Free Radical Biol. Med. 2005;39:327–335. doi: 10.1016/j.freeradbiomed.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Lacza Z., Horvath E. M., Pankotai E., Csordas A., Kollai M., Szabo C., Busija D. W. The novel red-fluorescent probe DAR-4M measures reactive nitrogen species rather than NO. J. Pharmacol. Toxicol. Methods. 2005;52:335–340. doi: 10.1016/j.vascn.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Freedman J. E., Loscalzo J., Benoit S. E., Valeri C. R., Barnard M. R., Michelson A. D. Decreased platelet inhibition by nitric oxide in two brothers with a history of arterial thrombosis. J. Clin. Invest. 1996;97:979–987. doi: 10.1172/JCI118522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenet G., Freedman J., Shenkman B., Regina E., Brok-Simoni F., Holzman F., Vavva F., Brand N., Michelson A., Trolliet M., Loscalzo J., Inbal A. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arterioscler., Thromb., Vasc. Biol. 1999;19:2017–2023. doi: 10.1161/01.atv.19.8.2017. [DOI] [PubMed] [Google Scholar]
- 28.Nemoto T., Shimma N., Horie S., Saito T., Okuma Y., Nomura Y., Murayama T. Involvement of the system L-amino acid transporter on uptake of S-nitroso-L-cysteine, an endogenous S-nitrosothiol, in PC12 cells. Eur. J. Pharmacol. 2003;458:17–24. doi: 10.1016/s0014-2999(02)02699-7. [DOI] [PubMed] [Google Scholar]
- 29.Zeng H., Spencer N. Y., Hogg N. Metabolism of S-nitrosoglutathione by endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H432–H439. doi: 10.1152/ajpheart.2001.281.1.H432. [DOI] [PubMed] [Google Scholar]
- 30.Zai A., Rudd M. A., Scribner A. W., Loscalzo J. Cell-surface protein disulfide isomerase catalyses transnitrosation and regulates intracellular transfer of nitric oxide. J. Clin. Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sliskovic I., Raturi A., Mutus B. Characterization of the S-denitrosation activity of protein disulfide isomerase. J. Biol. Chem. 2005;280:8733–8741. doi: 10.1074/jbc.M408080200. [DOI] [PubMed] [Google Scholar]
- 32.Espey M. G., Miranda K. M., Thomas D. D., Wink D. A. Ingress and reactive chemistry of nitroxyl-derived species within human cells. Free Radical Biol. Med. 2002;33:827–834. doi: 10.1016/s0891-5849(02)00978-4. [DOI] [PubMed] [Google Scholar]
- 33.Essex D. W., Li M., Miller A., Feinman R. D. Protein disulfide isomerase and sulphydryl-dependent pathways in platelet activation. Biochemistry. 2001;40:6070–6075. doi: 10.1021/bi002454e. [DOI] [PubMed] [Google Scholar]
- 34.Jordan P. A., Stevens J. M., Hubbard G. P., Barrett N. E., Sage T., Authi K. S., Gibbins J. M. A role for the thiol isomerase protein ERP5 in platelet function. Blood. 2005;105:1500–1507. doi: 10.1182/blood-2004-02-0608. [DOI] [PubMed] [Google Scholar]
- 35.Robinson A., O'Neill S., Kiernan A., O'Donoghue N., Moran N. Bacitracin reveals a role for multiple thiol isomerases in platelet function. Br. J. Haematol. 2006;132:339–348. doi: 10.1111/j.1365-2141.2005.05878.x. [DOI] [PubMed] [Google Scholar]