Abstract
We have investigated the ability of apoE (apolipoprotein E) to participate in the biogenesis of HDL (high-density lipoprotein) particles in vivo using adenovirus-mediated gene transfer in apoA-I−/− (apolipoprotein A-I) or ABCA1−/− (ATP-binding cassette A1) mice. Infection of apoA-I−/− mice with 2×109 pfu (plaque-forming units) of an apoE4-expressing adenovirus increased both HDL and the triacylglycerol-rich VLDL (very-low-density lipoprotein)/IDL (intermediate-density lipoprotein)/LDL (low-density lipoprotein) fraction and generated discoidal HDL particles. ABCA1−/− mice treated similarly failed to form HDL particles, suggesting that ABCA1 is essential for the generation of apoE-containing HDL. Combined infection of apoA-I−/− mice with a mixture of adenoviruses expressing both apoE4 (2×109 pfu) and human LCAT (lecithin:cholesterol acyltransferase) (5×108 pfu) cleared the triacylglycerol-rich lipoproteins, increased HDL and converted the discoidal HDL into spherical HDL. Similarly, co-infection of apoE−/− mice with apoE4 and human LCAT corrected the hypercholesterolaemia and generated spherical particles, suggesting that LCAT is essential for the maturation of apoE-containing HDL. Overall, the findings indicate that apoE has a dual functionality. In addition to its documented functions in the clearance of triacylglycerol-rich lipoproteins, it participates in the biogenesis of HDL-sized apoE-containing particles. HDL particles generated by this pathway may account at least for some of the atheroprotective functions of apoE.
Keywords: adenovirus-mediated gene transfer, apolipoprotein E (apoE), ATP-binding cassette transporter A1 (ABCA1), high-density lipoprotein (HDL), lecithin:cholesterol acyltransferase (LCAT)
Abbreviations: ABCA1, ATP-binding cassette A1; apoA-I, apolipoprotein A-I; apoE, apolipoprotein E; CMV, cytomegalovirus; GFP, green fluorescent protein; HDL, high-density lipoprotein; IDL, intermediate-density lipoprotein; LCAT, lecithin:cholesterol acyltransferase; LDL, low-density lipoprotein; pfu, plaque-forming units; POPC, 1-palmitoyl-2-oleoyl phosphatidylcholine; SR-BI, scavenger receptor B1; VLDL, very-low-density lipoprotein; WT, wild-type
INTRODUCTION
ApoE is an important protein of the lipoprotein transport system and plays a significant role in the protection from or the pathogenesis of atherosclerosis, dyslipidaemia and Alzheimer's disease [1–4].
The atheroprotective properties of apoE (apolipoprotein E) have been demonstrated in numerous studies, including bone marrow transplantation or retroviral gene transfer [5,6], and low levels of ectopic expression of apoE in the adrenals [7]. In mice bioengineered to express low levels of a mutant mouse apoE, an atherogenic diet promoted atherosclerosis and a switch to a normal diet and restoration of normal levels of apoE caused regression of atherosclerosis [8]. Inhibition of progression of early lesions and regression of advanced lesions were also observed by adenovirus-mediated apoE gene transfer in LDL (low-density lipoprotein) receptor-deficient mice, suggesting that other functions of apoE independent of LDL receptor recognition may also contribute to atheroprotection [9]. A well-documented function of apoE is its participation in the clearance of lipoprotein remnants of intestinal or hepatic origin by the liver [4]. ApoE also has been shown to promote cholesterol efflux in vitro [10], and thus may contribute to cell and tissue cholesterol homoeostasis and protection from atherosclerosis through different mechanisms [10].
ApoE at physiological concentrations maintains lipid homoeostasis and is atheroprotective [5–8,11–13]. However, increased expression of apoE increases the rate of hepatic VLDL (very-low-density lipoprotein) triacylglycerol secretion [14] and is associated with hypertriglyceridaemia [13,14]. In humans and experimental animals, plasma apoE levels are correlated with plasma triacylglycerol levels [13–15].
To gain insight into the opposing functions of apoE that lead either to lipoprotein clearance or to dyslipidaemia, we used adenovirus-mediated gene transfer in apoE−/− mice to correct the apoE deficiency [14,16,17]. These studies showed that apoE forms lacking the C-terminal domain can clear cholesterol efficiently from the plasma of apoE-deficient mice, whereas full-length apoE increased VLDL secretion [14,16], induced hypertriglyceridaemia and also affected the structure of HDL (high-density lipoprotein) [17]. Site-directed mutagenesis of apoE followed by in vivo gene transfer localized the residues responsible for hypertriglyceridaemia in the 261–269 region of apoE [17].
ApoE has striking structural [18] and functional [19,20] similarities with apoA-I (apolipoprotein A-I), a protein that is required for the biogenesis of HDL [21]. On the basis of these similarities, our hypothesis was that apoE, in addition to its known function in remnant clearance [4], may also participate in the biogenesis of HDL-like apoE-containing lipoprotein particles following a pathway similar to that of apoA-I [21]. To address this question, we used adenovirus-mediated apoE gene transfer in apoA-I−/− mice [22] or ABCA1−/− (ATP-binding cassette transporter A1) mice [23], which do not synthesize HDL. It was found that infection of apoA-I−/− with adenovirus expressing WT (wild-type) apoE4 promoted the formation of a large number of discoidal HDL particles and induced dyslipidaemia, whereas ABCA1−/− mice treated similarly failed to form HDL. Interestingly, infection of apoA-I−/− mice or apoE−/− mice with a combination of adenoviruses expressing apoE4 and human LCAT (lecithin:cholesterol acyltransferase) corrected the dyslipidaemia and converted the discoidal HDL into spherical particles.
It is important to note that infection of apoA-I−/− mice with adenovirus expressing a recently described apoE4 (L261A/W264A/F265A/L268A/V269A) mutant designated apoE4mut1 [17] did not induce dyslipidaemia and promoted the formation of spherical apoE-containing HDL particles, suggesting that this mutant has improved functions in lipoprotein clearance as well as in the biogenesis of apoE-containing HDL.
EXPERIMENTAL
Animal studies
The construction of the recombinant adenovirus expressing the WT human apoE4 form has been described previously [14].
Mice were purchased from Jackson Laboratories. ABCA1−/− mice were generated by crossing ABCA1+/− mice [23]. Female apoE−/− [24], apoA-I−/− [22] and ABCA1−/− [23] mice 4–6 weeks old were used in these studies. Groups were formed after determining the fasting cholesterol and triacylglycerol levels of the individual mice, to ensure similar average cholesterol and triacylglycerol levels among groups.
For the adenovirus infections, groups of at least six to eight mice were injected intravenously through the tail vein with doses of 2×109 pfu (plaque-forming units) of apoE4-expressing adenovirus in the presence of absence of 5×108 pfu of an adenovirus expressing human LCAT. Blood was obtained daily following a 4 h fasting period 4 days post-injection. Aliquots (20 μl) of plasma were stored at 4 and −20 °C.
RNA analysis
To assess the expression of apoE4 or apoE4mut1, at least six mice from each group were killed 4 days post-infection. Livers were collected from individual animals, frozen in liquid nitrogen and stored at −80 °C. Total RNA was isolated from the livers and analysed for apoE mRNA and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression by Northern blotting and quantified by phosphorimaging [14].
FPLC analysis and lipid determination
For FPLC analysis of serum samples, 12 μl of serum was diluted 1:5 with PBS, and loaded on to a Superose 6 column in a SMART micro-FPLC system (Amersham Biosciences), and eluted with PBS. A total of 25 fractions of 50 μl each were collected for further analysis. Triacylglycerols, total cholesterol and free (non-esterified) cholesterol were determined using the GPO-Trinder Kit (Sigma) and Infinity Cholesterol kit (Thermo Electron Corporation) and Free Cholesterol C Kit (Wako), according to the manufacturers' instructions. The triacylglycerol and cholesterol concentrations of the serum and the FPLC fractions were determined spectrophotometrically at 540 and 492 nm respectively, as described previously [14].
Density-gradient ultracentrifugation
To assess the ability of WT and mutant apoE forms to associate with different lipoproteins, 0.3 ml of serum from mice infected either with the control recombinant attenuated adenovirus expressing GFP (green fluorescent protein) under the control of the CMV (cytomegalovirus) promoter or adenoviruses expressing WT apoE4, apoE4mut1 or human LCAT were fractionated by density-gradient ultracentrifugation at 30000 rev./min for 22 h using a Beckman swinging-bucket SW41 rotor and analysed by SDS/PAGE (13% gels) [17].
Electron microscopy
Aliquots of the fractions from equilibrium density-gradient centrifugation after dialysis against ammonium acetate and carbonate buffer, were stained with sodium phosphotungstate, visualized using a Philips CM-120 electron microscope, and photographed as described previously [21]. The photomicrographs were taken at ×75000 magnification and enlarged three times. Size measurements of particles (n>200 from each population) were taken directly from image negatives (×75000) with a 7× measuring magnifier with metric scale of 0.1 mm divisions. Measured values were classified into 1.3 nm intervals and the diameter of each value within an interval was represented by the midpoint of that interval. Descriptive statistics of a measured population of at least 200 particles were prepared using the Analysis Tool Pack in Microsoft Excel 2002.
LCAT activation
POPC (1-palmitoyl-2-oleoyl phosphatidylcholine)/cholesterol/apoE and POPC/cholesterol/apoA-I particles containing [14C]cholesterol were generated by the sodium cholate dialysis method at a POPC/cholesterol/apoE or POPC/cholesterol/apoA-I proportion of 100:10:1. The rHDL (reconstituted HDL) particles thus produced were used as substrate in the activation of LCAT and the calculation of the apparent Vmax and Km values. Experiments were performed as described in [25]. The cholesterol esterification rate was expressed as nanomol of cholesteryl ester formed per h. To calculate the apparent Vmax and Km values, the rate of cholesteryl ester formation was plotted against the concentration of apoA-I or apoE4. The data were fitted to Michaelis–Menten kinetics using Prism (GraphPad Software). Owing to the low catalytic activity of apoE4, the kinetic parameters for apoE4 were obtained with 10-fold higher enzyme concentrations than those used to obtain the kinetic parameters of apoA-I and the Vmax (app) value for apoE4 was normalized by dividing the experimentally obtained Vmax (app) by 10.
Statistical analysis
Comparison of data from two groups of mice was carried out using Student's t test.
RESULTS
Plasma lipid, FPLC profiles and hepatic apoE mRNA levels of apoA-I−/− mice following adenovirus infections
In the present study, we selected to measure lipid, lipoprotein and apoE mRNA levels at 4 days post-infection based on a recent study which showed that plasma apoE and triacylglycerol levels reach a plateau between days 2 and 5 and decline rapidly between days 6 and 8 [13]. Treatment of apoA-I−/− mice with a control adenovirus expressing GFP did not change their plasma cholesterol and triacylglycerol levels (Table 1 and Figure 1A). Potential liver damage following adenovirus infection was assessed by measuring serum transaminases using the Reflotron Plus system (Roche). These analyses showed normal serum transaminase levels when mice were infected with 2×109 pfu of recombinant adenoviruses. Analysis of plasma lipids and apoE levels, and hepatic apoE mRNA levels at 4 days post-infection showed that, compared with the non-infected controls, apoA-I−/− mice infected with 2×109 pfu of an adenovirus expressing the WT apoE4 had increased total and free cholesterol and triacylglycerol levels (Table 1 and Figure 1B). The great majority of the cholesterol in mice expressing apoE4 was in the form of free cholesterol that was mainly distributed in the VLDL/IDL (intermediate-density lipoprotein) and HDL region (Table 1 and Figure 1B).
Table 1. Comparison of plasma lipid and apoE levels, and hepatic apoE mRNA levels of apoE−/− mice infected with recombinant adenoviruses expressing apoE4, apoE4mut1 and/or LCAT.
AdGFP, recombinant attenuated adenovirus expressing GFP under the control of the CMV promoter; AdGFP-E4, recombinant attenuated adenovirus expressing WT apoE4 and GFP under the independent control of two CMV promoters; AdGFP-E4mut1, recombinant attenuated adenovirus expressing apoE4-mut1 and GFP under the independent control of two CMV promoters; Ad-LCAT, recombinant attenuated adenovirus expressing LCAT under the independent control of the CMV promoter.
| Mice | Number | apoE (mg/dl) | Total cholesterol (mg/dl) | Free cholesterol (mg/dl) | Triacylglycerol (mg/dl) | Relative apoE mRNA (%) |
|---|---|---|---|---|---|---|
| ApoA-I−/− | ||||||
| Untreated | 10 | − | 26±7 | 18±3 | 41±9 | − |
| +AdGFP | 8 | − | 20±3 | 13±1 | 30±6 | − |
| +AdGFP-E4 | 6 | 154±4 | 561±6 | 518±5 | 1368±102 | 100 |
| +AdGFP-E4+Ad-LCAT | 6 | 89±2 | 347±8 | 94±11 | 77±11 | 218 |
| +AdGFP-E4mut1 | 8 | 100±9 | 61±6 | 30±4 | 83±4 | 120 |
| ABCA1−/− | ||||||
| +AdGFP | 6 | − | 33±10 | 14±5 | 23±5 | − |
| +AdGFP-E4 | 6 | 80±6 | 197±17 | 122±8 | 1227±45 | 137 |
| +Ad-LCAT | 6 | − | 14±2 | 11±5 | 27±3 | − |
| ApoE−/− | ||||||
| Untreated | 10 | − | 942±243 | 252±68 | 86±25 | − |
| +AdGFP | 10 | − | 877±152 | 213±76 | 68±30 | − |
| +AdGFP-E4 | 10 | 115±6 | 882±323 | 770±261 | 2061±851 | 152 |
| +AdLCAT | 6 | − | 1079±35 | 177±42 | 24±4 | − |
| +AdGFP-E4+Ad-LCAT | 6 | − | 140±53 | 81±23 | 217±82 | 295 |
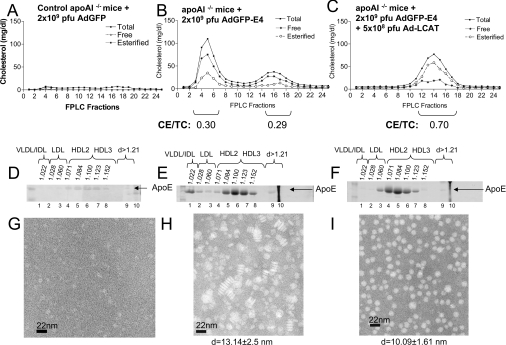
Figure 1. Cholesterol FPLC profiles, apoE distribution and electron microscopy analyses of HDL fractions of control apoA-I−/− mice and apoA-I−/− mice infected with recombinant adenoviruses expressing GFP or apoE4 alone or a combination of apoE4 and human LCAT.
(A–C) FPLC profiles. (D–F) ApoE distribution among different densities (d). (G–I) Electron micrographs of fractions 6–8 of HDL. Particle diameters (d) are given. (A, D, G) Analyses of samples obtained from apoA-I−/− mice infected with 2×109 pfu of control adenoviruses expressing GFP (AdGFP). (B, E, H) Analyses of samples obtained from apoA-I−/− mice infected with 2×109 pfu of adenoviruses expressing apoE4 (AdGFP-E4). (C, F, I) Analyses of samples obtained from apoA-I−/− mice infected with a mixture of adenoviruses expressing apoE4 (AdGFP-E4) (2×109 pfu) and human LCAT (Ad-LCAT) (5×108 pfu). CE/TC, cholesteryl ester/total cholesterol ratio.
Treatment of apoA-I−/− mice with adenoviruses expressing apoE4 (2×109 pfu) and human LCAT (5×108 pfu) eliminated the VLDL/IDL fraction and increased the HDL fraction (Figure 1C). Overall, this treatment decreased the total and free plasma cholesterol and increased the total HDL cholesterol and cholesteryl esters and shifted the HDL fraction slightly towards the LDL density region (Table 1 and Figure 1C).
Effect of apoE4 alone or the combination of apoE4 and LCAT on the distribution of apoE in different lipoproteins and the size and the shape of HDL
Analysis of the distribution of apoE by SDS/PAGE following density-gradient ultracentrifugation of plasma showed that in apoA-I−/− mice infected with the control adenovirus expressing GFP (Figure 1D), as well as in mice infected with apoE4-expressing adenoviruses, apoE was predominantly distributed in the HDL3 and to a lesser extent the HDL2 region. In mice expressing apoE4, a small amount of apoE was also found in the LDL, IDL and VLDL regions (Figure 1E). Electron microscopy analysis of the peak HDL fractions 6–8 showed that HDL consisted of discoidal particles with diameter 13.25±2.5 nm and thickness 5.69±0.21 nm (Figure 1H), whereas the HDL fraction of the control apoA-I−/− mice infected with the control adenovirus contained very few particles (Figure 1G). It has been shown previously that the HDL fraction of apoA-I−/− mice is enriched in apoE [16]. Treatment of the mice with a mixture of adenoviruses expressing apoE4 and human LCAT shifted the distribution of apoE towards the HDL2 region (Figure 1F) and resulted in the formation of spherical HDL particles with diameter 10.09±1.61 nm (Figure 1I).
In vitro LCAT activation experiments using POPC/cholesterol/apoE4 or POPC/cholesterol/apoA-I proteoliposomes showed that the catalytic efficiency of POPC/cholesterol/apoE particles is 14.8% of that of POPC/cholesterol/apoA-I particles [Vmax (app)/Km (app)=5.04 nmol of cholesteryl ester/h per μM for apoA-I proteoliposomes compared with 0.74 nmol of cholesteryl ester/h per μM for apoE proteoliposomes].
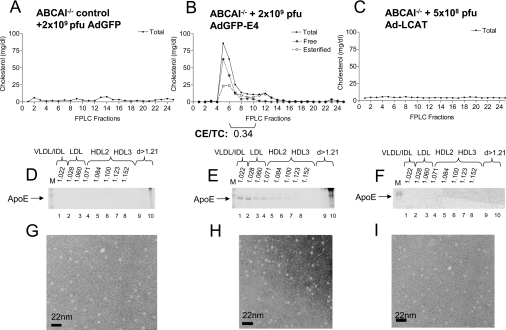
Lipid, lipoprotein, apoE and HDL profiles of ABCA1−/− mice infected with the apoE4-expressing adenovirus
To test the involvement of ABCA1 in the biogenesis of apoE-containing HDL, we treated ABCA1−/− mice with 2×109 pfu of adenovirus expressing apoE4. This treatment caused dyslipidaemia characterized by high plasma triacylglycerols and moderately elevated plasma cholesterol (Table 1). Lipid analysis and FPLC fractionation of plasma showed that control ABCA1−/− mice infected with adenoviruses expressing either GFP or LCAT had very low cholesterol levels (Table 1, and Figures 2A and 2C). FPLC fractionation of plasma showed that, in the ABCA1−/− mice expressing apoE4, the lipids were distributed in the VLDL/IDL/LDL that was enriched in free cholesterol and there was a virtual absence of an HDL peak (Figure 2B). The triacylglycerol distribution was similar to that of cholesterol (results not shown). Analysis of the distribution of apoE by SDS/PAGE following density-gradient ultracentrifugation of plasma showed that, in the ABCA1−/− mice infected with adenoviruses expressing GFP or LCAT, the endogenous apoE was undetectable in the HDL region (Figures 2D and 2F). In contrast, the ABCA1−/− mice infected with the apoE4-expressing adenovirus had increased levels of human apoE4 that was distributed in the VLDL/IDL/LDL region (Figure 2E). The accumulation of apoE in this density region was associated with severe hypertriglyceridaemia (Table 1). Electron microscopy analysis of HDL density fractions 6–8 showed the presence of very few particles both in the control and in the ABCA1−/− mice that were infected with the GFP-, LCAT- or the apoE4-expressing adenovirus (Figures 2G–2I). The electron microscopy patterns of the HDL fractions of these mice was similar to that obtained in apoA-I−/− mice infected with the control adenovirus expressing GFP (compare Figure 1G with Figures 2G–2I). A previous study showed that expression of apoA-I in apoA-I−/− mice promoted the formation of spherical HDL [25,26]. Furthermore, expression of LCAT in apoA-I−/− mice increases the apoE levels in the HDL2 region, suggesting formation of apoE-containing HDL particles [25]. These findings establish unequivocally that the discoidal particles observed after infection of apoA-I−/− mice with adenoviruses expressing apoE4 require the functions of ABCA1. As shown in Figure 2(H), mice that lack ABCA1 fail to form HDL, even when apoE is overexpressed following adenovirus-mediated gene transfer.
Figure 2. Cholesterol FPLC profiles, apoE distribution and electron microscopy analyses of HDL fractions of control ABCA1−/− mice and ABCA1−/− mice infected with recombinant adenoviruses expressing apoE4 or LCAT.
(A–C) Cholesterol FPLC profiles. (D–F) ApoE distribution among different densities (d). Lanes M, molecular-mass markers. (G–I) Electron micrographs of fractions 6–8 of HDL. (A, D, G) Analyses of samples obtained from ABCA1−/− mice infected with 2×109 pfu of the control adenovirus expressing GFP (AdGFP). (B, E, H) Analyses of samples obtained from ABCA1−/− infected with 2×109 pfu of adenoviruses expressing apoE4 (AdGFP-E4). (C, F, I) Analyses of samples obtained from ABCA1−/− mice infected with 5×108 pfu of adenoviruses expressing LCAT (Ad-LCAT). CE/TC, cholesteryl ester/total cholesterol ratio.
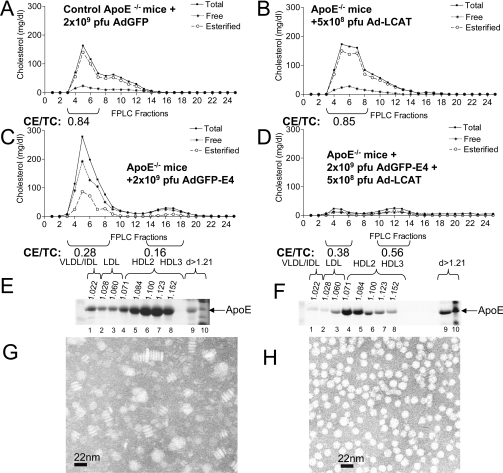
Effects of apoE, LCAT or a combination of apoE and LCAT on plasma cholesterol, apoE distribution and HDL formation in apoE−/− mice
To test further the role of LCAT in the biogenesis of HDL, we infected apoE−/− mice with adenoviruses expressing LCAT (5×108 pfu) or a combination of apoE4 (2×109 pfu) and human LCAT (5×108 pfu). Treatment of apoE−/− mice with the adenovirus expressing LCAT alone did not affect significantly the total cholesterol, free cholesterol or triacylglycerol levels in the infected mice (Table 1). The FPLC profiles of the LCAT-treated mice were similar to those of the apoE−/− mice (Figures 3A and 3B). In both cases, the cholesterol peak of the VLDL/LDL/IDL region was heavily enriched in cholesteryl esters and contained low levels of non-esterified cholesterol (Figures 3A and 3B). Treatment of apoE−/− with the adenovirus expressing apoE4 increased the level of total and free cholesterol and induced high plasma triacylglycerols (Table 1). FPLC fractionation of plasma showed that most of the cholesterol in mice treated with adenoviruses expressing apoE4 was distributed in the VLDL/IDL/LDL fraction and small amounts were distributed in the HDL fraction (Figure 3C). The triacylglycerol distribution was similar to that of total cholesterol (results not shown). Both the VLDL/IDL/LDL fractions and the HDL fraction of mice treated with the adenovirus expressing apoE4 were heavily enriched in free cholesterol and had a decreased cholesteryl ester/total cholesterol ratio (Figure 3C). This is in contrast with the VLDL/IDL/LDL peak of the untreated apoE−/− mice or apoE−/− mice treated with LCAT (compare Figures 3A and 3B with Figure 3C). Treatment of apoE−/− mice with a mixture of adenoviruses expressing apoE4 and LCAT corrected hypertriglyceridaemia and hypercholesterolaemia induced in apoE−/− by overexpression of apoE4. This treatment eliminated the cholesterol peak of VLDL and shifted the HDL towards lower density fractions and increased the cholesteryl ester/total cholesterol ratio of HDL (Table 1 and Figure 3D).
Figure 3. Cholesterol FPLC profiles, apoE distribution and electron microscopy analyses of HDL of apoE−/− mice and apoE−/− mice infected with recombinant adenoviruses expressing human LCAT or apoE4 or a combination of apoE4 and human LCAT.
(A–D) Cholesterol FPLC profiles of (A) apoE−/− mice treated with 2×109 pfu of control adenovirus expressing GFP (AdGFP), (B) apoE−/− mice treated with 5×108 pfu of adenoviruses expressing LCAT (Ad-LCAT), (C) apoE−/− mice treated with 2×109 pfu of adenoviruses expressing apoE4 (AdGFP-E4), and (D) apoE−/− mice treated with a mixture of 2×109 pfu of adenoviruses expressing apoE4 (AdGFP-E4) and 5×108 pfu of adenoviruses expressing human LCAT (Ad-LCAT). (E and F) ApoE distribution among different densities (d) of human apoE in apoE−/− mice infected with the adenoviruses expressing apoE4 alone (E), or a combination of apoE4 and human LCAT (F). (G and H) Electron micrographs of HDL obtained from mice infected with adenoviruses expressing apoE4 alone (G), or apoE4 and human LCAT (H). CE/TC, cholesteryl ester/total cholesterol ratio.
The LCAT treatment also changed the distribution of apoE and the shape of the HDL particles. In mice infected with the apoE4-expressing adenovirus, the majority of apoE was distributed in the HDL2 and HDL3 regions and to a lesser extent in the VLDL/IDL/LDL region (Figure 3E), and contained discoidal particles (Figure 3G). Treatment of mice with a combination of adenoviruses expressing apoE4 and human LCAT shifted the majority of apoE towards the HDL2 region (Figure 3F) and converted the discoidal HDL into spherical particles (Figures 3H).
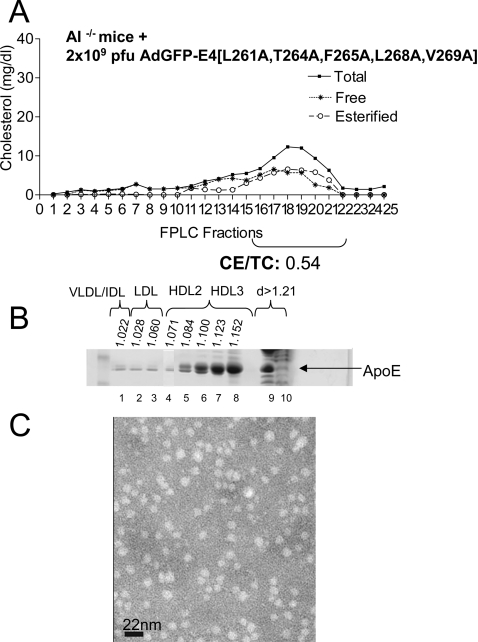
Substitution of alanine for hydrophobic residues in the region 261–269 enhances formation of spherical HDL and does not induce dyslipidaemia in apoA-I−/− mice
In another set of experiments, apoA-I−/− mice were infected with 2×109 pfu of an adenovirus expressing the apoE4 mutant (L261A/W264A/F265A/L268A/V269A) mutant designated apoE4mut1 [17]. This mutant was shown recently to clear cholesterol efficiently from the plasma of apoE−/− mice without induction of hypertriglyceridaemia [17]. This treatment increased plasma cholesterol of apoA-I−/− from 14 mg/dl before infection to 61 mg/dl following infection and did not induce hypertriglyceridaemia (Table 1). FPLC analysis showed that the great majority of cholesterol was in the HDL fraction. The cholesteryl ester/total cholesterol ratio of mice treated with apoE4mut1 was 0.54 (Figure 4A), as compared with mice treated with apoE4 alone, which had a cholesteryl ester/total cholesterol ratio of 0.29 (Figure 1B). Density-gradient ultracentrifugation of plasma and electron microscopy analysis of the HDL fraction showed that the great majority of cholesterol and apoE was in the HDL3 and, to a lesser extent, the HDL2 fractions (Figure 4B), and the HDL peak contained spherical particles (Figure 4C).
Figure 4. Cholesterol FPLC profile, apoE distribution and electron microscopy analysis of HDL fraction of apoE−/− mice infected with recombinant adenoviruses expressing apoE4mut1.
(A) FPLC profiles of total, free and esterified cholesterol of apoE−/− mice infected with 2×109 pfu of adenoviruses expressing apoE4mut1 (AdGFRP-E4[L261A,T264A,F265A,L268A,V269A]). (B) ApoE distribution among different densities (d). (C) Electron micrographs of fractions 6–8 of HDL obtained from mice infected with adenoviruses expressing apoE4mut1. CE/TC, cholesteryl ester/total cholesterol ratio.
Northern blot analysis for the experiments described in Figures 1–4 showed the hepatic mRNA of apoA-I−/−, apoE−/− or ABCA1−/− mice treated with adenoviruses expressing apoE4 were comparable, and that the combination of apoE4 and LCAT normalized the lipids and lipoprotein levels despite the increase in hepatic apoE mRNA levels (Table 1).
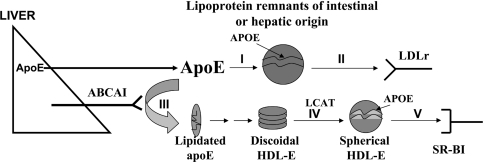
Figure 5 is a schematic representation depicting the role of apoE in the biogenesis of apoE-containing HDL and in remnant clearance. The HDL pathway is supported by the data shown in Figures 1–3, which showed that biogenesis of apoE-containing HDL particles requires the functions of ABCA1. Furthermore, the discoidal apoE-containing HDL particles that are intermediates in the biogenesis of spherical particles can accumulate under conditions of apoE overexpression, but can be converted into spherical particles in the presence of excess LCAT. Spherical HDL thus formed can interact with the HDL receptor SR-BI (scavenger receptor B1) (Figure 5; branches III–V of the pathway) [20]. Earlier reports in the literature have also established unequivocally that apoE is required for the receptor-mediated clearance of apoE-containing lipoprotein remnants [3,4,13], as depicted in Figure 5 (branches I and II of the pathway).
Figure 5. Participation of apoE in the biogenesis of apoE-containing HDL and the clearance of triacylglycerol-rich lipoproteins.
Schematic representation of the participation of apoE in the biogenesis of apoE-containing HDL (branches III–V) and the clearance of triacylglycerol-rich lipoproteins (branches I and II). LDLr, LDL receptor. The Figure is based on the data of Figures 1–3 and [3,4].
DISCUSSION
ApoE promotes the formation of discoidal HDL particles in apoA-I−/− mice which are converted into spherical particles by the action of LCAT
In the present study, the ability of apoE to drive the formation of apoE-containing HDL particles was initially tested by infection of apoA-I−/− mice, which have a normal ABCA1 gene, with an adenovirus expressing apoE4. This treatment caused combined dyslipidaemia characterized by high cholesterol and triacylglycerol levels and generated discoidal HDL particles. Experiments with apoE3- or apoE2-expressing adenoviruses gave similar results ([16,27], and results not shown). The discoidal particles formed were converted into spherical particles by co-infection of the apoA-I−/− mice with a mixture of adenoviruses expressing apoE4 and human LCAT. These experiments indicated that, under conditions of apoE overexpression, the endogenous activity of LCAT becomes rate-limiting and additional LCAT is required for the esterification of the cholesterol present in the discoidal particles and their conversion into spherical particles [19]. ApoE-containing discoidal HDL particles have been detected in the plasma of LCAT-deficient patients [28,29] and in liver perfusates that contained an LCAT inhibitor [28,30,31]. Previous cell culture studies also showed that ABCA1 interacts with apoE and promotes its lipidation [32–35]. However, the pathway of biogenesis of apoE-containing HDL in vivo and its impact on lipid and lipoprotein homoeostasis has not been documented previously.
A combination of apoE and LCAT corrects apoE-induced dyslipidaemia in apoE−/− mice
The question that arises is how the synergy between apoE and LCAT drives the concomitant clearance of the VLDL/IDL/IDL fraction and the conversion of the discoidal HDL into spherical HDL. To address this question, we performed a co-infection experiment in apoE−/− mice, which maintain the expression of mouse ABCA1 and apoA-I, with adenoviruses expressing apoE4 and human LCAT alone or in combination. Treatment of apoE−/− with LCAT did not correct their plasma lipid and lipoprotein profiles. In both the untreated and LCAT-treated mice, the VLDL/IDL/LDL fraction was highly enriched in cholesteryl esters. It has been shown previously that overexpression of apoE4 in apoE−/− mice following adenovirus infection increased the secretion of VLDL [14,16]. In the present study we demonstrate that apoE overexpression in apoE−/−, apoA-I−/− or ABCA1−/− mice also alters the composition of the newly secreted VLDL/IDL/LDL fraction by increasing dramatically the content of free cholesterol and triacylglycerol (Figures 1B, 2B and 3C). This change in VLDL/IDL/LDL composition was also associated with formation of discoidal HDL particles enriched in free cholesterol.
The apoE-induced dyslipidaemia that is characterized by accumulation of free cholesterol in VLDL/IDL/LDL and HDL, hypertriglyceridaemia and the formation of discoidal HDL was corrected by simultaneous treatment of mice with apoE4 and LCAT. This treatment promoted esterification of the HDL cholesterol as was evident by the increase in the cholesteryl ester/total cholesterol ratio of the HDL peak. The treatment also led to the formation of spherical HDL and the clearance of the VLDL cholesterol and triacylglycerol peak. It has been shown recently that apoE facilitates esterification of cholesterol of apoB-containing lipoproteins by activating LCAT [36]. The change in the composition of VLDL following cholesterol esterification appears to facilitate triacylglycerol hydrolysis and/or to promote LDL receptor-mediated remnant clearance, possibly by unmasking the receptor-binding domain of apoE [13,16]. These events can account for the correction of the hyperlipidaemia.
ABCA1 is required for the biogenesis of apoE-containing HDL
The requirement of ABCA1 for the generation of apoE-containing HDL was tested further by infection of ABCA1−/− mice with the adenovirus expressing apoE4. ABCA1−/− mice thus treated failed to form either discoidal or spherical particles, indicating that ABCA1 is required for the biogenesis of apoE-containing HDL in vivo.
A variant apoE form promotes exclusively the formation of spherical apoE-containing HDL in apoA-I−/− mice
In contrast with apoE4 that promoted dyslipidaemia and the formation of discoidal HDL, overexpression of apoE4mut1 [17] in apoA-I−/− mice resulted in normal lipid levels and formation of spherical HDL particles. It appears that the substitution of alanine for Leu261, Trp264, Phe265, Leu268 and Val269 in the apoE4mut1 altered the biological functions of apoE. The variant protein promoted efficient cholesterol esterification of the precursor discoidal HDL by the endogenous mouse LCAT and their conversion into spherical HDL as well as the concomitant clearance of the VLDL/IDL/LDL fraction.
Dual role of apoE in lipoprotein clearance and the generation of apoE-containing HDL particles
The findings shown in Figures 1–3 document the existence of a pathway of biogenesis of apoE-containing HDL and expanded the known function of apoE beyond the clearance of triacylglycerol-rich lipoproteins. Figure 5 outlines the functions of apoE in the clearance of triacylglycerol-rich lipoproteins as well as in the biogenesis of HDL. In the first step of the HDL biosynthesis pathway, lipid-free apoE or minimally lipidated apoE secreted by the liver or other tissues interacts functionally with the ABCA1 lipid transporter and acquires phospholipids and cholesterol. As documented in the present study, in the absence of ABCA1 or apoE, this pathway cannot proceed. Following intermediate steps that are poorly understood, lipidated apoE is converted into discoidal particles which accumulate under conditions of apoE overexpression and can be visualized by electron microscopy. In the presence of sufficient LCAT activity, the discoidal HDL particles are converted into spherical particles. In addition to the novel pathway that leads to the biogenesis of apoE-containing HDL, lipid-free and possibly lipid-bound apoE can be incorporated into lipoprotein remnants, which can then be cleared by the LDL receptor [13,16]. Normally, the two branches of the pathway are balanced. Under physiological conditions, the remnants are cleared efficiently by the LDL receptor [4,13] and apoE-containing HDL is generated. Under conditions of apoE overexpression, it appears that the endogenous mouse LCAT fails to esterify the cholesterol of the VLDL/IDL/LDL fraction, and thus contributes to the observed dyslipidaemia. This interpretation is consistent with the finding that both the apoE-induced dyslipidaemia and the accumulation of discoidal HDL are corrected by excess LCAT or excess lipoprotein lipase [29]. ApoE-induced dyslipidaemia is also prevented by substitution of alanine for Leu261, Trp264, Phe265, Leu268 and Val269 in the apoE4mut1.
ApoE present in HDL may perform additional functions similar to those of HDL that contain apoA-I [1,37–39]. This may include functional interactions with SR-BI which lead to the uptake of cholesteryl esters and cholesterol efflux [1,37]. They may also promote NO release [40] or have antioxidant and anti-inflammatory properties similar to those assigned to apoA-I-containing HDL [38,39].
The novel apoE pathway may account for some of the atheroprotective functions of apoE
Numerous studies (reviewed in [1]) indicate that the ability of apoE to protect from or to promote atherogenesis appears to be much more pronounced than that of apoA-I, which is the main protein component of HDL. Thus, in contrast with apoE−/− mice which develop atherosclerosis within 10 weeks [3], apoA-I−/− mice do not develop atherosclerosis when on normal or atherogenic diets [41]. Only when apoA-I−/− mice are crossed with apoB transgenic mice, they develop more atherosclerosis than the apoB transgenic mice [42]. In the absence of apoA-I, apoE may form apoE-containing HDL particles that may also confer atheroprotection (Figure 5, branches III, IV and V of the pathway). At the same time, apoE promotes the clearance of cholesteryl ester-rich, triacylglycerol-rich lipoproteins from plasma which are known to be atherogenic (Figure 5, branches I and II of the pathway). A similar rationalization can be offered for another important protein of HDL, the SR-BI. It has been shown that mice deficient in SR-BI do not develop atherosclerosis [43]; however, the double-deficient apoE−/−×SR-BI−/− mice, which express the endogenous apoA-I, develop occlusive atherosclerosis and die before reaching 6 weeks of age [43,44]. When both apoE and SR-BI are defective, both branches of the apoE pathway are eliminated, lipoprotein remnants cannot be cleared, and apoE-containing HDL cannot be formed. In this case, the abnormal HDL particles generated in the SR-BI−/−×apoE−/− mice [43,44] are not sufficient by themselves in the absence of apoE to protect the endothelium from atherosclerosis.
Overall, these studies establish that apoE participates in a pathway of biogenesis of apoE-containing HDL particles that requires the activities of the ABCA1 transporter and LCAT, and raises the possibility that the apoE-containing HDL fraction may contribute to atheroprotection.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL68216) and Kos Pharmaceuticals (Miami, FL, U.S.A.) to V.I.Z., and from the American Heart Association (SDG 0535443T) to K.E.K. We thank Anne Plunkett for preparing the manuscript, and George Konkos and Kostianna Sereti for technical assistance.
References
- 1.Zannis V. I., Kypreos K. E., Chroni A., Kardassis D., Zanni E. E. Lipoproteins and atherogenesis. In: Loscalzo J., editor. Molecular Mechanisms of Atherosclerosis. New York: Taylor & Francis; 2004. pp. 111–174. [Google Scholar]
- 2.Zannis V. I., Zanni E. E., Makrides S. C., Kardassis D., Aleshkov S. Role of apolipoprotein E in Alzheimer's disease. In: Catravas J. D., editor. NATO ASI Series, Life Sciences. New York: Plenum Press; 1998. pp. 179–209. [Google Scholar]
- 3.Plump A. S., Smith J. D., Hayek T., Aalto-Setala K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer E. J., Gregg R. E., Ghiselli G., Forte T. M., Ordovas J. M., Zech L. A., Brewer H. B., Jr Familial apolipoprotein E deficiency. J. Clin. Invest. 1986;78:1206–1219. doi: 10.1172/JCI112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linton M. F., Fazio S. Macrophages, lipoprotein metabolism, and atherosclerosis: insights from murine bone marrow transplantation studies. Curr. Opin. Lipidol. 1999;10:97–105. doi: 10.1097/00041433-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Hasty A. H., Linton M. F., Brandt S. J., Babaev V. R., Gleaves L. A., Fazio S. Retroviral gene therapy in ApoE-deficient mice: ApoE expression in the artery wall reduces early foam cell lesion formation. Circulation. 1999;99:2571–2576. doi: 10.1161/01.cir.99.19.2571. [DOI] [PubMed] [Google Scholar]
- 7.Thorngate F. E., Rudel L. L., Walzem R. L., Williams D. L. Low levels of extrahepatic nonmacrophage ApoE inhibit atherosclerosis without correcting hypercholesterolemia in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2000;20:1939–1945. doi: 10.1161/01.atv.20.8.1939. [DOI] [PubMed] [Google Scholar]
- 8.Raffai R. L., Loeb S. M., Weisgraber K. H. Apolipoprotein E promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2005;25:436–441. doi: 10.1161/01.ATV.0000152613.83243.12. [DOI] [PubMed] [Google Scholar]
- 9.Tsukamoto K., Tangirala R. K., Chun S., Usher D., Pure E., Rader D. J. Hepatic expression of apolipoprotein E inhibits progression of atherosclerosis without reducing cholesterol levels in LDL receptor-deficient mice. Mol. Ther. 2000;1:189–194. doi: 10.1006/mthe.2000.0028. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y., von Eckardstein A., Wu S., Maeda N., Assmann G. A plasma lipoprotein containing only apolipoprotein E and with γ mobility on electrophoresis releases cholesterol from cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1834–1838. doi: 10.1073/pnas.91.5.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimano H., Ohsuga J., Shimada M., Namba Y., Gotoda T., Harada K., Katsuki M., Yazaki Y., Yamada N. Inhibition of diet-induced atheroma formation in transgenic mice expressing apolipoprotein E in the arterial wall. J. Clin. Invest. 1995;95:469–476. doi: 10.1172/JCI117687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukamoto K., Tangirala R., Chun S. H., Pure E., Rader D. J. Rapid regression of atherosclerosis induced by liver-directed gene transfer of ApoE in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 1999;19:2162–2170. doi: 10.1161/01.atv.19.9.2162. [DOI] [PubMed] [Google Scholar]
- 13.Kypreos K. E., Zannis V. I. LDL receptor deficiency or apoE mutations prevent remnant clearance and induce hypertriglyceridemia in mice. J. Lipid Res. 2006;47:521–529. doi: 10.1194/jlr.M500322-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Kypreos K. E., Van Dijk K. W., van Der Z. A., Havekes L. M., Zannis V. I. Domains of apolipoprotein E contributing to triglyceride and cholesterol homeostasis in vivo: carboxyl-terminal region 203–299 promotes hepatic very low density lipoprotein–triglyceride secretion. J. Biol. Chem. 2001;276:19778–19786. doi: 10.1074/jbc.M100418200. [DOI] [PubMed] [Google Scholar]
- 15.Havel R. J., Kotite L., Vigne J. L., Kane J. P., Tun P., Phillips N., Chen G. C. Radioimmunoassay of human arginine-rich apolipoprotein, apoprotein E: concentration in blood plasma and lipoproteins as affected by apoprotein E-3 deficiency. J. Clin. Invest. 1980;66:1351–1362. doi: 10.1172/JCI109988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zannis V. I., Chroni A., Kypreos K. E., Kan H. Y., Cesar T. B., Zanni E. E., Kardassis D. Probing the pathways of chylomicron and HDL metabolism using adenovirus-mediated gene transfer. Curr. Opin. Lipidol. 2004;15:151–166. doi: 10.1097/00041433-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Kypreos K. E., Van Dijk K. W., Havekes L. M., Zannis V. I. Generation of a recombinant apolipoprotein E variant with improved biological functions: hydrophobic residues (Leu-261, Trp-264, Phe-265, Leu-268, Val-269) of apoE can account for the apoE-induced hypertriglyceridemia. J. Biol. Chem. 2005;280:6276–6284. doi: 10.1074/jbc.M413458200. [DOI] [PubMed] [Google Scholar]
- 18.Nolte R. T., Atkinson D. Conformational analysis of apolipoprotein A-I and E-3 based on primary sequence and circular dichroism. Biophys. J. 1992;63:1221–1239. doi: 10.1016/S0006-3495(92)81698-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinmetz A., Kaffarnik H., Utermann G. Activation of phosphatidylcholine-sterol acyltransferase by human apolipoprotein E isoforms. Eur. J. Biochem. 1985;152:747–751. doi: 10.1111/j.1432-1033.1985.tb09256.x. [DOI] [PubMed] [Google Scholar]
- 20.Chroni A., Nieland T. J., Kypreos K. E., Krieger M., Zannis V. I. SR-BI mediates cholesterol efflux via its interactions with lipid-bound ApoE: structural mutations in SR-BI diminish cholesterol efflux. Biochemistry. 2005;44:13132–13143. doi: 10.1021/bi051029o. [DOI] [PubMed] [Google Scholar]
- 21.Chroni A., Liu T., Gorshkova I., Kan H. Y., Uehara Y., von Eckardstein A., Zannis V. I. The central helices of apoA-I can promote ATP-binding cassette transporter A1 (ABCA1)-mediated lipid efflux: amino acid residues 220–231 of the wild-type apoA-I are required for lipid efflux in vitro and high density lipoprotein formation in vivo. J. Biol. Chem. 2003;278:6719–6730. doi: 10.1074/jbc.M205232200. [DOI] [PubMed] [Google Scholar]
- 22.Williamson R., Lee D., Hagaman J., Maeda N. Marked reduction of high density lipoprotein cholesterol in mice genetically modified to lack apolipoprotein A-I. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7134–7138. doi: 10.1073/pnas.89.15.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeish J., Aiello R. J., Guyot D., Turi T., Gabel C., Aldinger C., Hoppe K. L., Roach M. L., Royer L. J., de Wet J., et al. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 25.Chroni A., Duka A., Kan H. Y., Liu T., Zannis V. I. Point mutations in apolipoprotein a-I mimic the phenotype observed in patients with classical lecithin: cholesterol acyltransferase deficiency. Biochemistry. 2005;44:14353–14366. doi: 10.1021/bi050962o. [DOI] [PubMed] [Google Scholar]
- 26.Chroni A., Kan H. Y., Shkodrani A., Liu T., Zannis V. I. Deletions of helices 2 and 3 of human ApoA-I are associated with severe dyslipidemia following adenovirus-mediated gene transfer in ApoA-I-deficient mice. Biochemistry. 2005;44:4108–4117. doi: 10.1021/bi047998l. [DOI] [PubMed] [Google Scholar]
- 27.Kypreos K. E., Li X., Van Dijk K. W., Havekes L. M., Zannis V. I. Molecular mechanisms of Type III hyperlipoproteinemia: the contribution of the carboxy-terminal domain of ApoE can account for the dyslipidemia that is associated with the E2/E2 phenotype. Biochemistry. 2003;42:9841–9853. doi: 10.1021/bi0271796. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell C. D., King W. C., Applegate K. R., Forte T., Glomset J. A., Norum K. R., Gjone E. Characterization of apolipoprotein E-rich high density lipoproteins in familial lecithin:cholesterol acyltransferase deficiency. J. Lipid Res. 1980;21:625–634. [PubMed] [Google Scholar]
- 29.Assmann G., von Eckardstein A., Brewer H. B. Familial analphalipoproteinemia: Tangier disease. In: Scriver C. R., Beaudet A. L., Sly W. S., Valle D., editors. The Metabolic and Molecular Basis of Inherited Disease. New York: McGraw-Hill; 2001. pp. 2937–2960. [Google Scholar]
- 30.Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J. Clin. Invest. 1976;58:667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong E. L., Nichols A. V., Weisgraber K. H., Forte T. M., Shore V. G., Blanche P. J. Discoidal complexes containing apolipoprotein E and their transformation by lecithin-cholesterol acyltransferase. Biochim. Biophys. Acta. 1989;1006:317–328. doi: 10.1016/0005-2760(89)90019-2. [DOI] [PubMed] [Google Scholar]
- 32.Wahrle S. E., Jiang H., Parsadanian M., Legleiter J., Han X., Fryer J. D., Kowalewski T., Holtzman D. M. ABCA1 is required for normal central nervous system ApoE levels and for lipidation of astrocyte-secreted apoE. J. Biol. Chem. 2004;279:40987–40993. doi: 10.1074/jbc.M407963200. [DOI] [PubMed] [Google Scholar]
- 33.Krimbou L., Denis M., Haidar B., Carrier M., Marcil M., Genest J., Jr Molecular interactions between apoE and ABCA1: impact on apoE lipidation. J. Lipid Res. 2004;45:839–848. doi: 10.1194/jlr.M300418-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Koldamova R., Staufenbiel M., Lefterov I. Lack of ABCA1 considerably decreases brain ApoE level and increases amyloid deposition in APP23 mice. J. Biol. Chem. 2005;280:43224–43235. doi: 10.1074/jbc.M504513200. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch-Reinshagen V., Maia L. F., Burgess B. L., Blain J. F., Naus K. E., McIsaac S. A., Parkinson P. F., Chan J. Y., Tansley G. H., Hayden M. R., et al. The absence of ABCA1 decreases soluble ApoE levels but does not diminish amyloid deposition in two murine models of Alzheimer disease. J. Biol. Chem. 2005;280:43243–43256. doi: 10.1074/jbc.M508781200. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y., Thorngate F. E., Weisgraber K. H., Williams D. L., Parks J. S. Apolipoprotein E is the major physiological activator of lecithin-cholesterol acyltransferase (LCAT) on apolipoprotein B lipoproteins. Biochemistry. 2005;44:1013–1025. doi: 10.1021/bi0481489. [DOI] [PubMed] [Google Scholar]
- 37.Liu T., Krieger M., Kan H. Y., Zannis V. I. The effects of mutations in helices 4 and 6 of apoA-I on scavenger receptor class B type I (SR-BI)-mediated cholesterol efflux suggest that formation of a productive complex between reconstituted high density lipoprotein and SR-BI is required for efficient lipid transport. J. Biol. Chem. 2002;277:21576–21584. doi: 10.1074/jbc.M112103200. [DOI] [PubMed] [Google Scholar]
- 38.Rader D. J. High-density lipoproteins and atherosclerosis. Am. J. Cardiol. 2002;90:62i–70i. doi: 10.1016/s0002-9149(02)02635-8. [DOI] [PubMed] [Google Scholar]
- 39.Navab M., Hama S. Y., Cooke C. J., Anantharamaiah G. M., Chaddha M., Jin L., Subbanagounder G., Faull K. F., Reddy S. T., Miller N. E., Fogelman A. M. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J. Lipid Res. 2000;41:1481–1494. [PubMed] [Google Scholar]
- 40.Yuhanna I. S., Zhu Y., Cox B. E., Hahner L. D., Osborne-Lawrence S., Lu P., Marcel Y. L., Anderson R. G., Mendelsohn M. E., Hobbs H. H., Shaul P. W. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 41.Li H., Reddick R. L., Maeda N. Lack of apoA-I is not associated with increased susceptibility to atherosclerosis in mice. Arterioscler. Thromb. 1993;13:1814–1821. doi: 10.1161/01.atv.13.12.1814. [DOI] [PubMed] [Google Scholar]
- 42.Hughes S. D., Verstuyft J., Rubin E. M. HDL deficiency in genetically engineered mice requires elevated LDL to accelerate atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1997;17:1725–1729. doi: 10.1161/01.atv.17.9.1725. [DOI] [PubMed] [Google Scholar]
- 43.Trigatti B., Rayburn H., Vinals M., Braun A., Miettinen H., Penman M., Hertz M., Schrenzel M., Amigo L., Rigotti A., Krieger M. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun A., Trigatti B. L., Post M. J., Sato K., Simons M., Edelberg J. M., Rosenberg R. D., Schrenzel M., Krieger M. Loss of SR-BI expression leads to the early onset of occlusive atherosclerotic coronary artery disease, spontaneous myocardial infarctions, severe cardiac dysfunction, and premature death in apolipoprotein E-deficient mice. Circ. Res. 2002;90:270–276. doi: 10.1161/hh0302.104462. [DOI] [PubMed] [Google Scholar]