Abstract
Insulin stimulation of the trafficking of the glucose transporter GLUT4 to the plasma membrane is controlled in part by the phosphorylation of the Rab GAP (GTPase-activating protein) AS160 (also known as Tbc1d4). Considerable evidence indicates that the phosphorylation of this protein by Akt (protein kinase B) leads to suppression of its GAP activity and results in the elevation of the GTP form of a critical Rab. The present study examines a similar Rab GAP, Tbc1d1, about which very little is known. We found that the Rab specificity of the Tbc1d1 GAP domain is identical with that of AS160. Ectopic expression of Tbc1d1 in 3T3-L1 adipocytes blocked insulin-stimulated GLUT4 translocation to the plasma membrane, whereas a point mutant with an inactive GAP domain had no effect. Insulin treatment led to the phosphorylation of Tbc1d1 on an Akt site that is conserved between Tbc1d1 and AS160. These results show that Tbc1d1 regulates GLUT4 translocation through its GAP activity, and is a likely Akt substrate. An allele of Tbc1d1 in which Arg125 is replaced by tryptophan has very recently been implicated in susceptibility to obesity by genetic analysis. We found that this form of Tbc1d1 also inhibited GLUT4 translocation and that this effect also required a functional GAP domain.
Keywords: adipocyte, GLUT4, GTPase-activating protein (GAP), insulin, Rab, Tbc1d1
Abbreviations: AMPK, AMP-activated protein kinase; GAP, GTPase-activating protein; GFP, green fluorescent protein; GST, glutathione S-transferase; HA, haemagglutinin; HEK, human embryonic kidney; PAS, phospho-Akt substrate; PI3K, phosphoinositide 3-kinase; PTB, phosphotyrosine-binding
INTRODUCTION
Insulin treatment causes a rapid increase in the amount of the glucose transporter GLUT4 at the cell surface in fat and muscle cells. This effect, which is referred to as GLUT4 translocation, is due to the movement of intracellular vesicles containing GLUT4 to the plasma membrane followed by fusion of the vesicles with the plasma membrane [1,2]. A signal transduction pathway critical for GLUT4 translocation proceeds from the insulin receptor to the activation of PI3K (phosphoinositide 3-kinase) and the protein kinase Akt (protein kinase B) [1–3]. Akt then phosphorylates a 160 kDa substrate known as AS160 (also called Tbc1d4) [3–5]. AS160 has a Rab GAP (GTPase-activating protein) domain [6]. Considerable evidence now supports the following proposed role for AS160 in GLUT4 translocation [3–11]: in the absence of insulin, the GAP domain of AS160 keeps a critical Rab in its inactive GDP form; phosphorylation of AS160 leads to suppression of its GAP activity toward this Rab; as a consequence, the GTP form of the Rab increases and this active form of the Rab triggers movement to, and/or docking with, the plasma membrane of GLUT4-containing vesicles.
In addition to AS160, the animal genome encodes a closely related protein known as Tbc1d1. Tbc1d1 is a protein of roughly the same size as AS160 that is approx. 50% identical with AS160 over its entire length. The GAP domain of Tbc1d1 is especially similar to that of AS160. At the time that we began the present study, there was no information in the literature about Tbc1d1 other than descriptions of the cloning of its cDNA and the relative levels of its mRNA in various tissues [12,13]. In the present study, in order to learn more about the function of Tbc1d1, we first examined the Rab specificity of the GAP domain of Tbc1d1. Since this specificity proved to be identical with that of AS160, we then determined the effect of Tbc1d1 on GLUT4 translocation in 3T3-L1 adipocytes. Ectopic expression of Tbc1d1 markedly inhibited GLUT4 translocation, whereas expression of a point mutant with a catalytically inactive GAP domain did not. These results indicate that Tbc1d1 can regulate GLUT4 translocation through its GAP activity. While this study was in progress, it was reported that the R125W mutant of Tbc1d1 is linked to obesity susceptibility in humans [14]. Consequently, we also examined the effect of Tbc1d1 with the R125W mutation on GLUT4 translocation.
EXPERIMENTAL
Plasmids
The cDNA encoding human Tbc1d1 (NCBI gi 29792154 for cDNA, corresponding to NCBI gi 54887445 for the protein) in the pBluescript® vector was obtained from Open Biosystems. The cDNA encoding the protein, except for the start methionine residue, was amplified by PCR and ligated into the KpnI site of the 3XFLAG-CMV-7.1 vector from Sigma. The GAP domain of Tbc1d1, encoding amino acids 746–1168, was amplified by PCR and ligated into the SacI and EcoRI sites of the pGEX-KText vector [15] for expression as a GST (glutathione S-transferase)-fusion protein. Point mutations were introduced through the use of the QuikChange® II XL site-directed mutagenesis kit from Stratagene, and verified by DNA sequencing. pGEX plasmids for the expression of GST-fusion proteins with Rab8B and Rab37 were a gift from Dr Mitsunori Fukuda (Fukuda Initiative Research Unit, RIKEN, Wako, Saitama, Japan). Plasmids for the expression of other GST–Rab fusion proteins were those given in [6].
Rab GAP assay
GST-fusion proteins of the Rabs and of the Tbc1d1 GAP domain were prepared as described in [6]. The GAP assay was performed exactly as described previously [6]. In brief, the GST–Rab was loaded with [α-32P]GTP, and then incubated at 30 °C either alone or with the GST–GAP domain or the R854K mutant thereof. Before the addition of the GAP and at 15 and 30 min thereafter, aliquots of the reaction mixture were removed, the GDP and GTP in the aliquots were separated by TLC, and the radioactivity in each was measured by phosphoimaging.
3T3-L1 culture, transfection and assay of cell-surface GLUT4
3T3-L1 fibroblasts were maintained in culture and differentiated into adipocytes as described in [16]. The relative amount of GLUT4 at the cell surface in basal and insulin-treated adipocytes was measured by a single-cell fluorescence assay with a reporter construct of GLUT4 containing the HA (haemagglutinin) epitope tag in an extracellullar loop and fused to GFP (green fluorescent protein) at its C-terminus [5]. This method, which is described in detail in [5], was briefly as follows. Adipocytes on day 4 of differentiation were co-transfected by electroporation with the plasmids for HA–GLUT4–GFP and Tbc1d1. After 24 h, the cells were serum-starved for 2 h and then treated or not with 160 nM insulin for 30 min. Subsequently, the cells were fixed with formaldehyde and stained with an antibody against the HA epitope and then with a Cy3-conjugated secondary antibody. Individual transfected cells were quantified for both Cy3 and GFP fluorescence. After correction of the fluorescence values for background, the ratio of the Cy3 to GFP fluorescence was calculated. This ratio is a measure of the fraction of GLUT4 at the cell surface normalized to the level of GLUT4 expression in that cell. For each condition, the ratio was measured for approx. 50 cells.
Transfection of cells, immunoprecipitation and immunoblotting
Plates of HEK-293 (human embryonic kidney 293) cells (35 mm) were transfected with 2 μg of Tbc1d1 plasmid through the use of the Lipofectamine™ 2000 reagent (Invitrogen) according to the manufacturer's instructions. After 48 h, the cells were serum-starved for 2 h. During this period the plates were either treated with 200 nM wortmannin for the last 1 h or 1 μM insulin for the last 10 min. Each plate was then washed with cold PBS, and solubilized in 0.5 ml of SDS sample buffer. In the case of 3T3-L1 adipocytes, 10-cm-diameter plates were electroporated with 100 μg of Tbc1d1 plasmid and re-plated on four 35-mm-diameter plates. After 24 h cells were serum-starved for 2 h, and two plates were treated with 160 nM insulin for 10 min and two were left untreated. Each plate was washed with PBS and solubilized in 0.1 ml of a solution containing 40 mg/ml SDS, 10 mM dithiothreitol, 300 mM NaCl and 100 mM Hepes, pH 7.5, with protease inhibitors (10 μM leupeptin, 10 μM EP475, 1 μM pepstatin and 10 μg/ml aprotinin). The combined lysates from two plates were held at 100 °C for 5 min, and then the thiol groups were alkylated by the addition of 25 mM N-ethylmaleimide. These SDS lysates (0.2 ml) were mixed with 0.8 ml of 40 mg/ml nonaocta(ethylene glycol) dodecyl ether, 150 mM NaCl and 50 mM Hepes, pH 7.5, and centrifuged at 6000 g for 15 min. The supernatants were immunoadsorbed on anti-FLAG conjugated to agarose (A2220; Sigma) for 16 h at 4 °C; the immunoadsorbates were washed four times with 5 mg/ml nonaocta(ethylene glycol) dodecyl ether, 150 mM NaCl and 50 mM Hepes, pH 7.5, and solubilized in SDS sample buffer. SDS samples of lysates or immunoprecipitates were immunoblotted with the anti-PAS (phospho-Akt substrate) antibody (9611; Cell Signaling Technology), anti-FLAG conjugated to horseradish peroxidase (A8592; Sigma), and anti-phospho-Ser473 on activated Akt (9271; Cell Signaling Technology), as described previously [4].
RESULTS
Comparison of Tbc1d1 and AS160
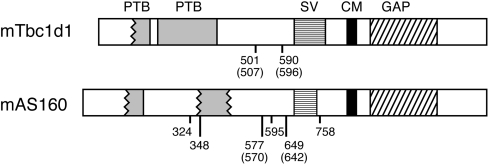
Tbc1d1 shows considerable similarity to AS160. The two proteins from mice are 47% identical and 61% similar over their entire length. Figure 1 presents schematic diagrams of the two mouse proteins. As expected, the predicted domains in both proteins are similar. There are two partial or full PTB (phospho-tyrosine-binding) domains at the N-terminus and a complete Rab GAP domain near the C-terminus. The degree of sequence homology between the Rab GAP domains of the two proteins is especially high; the GAP domains are 79% identical and 91% similar. This fact suggested that the Rab specificity of the GAP domain of Tbc1d1 might be similar to that of AS160.
Figure 1. Schematic representations of mouse Tbc1d1 and AS160.
The amino acid sequences used are NCBI gi 37538012 for mouse Tbc1d1 and NCBI gi 67462068 for mouse AS160. Tbc1d1 and AS160 are proteins of 1255 and 1307 amino acids respectively. The two PTB domains and the GAP domains are those predicted by analysis with the Pfam program (http://pfam.wustl.edu). The wavy line at the border of a domain indicates that the domain alignment is incomplete at that end of the domain. SV designates the section deleted from the shorter slice variant. CM designates the calmodulin-binding motif. The sites of insulin-stimulated phosphorylation of AS160, most likely by the protein kinase Akt, are numbered, as are the two such sites conserved in Tbc1d1. The numbers in parentheses are the numbers of the same Akt sites in the human versions of Tbc1d1 (NCBI gi 54887445) and AS160 (NCBI gi 114688046). Thr590 (Thr596 in humans) of Tbc1d1 corresponds to Thr649 (Thr642 in humans) of AS160; Ser501 (Ser507 in humans) of Tbc1d1 corresponds to Ser577 (Ser570 in humans) of AS160.
Tbc1d1 and AS160 from mouse also show two other similarities. AS160 has a motif that binds calmodulin between amino acids 842 and 865 [17]. The corresponding sequence in Tbc1d1 is 79% identical, and thus is very likely also to bind calmodulin (Figure 1). Secondly, sequences in the mouse protein database show that there is a short splice variant in mouse Tbc1d1 that lacks amino acids 631–724, which are encoded by two adjacent exons. The same case holds for AS160, where the short splice variant lacks amino acids 685–739, which are encoded by a single exon (Figure 1). We have found that 3T3-L1 adipocytes express mainly the shorter version of AS160 (H. Sano and G. E. Lienhard, unpublished work). One major difference between Tbc1d1 and AS160 is the number of potential sites on Tbc1d1 for phosphorylation by the protein kinase Akt. AS160 has been shown to undergo insulin-stimulated phosphorylation on five standard Akt motifs (RXRXXS/T) and one partial one (RXXXXS/T) [5]. Only one of the five standard motifs and the single partial motif are conserved in Tbc1d1; these are at Thr590 and Ser501, respectively, corresponding to Thr649 and Ser577 in AS160 (Figure 1).
Human Tbc1d1 and AS160 are approx. 90% identical with the corresponding mouse proteins, and all of the features described above are also found in the human proteins. We have presented the mouse proteins here, rather than the human ones, because, at this time, the human protein and EST (expressed sequence tag) databases contain only the short splice variant of human Tbc1d1 (NCBI gi 54887445) and the long splice variant of AS160 (NCBI gi 114688046). It is very likely that the other splice variants are also expressed in humans. The human Tbc1d1 gene contains two adjacent potential exons corresponding to the ones present in the long splice variant of mouse Tbc1d1 and absent in the short version. The human AS160 gene contains an exon corresponding to the one present in the long splice variant of mouse AS160 and deleted in the short version. The present study has been carried out with the short splice variant of human Tbc1d1 (NCBI gi 54887445). In this human variant, the residues of the two possible Akt phosphorylation sites conserved between Tbc1d1 and AS160 are Thr596 and Ser507, corresponding to Thr590 and Ser501 in mouse Tbc1d1 (see above and Figure 1).
Rab specificity of the Tbc1d1 GAP domain
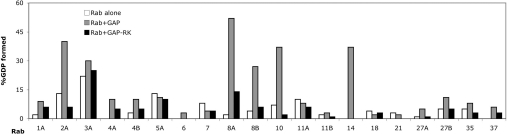
Previously, we measured the activity of the GAP domain of human AS160 against a panel of 18 Rabs [6]. Rabs 2, 8A, 10 and 14 were found to be substrates. More recently, we have also tested Rab8B and found it to be a substrate for the AS160 GAP domain (results not shown). In the present study, we examined the activity of the GAP domain of Tbc1d1 against the same group of 18 Rabs, and, in addition, Rab8B and Rab37. Figure 2 summarizes the results. For each Rab it presents the percentage of the [α-32P]GTP converted into GDP after 15 min, with the Rab alone, with the wild-type GAP domain, or with the GAP domain containing the R854K mutation. In other Rab GAPs, including AS160, mutation of the corresponding arginine residue, which crystallography has shown to participate directly in catalysis [18], inactivates the GAP activity [6]. A subgroup of the Rabs, namely 2A, 8A, 8B, 10 and 14, show enhanced GTP hydrolysis in the presence of the wild-type GAP domain, and are thus substrates for it. As expected, the R854K mutant of the GAP domain showed no activity towards these substrate Rabs. It is notable that this specificity of the Tbc1d1 GAP domain is identical with that of the GAP domain of AS160 [6]. The catalytic efficiency of the two GAP domains is also very similar. The Tbc1d1 GAP domain catalysed the hydrolysis of 27, 50, 30, 24 and 37% of the GTP bound to Rab 2A, 8A, 8B, 10 and 14 respectively in 15 min, after correction for the intrinsic GTPase activity of the Rab (Figure 2). Under similar conditions, the corresponding values for the GAP domain of AS160 are 40, 50, 32, 32 and 38% (Figure 2 of [6] and data for Rab8B).
Figure 2. Activity of Tbc1d1 GAP domain against 20 Rab proteins.
The GAP assay was carried out as described in the Experimental section. The data show the increase in the percentage of the total radioactivity in the GDP form between 0 and 15 min for the Rab alone, the Rab plus the GAP domain, and the Rab plus the GAP domain with the R854K mutation. In the case of Rabs 3A and 5A the data are for 6 min and 10 min respectively, rather than 15 min, due to the higher intrinsic GTPase activity of these Rabs. These data were taken from the time courses of GDP formation over the period from 0 to 30 min, with a second time point taken at 30 min (except for Rabs 3A and 5A where the second time point was at 12 or 20 min respectively); the results at the longer time point again showed significant GAP activity against only Rabs 2A, 8A, 8B, 10 and 14. Separate replicate determinations of the times courses for Rabs 3A, 10 and 14 yielded the same results for these Rabs as shown here.
Effect of Tbc1d1 on insulin-stimulated GLUT4 translocation
Previously, we found a role for AS160 in insulin-stimulated GLUT4 translocation through examining the effect of wild-type and mutant forms of human AS160 on the process in 3T3-L1 adipocytes [5]. Overexpression of wild-type AS160 or of AS160 with the inactivating R→K mutation in the GAP domain had no effect on GLUT4 translocation. On the other hand, overexpression of mutants in which one to six of the Akt phosphorylation sites were changed to alanine and so not phosphorylatable inhibited GLUT4 translocation to various extents. For example, the single-site T642A mutant of human AS160 inhibited GLUT4 translocation by 60%, and a four-site phosphorylation mutant (S318A/S588A/T642A/S751A) inhibited by 80%. This inhibition by the four-site mutant did not occur with AS160 containing both the four phosphorylation site mutations and the inactivating R→K mutation in the GAP domain. Hence, an active GAP domain is necessary for the inhibition by the nonphosphorylatable mutant AS160. These findings led to the hypothesis for AS160 function described in the Introduction.
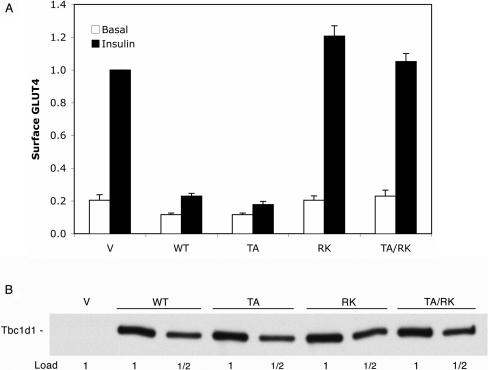
In the present study, we examined the effect of a similar set of Tbc1d1 constructs on insulin-stimulated GLUT4 translocation by means of the single-cell fluorescence assay with HA–GLUT4–GFP. The mutants examined were: (i) Tbc1d1 T596A (designated TA); Thr596 is in a motif that is very similar to that of the Thr642 Akt site in human AS160 (see Figure 1 and below); (ii) Tbc1d1 R854K (designated RK), which, as shown in the GAP assay, inactivates the GAP activity; and (iii) the double mutant T596A/R854K (designated TA/RK). Figure 3(A) summarizes the results. In the vector control, insulin caused a 5-fold increase in GLUT4 at the cell surface. Both the wild-type Tbc1d1 and the T596A mutant markedly inhibited insulin-stimulated GLUT4 translocation. The inhibition by the wild-type Tbc1d1 was unexpected, since wild-type AS160 does not inhibit GLUT4 translocation. Inhibition by either wild-type Tbc1d1 or the T596A mutant required a functional GAP domain, since the R854K and T596A/R854K mutants had no significant effect on GLUT4 translocation. In order to be sure that the different effects of the constructs were not due to different levels of expression, we immunoblotted SDS lysates of the cells for the FLAG tag present on the constructs. Figure 3(B) shows that there was equal expression of the various forms of Tbc1d1. Also, as judged by microscopy, co-expression of Tbc1d1 and its mutants with HA–GLUT4–GFP did not alter the cell morphology of the adipocytes or the subcellular distribution of HA–GLUT4–GFP, which showed its typical concentration in perinuclear vesicles [5] (results not shown).
Figure 3. Effects of Tbc1d1 on GLUT4 translocation.
(A) 3T3-L1 adipocytes were transfected with HA–GLUT4–GFP and vector (V), wild-type Tbc1d1 (WT) or one of the mutant forms of Tbc1d1 (TA, T596A; RK, R854K; TA/RK, T596A/R854K). The relative amount of HA–GLUT4–GFP at the cell surface in basal and insulin-stimulated 3T3-L1 adipocytes was measured using the single-cell fluorescence assay, as described in the Experimental section. Results are means±S.E.M. for three separate experiments. The values in each experiment were normalized to a value of 1.0 for the vector in the insulin state. (B) SDS lysates were made of the transfected cells described in (A) and immunoblotted for the FLAG epitope. The 1× load per lane was 5 μg of protein.
Phosphorylation on Thr596 of human Tbc1d1
Thr596 on human Tbc1d1 and the corresponding Thr590 on mouse Tbc1d1 are in a standard Akt phosphorylation motif of the sequence RRRANTL. According to analysis with the Scansite program for phosphorylation sites (http://scansite.mit.edu), this threonine residue is predicted to be an excellent Akt site and is by far the best Akt site in Tbc1d1. The sequence surrounding Thr596/Thr590 is very similar to the sequence surrounding Thr642 in human AS160 and the corresponding Thr649 in mouse AS160, which is RRRAHTF (Figure 1). In 3T3-L1 adipocytes, Thr649 is phosphorylated in response to insulin, almost certainly by Akt [3–5]. Thus it seemed likely that insulin-activated Akt would also phosphorylate Thr596 in human Tbc1d1.
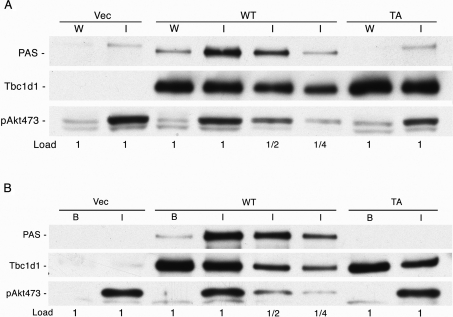
In order to test for phosphorylation of Thr596 in human Tbc1d1, we first examined the phosphorylation of Tbc1d1 and its T596A mutant overexpressed in HEK-293 cells through immunoblotting with an antibody specific for the phosphomotif RXRXXpS/T generated by Akt phosphorylation (anti-PAS antibody). The cells were treated either with wortmannin, which inactivates PI3K and thereby also Akt, or with insulin, which activates Akt. SDS lysates of the cells were immunoblotted directly with the anti-PAS antibody. In response to insulin treatment, wild-type Tbc1d1 was phosphorylated on an Akt motif, whereas no signal was detected for the T596A mutant (Figure 4A, top panel). Hence, in HEK-293 cells, Thr596 is a site of insulin-stimulated phosphorylation, most probably by the protein kinase Akt. Immunoblotting for the Tbc1d1 protein showed that the expression levels of the wild-type and T596A mutant were the same, and immunoblotting for Akt phosphorylated on Ser473 showed that insulin activated Akt compared with the wortmannin control (Figure 4A, bottom panel). The PAS blot of the insulin-treated vector control cells and the T596A-expressing cells showed a faint band corresponding to a slightly greater size than the strong band given by insulin-treated cells containing wild-type Tbc1d1 (Figure 4A, top panel). We attribute this weak band to phosphorylation of AS160, which is an endogenous protein in HEK-293 cells. In the insulin-treated cells expressing wild-type Tbc1d1, this weak band is not evident, probably because the strong PAS band from the wild-type Tbc1d1 obscures it.
Figure 4. Phosphorylation on Thr596 of Tbc1d1.
(A) HEK-293 cells were transfected with vector (Vec) or the various forms of Tbc1d1 and treated with wortmannin (W) for or insulin (I), as described in the Experimental section. SDS lysates were prepared and immunoblotted for phosphorylation with the anti-PAS antibody, for Tbc1d1 with antibody against its FLAG epitope tag, and for Akt activation with antibody for the Ser473 phosphorylation site on Akt. (B) 3T3-L1 adipocytes were transfected with vector or the various forms of Tbc1d1 and treated with 160 nM insulin for 10 min (I) or left in the basal state (B). SDS/non-ionic detergent lysates were prepared and the FLAG-tagged Tbc1d1 was immunoprecipitated, as described in the Experimental section. The immunoprecipitate was blotted with the anti-PAS antibody and with anti-FLAG. SDS samples of the total cell lysates were immunoblotted for activation of Akt as in (A). The experiments in (A) and (B) were repeated and gave similar results.
In a similar way, we examined whether Tbc1d1 underwent phosphorylation in response to insulin in 3T3-L1 adipocytes. In this case, the Tbc1d1 was first isolated from SDS/non-ionic detergent lysates of the cells by immunoprecipitation with antibody against the FLAG epitope tag. This isolation step was included so that the interpretation of the PAS immunoblot was not complicated by the signal from endogenous AS160, which would have been more significant in the adipocytes due to the lower ectopic expression of Tbc1d1 in these cells. In the adipocytes, as in the HEK-293 cells, wild-type Tbc1d1 showed marked insulin-elicited phosphorylation with the anti-PAS antibody, whereas no signal was detected for the T596A mutant (Figure 4B, top panel). Immunoblots for the FLAG tag on Tbc1d1 and for Akt activation showed approximately equal expression of the Tbc1d1 forms and insulin-stimulated activation of Akt (Figure 4B, bottom panel).
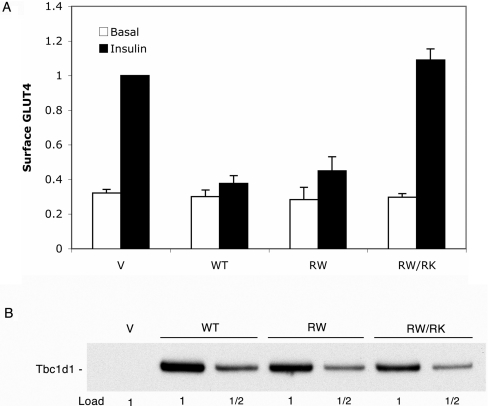
Functional effect of R125W mutation in Tbc1d1
The R125W mutation in Tbc1d1 is associated with susceptibility to obesity in humans [14]. Consequently, we examined whether this mutation affected the ability of Tbc1d1 to inhibit GLUT4 translocation. The R125W mutant of Tbc1d1 inhibited GLUT4 translocation to the same marked extent as did wild-type Tbc1d1 (Figure 5A). The R125W/R854K double mutant had no inhibitory effect (Figure 5A). This latter result again demonstrates that inhibition depends upon functional GAP activity. Immunoblotting of SDS lysates of the transfected adipocytes showed that the expression levels for wild-type Tbc1d1 and the mutants were similar (Figure 5B). The similarity of expression levels indicates that the R125W mutation does not alter the stability of Tbc1d1.
Figure 5. Effects of the R125W mutation on Tbc1d1 inhibition of GLUT4 translocation.
(A) 3T3-L1 adipocytes were transfected with HA–GLUT4–GFP and vector (V), wild-type Tbc1d1 (WT), the R125W mutant (RW), or the R125W/R854K mutant (RW/RK), and assayed for GLUT4 at the cell surface, as described in the Experimental section and the legend to Figure 3. Results are means±S.E.M. for four experiments, normalized to a value of 1.0 for the vector plus insulin. (B) SDS lysates of the transfected cells were immunoblotted for the FLAG epitope. The 1× load was 5 μg of protein.
DISCUSSION
The present study shows that the GAP domain of Tbc1d1 is functional and has a Rab specificity that is almost identical with that of the highly similar GAP domain of AS160. Moreover the GAP activity toward these substrate Rabs is approximately the same as that of AS160. These findings led us to examine the effect of Tbc1d1 on insulin-stimulated GLUT4 translocation. Expression of wild-type Tbc1d1 in 3T3-L1 adipocytes markedly inhibited GLUT4 translocation. This inhibition required GAP domain activity, since the R854K mutant that lacks GAP activity was not inhibitory. The likely explanation for the inhibitory effect of ectopically expressed Tbc1d1 on GLUT4 translocation is that Tbc1d1 maintains the Rab(s) required for GLUT4 translocation in its inactive GDP form. Hence, these results provide further support for the proposal that insulin signalling of GLUT4 translocation leads to the elevation of the active GTP form of one or more Rabs. Recently, in a collaborative study with the research group of Dr T. E. McGraw (Department of Biochemistry, Weill Medical College of Cornell University, New York, NY, U.S.A.), we have obtained evidence that activation of Rab10, a substrate for both AS160 and Tbc1d1, contributes to GLUT4 translocation (H. Sano, L. Equez, M. N. Teruel, M. Fukuda, T. D. Chuang, J. A. Chavez, G. E. Lienhard and T. E. McGraw, unpublished work).
The behaviour of Tbc1d1 differs from AS160 in a key way. Overexpression of wild-type AS160 in 3T3-L1 adipocytes does not inhibit insulin-stimulated GLUT4 translocation; inhibition is observed only through overexpression of a non-phosphorylatable mutant of AS160. Hence, wild-type Tbc1d1 does not show the same regulation through phosphorylation as does AS160. One possible explanation for this difference is that the GAP activity of Tbc1d1 is inhibited by its phosphorylation, but that phosphorylation of the overexpressed Tbc1d1 was incomplete. Hence, under the conditions of our assay, no regulation by phosphorylation was seen. The results of immunoblotting with the anti-PAS antibody show that insulin elicits the phosphorylation of Tbc1d1 on Thr596, most probably by Akt. However, they do not establish the stoichiometry of its phosphorylation, which may be only a modest percentage of the total Tbc1d1. A second possible explanation for the difference between Tbc1d1 and AS160 is that phosphorylation of Tbc1d1 may not lead to inhibition of its GAP activity. There is evidence suggesting that the inhibition of the GAP activity of AS160 upon phosphorylation requires the ensuing binding of a dimeric 14-3-3 protein [19]. Since regulation by 14-3-3 protein binding may require both subunits of the 14-3-3 dimer to engage the target protein [20], and since many of the Akt sites in AS160 are not conserved in Tbc1d1, it may be that 14-3-3 protein cannot bind to Tbc1d1 in a similar way to AS160.
3T3-L1 adipocytes almost certainly contain some endogenous Tbc1d1. It has been reported that mRNA for Tbc1d1 increases upon differentiation of 3T3-L1 fibroblasts into adipocytes [21]. Also, the mRNA for Tbc1d1 is relatively abundant in human fat [14]. Finally, we have detected peptides from Tbc1d1 in mass spectrometric analysis of AS160 immunoprecipitated from a non-ionic detergent lysate of 3T3-L1 adipocytes. In this analysis, two tryptic peptides unique to Tbc1d1 were found together with 92 peptides from AS160 (G. E. Lienhard and W. D. Lane, unpublished work). The presence of these peptides may be due to contamination of the AS160 immunoprecipitate or may indicate an interaction of Tbc1d1 with AS160. In the future, it will be important to examine the endogenous Tbc1d1 in 3T3-L1 adipocytes. The amount of Tbc1d1 relative to AS160 and the phosphorylation state of Thr596 after insulin treatment are of considerable interest. Unfortunately, to date, we have not been able to develop an antibody against Tbc1d1 that reliably detects the protein on an immunoblot of a 3T3-L1 adipocyte SDS lysate or that immunoprecipitates the protein from an SDS/non-ionic detergent lysate of 3T3-L1 adipocytes. The explanation may be that the amount of Tbc1d1 in 3T3-L1 adipocytes is relatively low.
The above considerations, and the previous studies on AS160 [3–11], suggest the following proposal for the action of Tbc1d1 and AS160 in GLUT4 translocation. In the absence of insulin, both Tbc1d1 and AS160 act upon one or more Rabs critical for GLUT4 translocation, including Rab10. This action maintains the Rab(s) in its inactive GDP form. As a consequence, GLUT4 is retained in its intracellular location. Insulin treatment results in the activation of Akt, which phosphorylates both AS160 and Tbc1d1. If the GAP activity of Tbc1d1 is inhibited by phosphorylation, then the inhibition of both its activity and that of AS160 will contribute to the elevation of the active GTP form of the Rab and so GLUT4 translocation. If the GAP activity of Tbc1d1 is not inhibited by its phosphorylation in response to insulin, then the extent of activation of the Rab and possibly GLUT4 translocation will be limited to some extent by the continued GAP action of Tbc1d1. In the future, it will be important to distinguish between these possibilities. Moreover, since insulin-stimulated GLUT4 translocation also occurs in muscle [1,2,11], it will be important to assess the role of Tbc1d1 in muscle. One study reports that skeletal muscle is the human tissue in which the mRNA for Tbc1d1 is most abundant [14], whereas two other studies have found its mRNA to be most abundant in kidney [12,13]. Contraction also stimulates GLUT4 translocation in muscle, and the phosphorylation of AS160 by AMPK (AMP-activated protein kinase) has been implicated in signalling this process [11]. Hence, Tbc1d1 may also be involved in contraction-elicited GLUT4 translocation. In this regard, it is of interest that Tbc1d1 has a potential AMPK site (Ser237 in human Tbc1d1) that is not present in AS160 [14].
While this study was in progress, it was reported that the R125W of Tbc1d1 is highly linked to a predisposition to obesity in the context of a specific gene–gene interaction [14]. The R125W mutation is relatively common in the general population, with an allele frequency of 0.09 [14]. Since this study is the first one to describe a function for Tbc1d1, it enabled us to test whether this mutation affected Tbc1d1 function. Even though the R125W mutation occurs in a predicted PTB domain and substitutes a hydrophobic amino acid for a positively charged one, it had no effect on the inhibition of GLUT4 translocation by Tbc1d1. Thus the effect of the R125W mutation on Tbc1d1 function is likely to be a subtle one.
Acknowledgments
This study was supported by a fellowship from the National Institutes of Health (training grant DK07508 to W. G. R.) and by a National Institutes of Health grant DK25336 to G. E. L.
References
- 1.Watson R. T., Kanzaki M., Pessin J. E. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr. Rev. 2004;25:177–204. doi: 10.1210/er.2003-0011. [DOI] [PubMed] [Google Scholar]
- 2.Thong F. S. L., Dugani C. B., Klip A. Turning signals on and off: GLUT4 traffic in the insulin signaling highway. Physiology. 2005;20:271–284. doi: 10.1152/physiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez E., McGraw T. E. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and fusion of GLUT4 vesicles to the plasma membrane. Mol. Biol. Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane S., Sano H., Liu S. C., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. A method to identify serine kinase substrates: Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 2002;277:22115–22118. doi: 10.1074/jbc.C200198200. [DOI] [PubMed] [Google Scholar]
- 5.Sano H., Kane S., Sano E., Mîinea C. P., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 6.Mîinea C. P., Sano H., Kane S., Sano E., Fukuda M., Peranen J., Lane W. S., Lienhard G. E. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeigerer A., McBrayer M. K., McGraw T. E. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent upon RabGAP AS160. Mol. Biol. Cell. 2004;15:4006–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Equez L., Lee A., Chavez J. A., Mîinea C. P., Kane S., Lienhard G. E., McGraw T. E. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Larance M., Ramm G., Stockli J., van Dam E. M., Winata S., Wasinger V., Simpson F., Graham M., Junutula J. R., Guillhaus M., James D. E. Characterization of the role of the RabGTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 10.Peck G. R., Ye S, Pham V., Fernando R. N., Macaulay S. L., Chai S. Y., Albiston A. L. Interaction of the Akt substrate, AS160, with the glucose transporter 4 vesicle marker protein, insulin-regulated aminopeptidase. Mol. Endocrinol. 2006;20:2576–2583. doi: 10.1210/me.2005-0476. [DOI] [PubMed] [Google Scholar]
- 11.Kramer H. F., Witczak C. A., Taylor E. B., Fujii N., Hirshman M. F., Goodyear L. J. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J. Biol. Chem. 2006;281:31488–31485. doi: 10.1074/jbc.M605461200. [DOI] [PubMed] [Google Scholar]
- 12.Richardson P. M., Zon L. I. Molecular cloning of a cDNA with a novel domain present in the tre-2 oncogene and the yeast cell cycle regulators BUB2 and cdc16. Oncogene. 1995;11:1139–1148. [PubMed] [Google Scholar]
- 13.Kikuno R., Nagase T., Ishikawa K., Hirosawa M., Miyajima N., Tanaka A., Kotani H., Nomura N., Ohara O. Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 1999;6:197–205. doi: 10.1093/dnares/6.3.197. [DOI] [PubMed] [Google Scholar]
- 14.Stone S., Abkevich V., Russell D. L., Riley R., Timms K., Tran T., Trem D., Frank D., Jammulapati S., Neff C. D., et al. TBC1D1 is a candidate for a severe obesity gene and evidence for gene/gene interaction in obesity predisposition. Hum. Mol. Genet. 2006;15:2709–2720. doi: 10.1093/hmg/ddl204. [DOI] [PubMed] [Google Scholar]
- 15.Guan K., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- 16.Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J. Biol. Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- 17.Kane S., Lienhard G. E. Calmodulin binds to the RabGTPase activating protein required for insulin-stimulated GLUT4 translocation. Biochem. Biophys. Res. Commun. 2005;335:175–180. doi: 10.1016/j.bbrc.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 18.Pan X., Eathiraj S., Munson M., Lambright D. G. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature. 2006;442:303–306. doi: 10.1038/nature04847. [DOI] [PubMed] [Google Scholar]
- 19.Ramm G., Larance M., Guilhaus M., James D. E. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J. Biol. Chem. 2006;281:29174–29180. doi: 10.1074/jbc.M603274200. [DOI] [PubMed] [Google Scholar]
- 20.Yaffe M. How do 14-3-3 proteins work? Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002;513:53–57. doi: 10.1016/s0014-5793(01)03288-4. [DOI] [PubMed] [Google Scholar]
- 21.Guo X., Liao K. Analysis of gene expression profile during 3T3-L1 preadipocyte differentiation. Gene. 2000;251:45–53. doi: 10.1016/s0378-1119(00)00192-x. [DOI] [PubMed] [Google Scholar]