Abstract
Establishing a complete pathway which links occupancy of the insulin receptor to GLUT4 translocation has been particularly elusive because of the complexities involved in studying both signalling and membrane trafficking processes. However, Lienhard's group has now discovered two related molecules that could function in this linking role. These proteins, Tbc1d4 (also known as AS160) and now Tbc1d1, as reported in this issue of the Biochemical Journal, have been demonstrated to be Rab GAPs (GTPase-activating proteins) that link upstream to Akt (protein kinase B) and phosphoinositide 3-kinase and downstream to Rabs involved in trafficking of GLUT4 vesicles. The data from Leinhard and colleagues suggest that high levels of Rab GAP activity lead to suppression of GLUT4 translocation and this observation has wide significance and is likely to be relevant to the recent discovery that mutations in the Tbc1d1 gene lead to some cases of severe human obesity.
Keywords: GLUT4 translocation, insulin signalling, obesity-susceptibility gene, Rab GTPase-activating protein (Rab GAP), Tbc1d1
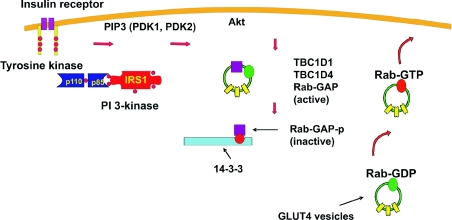
Studies on insulin regulation and GLUT4 trafficking have historically been studied in partial isolation. Much of the focus has been on early events in insulin signalling, such as the coupling of insulin receptor activity to tyrosine phosphorylation of IRS (insulin receptor substrate) molecules 1 and 2 and then to PDK (phosphoinositide-dependent kinase) 1 and 2 [mTOR (mammalian target of rapamycin)–rictor (rapamycin-insensitive companion of mTOR)] and leading to the phosphorylation of Akt (protein kinase B) at two regulatory sites. However, equal focus has surrounded the defining of machinery and compartments involved in GLUT4 subcellular trafficking. Emphasis has been placed on identification of targeting domains in GLUT4 that lead to its sequestration in its intracellular reservoir compartment. In addition, studies have revealed details of the translocation apparatus that facilitates GLUT4 vesicle movement from a perinuclear compartment outwards (often associated with microtubules) and towards localized sites on the plasma membrane that promote docking and fusion of the vesicles with the plasma membrane. The ways in which these two nodes (the beginning and the end of the signalling to trafficking pathway) are connected has been extremely elusive. Now, however, a plausible linked pathway has emerged based on the discoveries from Lienhard's group that demonstrate that Rab GAPs (GTPase-activating proteins) are Akt substrates. First, Sano et al. [1] reported that an Akt substrate of 160 kDa was a Rab GAP and that its phosphorylation by Akt was required for GLUT4 translocation. This protein was called AS160, but is also known as Tbc1d4. Tbc1d4 has GAP activity with the Rabs 2A, 8A, 8B, 10 and 14. Rabs 10 and 14 have been shown to be present on GLUT4 vesicles and therefore these may be the important target Rabs that lie between Tbc1d4 and regulated GLUT4 vesicle activity [2,3]. Lienhard's hypothesis that links Tbc1d4 activity to both insulin signalling and GLUT4 vesicle activity is shown in Figure 1. In outline, it is suggested that Akt phosphorylation of Tbc1d4 leads to suppression of its Rab GAP activity and that this leads to the relevant Rab being converted into a functionally active GTP-loaded form. This may occur through a direct conformational change that inhibits GAP activity or may be due to a change of localization such that the protein no longer has access to its Rab. It cannot then act on it to convert it into its non-functional GDP form. It has been shown that Tbc1d4 may be localized to GLUT4 vesicles by virtue of its binding to cargo proteins within the GLUT4 vesicles [3,4]. The change in localization of Tbc1d4 initiated by phosphorylation may involve its association with a 14-3-3 protein and subsequent dissociation from the GLUT4 vesicles and into the cytoplasm [5].
Figure 1. Lienhard's hypothesis for a role of the Tbc1d1 and Tbc1d4 proteins as links between insulin signalling and GLUT4 vesicle trafficking.
Insulin leads to autophosphorylation of its receptor which in turn leads to tyrosine phosphorylation of IRS1. This couples to activation of PI 3-kinase (phosphoinositide 3-kinase) which produces PIP3 (phosphatidylinositol trisphosphate) in the plasma membrane. PDK1, PDK2 and Akt become associated with PIP3 and together lead to the phosphorylation and activation of Akt. Activated Akt then phosphorylates Tbc1d1 and Tbc1d4 and this leads to suppression of their GTPase-activating activity. This may occur directly or indirectly through association of the phosphorylated proteins (Rab-GAP-p) with 14-3-3 and relocation to the cytoplasm. Following the reduction in GLUT4-vesicle-associated Rab GAP activity, the vesicle-associated Rabs are converted into their active GTP-loaded forms and this switch facilitates trafficking of the vesicles to the plasma membrane and possibly enhanced docking at the plasma membrane.
Studies in which Tbc1d4 has been knocked down by silencing RNA techniques indicate that the protein is involved in the exocytosis limb of the GLUT4 trafficking pathway [3,6]. Recent studies have highlighted the importance of docking and fusion of GLUT4 vesicles with the plasma membrane as being a highly insulin regulated site of action and possibly the rate-limiting step in the trafficking [7,8]. Tbc1d4 has been shown to be more important for a pre-fusion, possibly docking, step at the plasma membrane [8,9]. Whether Tbc1d1 controls the same or a different step (and an associated same or different Rab) will be an important area of further investigation.
The reported properties of Tbc1d1 reveal some similarities to those of Tbc1d4 (the proteins are 50% identical) and some interesting differences. The GAP domains are 79% identical, and, in this issue of the Biochemical Journal, Roach et al. [10] demonstrate that the Rabs identified as substrates (from the panel of Rabs that they tested) are essentially identical. Furthermore, Tbc1d1 has also been demonstrated to be phosphorylated by insulin signalling through activated Akt [10]. The domain architectures of Tbc1d1 and Tbc1d4 are also similar. Both have two N-terminally located PTB (phosphotyrosine-binding) domains, a central calmodulin-binding domain and a C-terminal GAP domain. There are centrally located Akt phosphorylation sites, the most important one being conserved between Tbc1d1 and Tbc1d4. The main differences between the two proteins are in the location and sequences in the PTB domains and in the occurrence of a predicted AMPK (AMP-activated protein kinase) phosphorylation site between the two PTB domains of Tbc1d1. These differences are likely to have functional consequences. One possibility is that Tbc1d1 can more effectively link both insulin signalling and metabolic/exercise signalling to GLUT4 translocation. Changes in the energy status of the cell that increase the AMP/ATP ratio lead to an associated activation of AMPK. In some muscle types, contraction leads to increased AMPK phosphorylation and activation, and this in turn leads to increased GLUT4 translocation. However, this may not be the case in all muscle types, and more complex patterns of regulation involving interplay between Akt phosphorylation, AMPK phosphorylation and calcium-dependent regulation take place [11,12]. Tbc1d1 with its Akt, AMPK and calmodulin-binding domains is therefore an attractive candidate molecule to act as a point of convergence between insulin action, exercise and metabolic stress pathways, but this role has yet to be established.
The potential importance of Tbc1d1 in linking insulin, exercise and energy status signalling with GLUT4 membrane traffic is heightened by the additional recent discovery by Stone et al. [13]. This group has reported that the gene coding for Tbc1d1 is a candidate severe obesity gene [13]. A defect in this gene (leading to substitution of tryptophan for arginine) is present in some cases of severe obesity in females and gene–gene interaction studies have provided evidence that Tbc1d1 may give rise to obesity predisposition.
The mutation occurs in the first PTB domain of Tbc1d1 and might be expected to alter the targeting of the protein within the cell. Clearly, further studies are required to identify the PTB-binding target. Such studies would allow an assessment to be made of whether mistargeting leads to an inability of the protein to function as a GAP towards the Rab involved in GLUT4 trafficking. However, one can speculate that the mutation may lead to some loss of normal localization of Rab GAP activity in the basal state. The ability of the protein to facilitate retention of GLUT4 vesicles in their basal location would then be lost and the cell would translocate more GLUT4 to the cell surface and consequently take up more glucose in the absence of any insulin stimulus. Roach et al. [10] have demonstrated that Tbc1d1 reverses insulin action on GLUT4 trafficking and that insulin action suppresses Tbc1d1 activity. Therefore active Tbc1d1 would normally suppress peripheral glucose uptake and inactive Tbc1d1 would promote peripheral glucose uptake. In this sense, the Tbc1d1 gene could be thought of as a ‘thrifty’ gene. That is, it may have been conserved in evolution to function in suppression of peripheral glucose uptake so that the blood glucose could be conserved when necessary. This would enable blood glucose levels to be maintained for the overriding requirement of sustaining brain glucose utilization at times when nutrients are scarce, a condition no longer pertaining in most 21st century human societies [14]. In this scenario, mutations in the thrifty Tbc1d1 gene would have high potential for leading to obesity susceptibility. Of course, this is likely to be an oversimplification and more complex pathophysiology [14] that includes multiple tissues, multiple inflammatory molecules and adipokines and possibly central control of appetite could be involved in the obesity occurring in persons with the Tbc1d1 R→W mutation. Clearly, more experiments on the thrifty link proteins Tbc1d1 and Tbc1d4 are required, and are eagerly awaited because of their possible significance in the growing epidemics of obesity and Type 2 diabetes.
Acknowledgments
Work in our laboratory is supported by the MRC (Medical Research Council) (U.K.) and by the Wellcome Trust.
References
- 1.Sano H., Kane S., Sano E., Mîinea C. P., Asara J. M., Lane W. S., Garner C. W., Lienhard G. E. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 2.Mîinea C. P., Sano H., Kane S., Sano E., Fukuda M., Peranen J., Lane W. S., Lienhard G. E. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larance M., Ramm G., Stockli J., van Dam E. M., Winata S., Wasinger V., Simpson F., Graham M., Junutula J. R., Guilhaus M., James D. E. Characterization of the role of the RabGAP AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 4.Peck G. R., Ye S., Pham V., Fernando R. N., Lance M. S., Yeen C. S., Albiston A. L. Interaction of the Akt substrate, AS160, with the GLUT4 vesicle marker protein, IRAP. Mol. Endocrinol. 2006;20:2576–2583. doi: 10.1210/me.2005-0476. [DOI] [PubMed] [Google Scholar]
- 5.Ramm G., Larance M., Guilhaus M., James D. E. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J. Biol. Chem. 2006;281:29174–29180. doi: 10.1074/jbc.M603274200. [DOI] [PubMed] [Google Scholar]
- 6.Zeigerer A., McBrayer M. K., McGraw T. E. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell. 2004;15:4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koumanov F., Jin B., Yang J., Holman G. D. Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab. 2005;2:179–189. doi: 10.1016/j.cmet.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Bai L., Wang Y., Fan J., Chen Y., Ji W., Qu A., Xu P., James D. E., Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez E., McGraw T. E. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol. Biol. Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roach W. G., Chavez J. A., Mîinea C. P., Lienhard G. E. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase activating protein Tbc1d1. Biochem. J. 2007;403:353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer H. F., Witczak C. A., Fujii N., Jessen N., Taylor E. B., Arnolds D. E., Sakamoto K., Hirshman M. F., Goodyear L. J. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson H. K., Zierath J. R., Kane S., Krook A., Lienhard G. E., Wallberg-Henriksson H. Insulin-stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes. 2005;54:1692–1697. doi: 10.2337/diabetes.54.6.1692. [DOI] [PubMed] [Google Scholar]
- 13.Stone S., Abkevich V., Russell D. L., Riley R., Timms K., Tran T., Trem D., Frank D., Jammulapati S., Neff C. D., et al. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum. Mol. Genet. 2006;15:2709–2720. doi: 10.1093/hmg/ddl204. [DOI] [PubMed] [Google Scholar]
- 14.Lazar M. A. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]