Abstract
SFKs (Src family kinases) contribute importantly to platelet function in haemostasis. SFK activity is controlled by Csk (C-terminal Src kinase), which phosphorylates a C-terminal tyrosine residue on SFKs, resulting in inhibition of SFK activity. Csk is recruited to sites of SFK activity by tyrosine-phosphorylated Csk-binding proteins. Paxillin, a multidomain adaptor protein, has been shown to act as a Csk-binding protein and to inhibit Src activity during growth factor signalling. Human platelets express Hic-5, a member of the paxillin family; however, its ability to act as a Csk-binding protein has not been characterized. We sought to identify and characterize the ability of paxillin family members to act as Csk-binding proteins during platelet activation. We found that murine and human platelets differ in the complement of paxillin family members expressed. Human platelets express Hic-5, whereas murine platelets express paxillin and leupaxin in addition to Hic-5. In aggregating human platelets, Hic-5 was tyrosine phosphorylated and recruited Csk via its SH2 domains. In aggregating murine platelets, however, Csk bound preferentially to paxillin, even though both paxillin and Hic-5 were abundantly present and became tyrosine phosphorylated. The SFK Lyn, but not Src or Fyn, was associated with paxillin family members in resting and aggregated human and murine platelets. Lyn, however, was phosphorylated on its C-terminal inhibitory tyrosine residue only following platelet aggregation, which was coincident with recruitment of Csk to paxillin and/or Hic-5 in a manner dependent on prior αIIbβ3 engagement. These observations support the notion that Hic-5 and paxillin function as negative feedback regulators of SFKs in aggregated platelets and that, when both are present, paxillin is preferentially used.
Keywords: Csk (C-terminal Src kinase)-binding protein, platelet, Src family kinase (SFK)
Abbreviations: Chk, Csk homologous kinase; CRP, collagen-related peptide; Csk, C-terminal Src kinase; GPCR, G-protein-coupled receptor; GST, glutathione S-transferase; Hic-5, hydrogen peroxide-inducible clone-5; HRP, horseradish peroxidase; SH, Src homology; SHIP, SH2-containing inositol phosphatase-1; SFK, Src family kinase; TXA2, thromboxane A2; VWF, von Willebrand factor
INTRODUCTION
Normal haemostasis requires proper regulation of platelet activation and subsequent adhesion of platelets to each other and to the extracellular matrix; dysregulation of these events results in bleeding or predisposes for the development of thrombosis [1]. Platelets can be activated by several different classes of agonist receptor, including heterotrimeric GPCRs (G-protein-coupled receptors) for ADP, epinephrine, serotonin, thrombin and TXA2 (thromboxane A2), and receptors that are coupled to the ITAM (immunoreceptor tyrosine-based activation motif)-containing FcR γ-chain, which include the GPIb/V/IX receptor for VWF (von Willebrand factor), the GPVI collagen receptor, and the Fcγ receptor for IgG immune complexes [2–6]. Agonist-induced platelet activation results in activation of the platelet-specific integrin, αIIbβ3, which binds fibrinogen and VWF, and enables platelet aggregation [7]. SFKs (Src family kinases) have been shown to play important roles in platelet activation, aggregation and adhesion [8–11]. Platelets express six SFKs, including p60Src [12–15], p59Fyn [14–17], p56Lck [15,17], p62Yes [15,18], p55Fgr [15] and p53/56Lyn [16,17]. Among these, p59Fyn and p53/56Lyn are involved in signal transduction by the GPVI–FcR γ-chain collagen receptor complex [8], whereas p60Src plays an important role in αIIbβ3-mediated signal transduction [11].
SFKs are composed of an N-terminal SH (Src homology) 3 domain, an SH2 domain, a kinase (SH1) domain and a C-terminal regulatory domain [19]. In resting cells, SFKs are maintained in an inactive conformation via two intramolecular interactions that include binding of the SH3 domain to a polyproline type II helix [20], and binding of the SH2 domain to a phosphotyrosine residue in the C-terminal regulatory domain [21–24]. SFKs are primed by dephosphorylation of the C-terminal inhibitory phosphotyrosine residue, which is carried out by CD45 in most haematopoietic cells and by receptor protein tyrosine phosphatase α in platelets and non-haematopoietic cells [25,26]. Primed SFKs are fully activated by auto-phosphorylation (in trans) of a tyrosine residue located within the catalytic domain [26]. Maintenance of SFKs in, or their return to, an inactive state requires phosphorylation of the C-terminal inhibitory tyrosine residue in the SFK by Csk (C-terminal Src kinase), or the related kinase Chk (Csk homologous kinase), causing the SFK to fold back up on itself in an inactive conformation [27,28]. Csk, which is normally present in the cytosol of resting cells, is brought into proximity with the SFKs it regulates via binding of its SH2 domain to a phosphotyrosine residue in a Csk-binding protein.
The identities of Csk-binding proteins and their roles in regulating SFK activity in platelets have not been characterized completely. Previous studies in other cell systems have shown that the adapter protein, paxillin, can participate in feedback inhibition of nearby SFKs by recruiting Csk [29–34]. Paxillin is a member of a family of proteins that includes three alternatively spliced forms of paxillin (paxillin α, β and γ), Hic-5 (hydrogen peroxide-inducible clone-5) and leupaxin [35,36]. Interestingly, whereas human megakaryocytes express paxillin but not other members of the paxillin family, mature human platelets express only Hic-5 [37,38]. Previous studies have shown that tyrosine phosphorylation of Hic-5 supports the binding of the SH2 domain of Csk [38,39] and that Hic-5 becomes tyrosine phosphorylated in activated human platelets [38]; nevertheless, the extent to which tyrosine phosphorylated Hic-5 binds Csk and regulates SFK activity in platelets is not known. Consequently, in the present study, we sought to characterize the conditions under which Hic-5 becomes tyrosine phosphorylated and recruits Csk in human platelets, to identify the SFKs that are regulated by Hic-5–Csk complexes and to elucidate the extent to which similar events regulate SFK activity in murine platelets. Our results show that Hic-5 in human platelets, and paxillin in murine platelets, function as Csk-binding proteins that regulate the activity of Lyn upon induction of platelet aggregation.
MATERIALS AND METHODS
Materials
All chemicals were obtained from Sigma unless indicated otherwise. CRP (collagen-related peptide) and RGDW peptide were synthesized in the Protein Chemistry Laboratory of the Blood Research Institute. The antibodies used for immunoprecipitation and Western blotting experiments were as follows: mouse anti-chicken paxillin (clone 349; BD Biosciences), rabbit anti-(phospho-Lyn-Tyr507) (Cell Signaling Technology), rabbit anti-Csk (C-20), rabbit anti-Hic-5 (H75), rabbit anti-Src (N-16), rabbit anti-Fyn (Fyn-3) and rabbit anti-Lyn (44) from Santa Cruz Biotechnology. HRP (horseradish peroxidase)-conjugated mouse anti-phosphotyrosine (clone PY20) antibody was purchased from Zymed Laboratories. HRP-conjugated, whole IgG and Fc-specific donkey anti-mouse and anti-rabbit secondary antibodies were purchased from Jackson Immunoresearch Laboratories.
Preparation of platelets
Washed human platelets from healthy volunteers and murine platelets from C57/Bl6 mice were obtained and prepared essentially as described previously [40]. White blood cell contamination in washed platelet preparations was undetectable using an automated cell counter, which can detect a single white blood cell per 104 cells/ml.
Stimulation of platelets under stirring conditions
Washed human and murine platelets were resuspended at a concentration of 5×108/ml in Tyrodes buffer [20 mM Hepes, pH 7.4, 137 mM NaCl, 2.5 mM KCl, 13.8 mM NaHCO3, 0.36 mM NaH2PO4, 5.5 mM glucose and 0.25% BSA (ICN Biomedicals)]. Platelet suspensions (500 μl) were stimulated with 1 μg/ml of CRP (for human platelets) and 0.25 μg/ml of CRP (for murine platelets) for the indicated times under stirring conditions at 37 °C in the presence of 1 mM CaCl2. Platelets stirred under the same conditions without any agonist served as a control. RGDW peptide (2 mM for human platelets and 4 mM for murine platelets) and 5 mM EDTA were included to block binding of released fibrinogen to the platelet-specific integrin, αIIbβ3, on CRP-stimulated platelets. Reactions were stopped by adding an equal volume of 2× lysis buffer [100 mM Tris/HCl, pH 7.6, 300 mM NaCl, 2% (v/v) Triton X-100] containing 4× protease- and phosphatase-inhibitor cocktail sets I and II respectively (EMD Biosciences).
Immunoprecipitation and Western blotting
Immunoprecipitation, Western blotting and detection of proteins were performed essentially as described previously [40]. Briefly, immunoprecipitated proteins were resolved by SDS/PAGE (7.5% gels) under reducing conditions, electroblotted on to PVDF membranes (Millipore) and detected by Western blotting using Super-signal West Pico chemiluminescent reagent (Pierce). For re-probing, the membranes were stripped by prewarmed (56 °C) 200 mM glycine/HCl buffer (pH 3.0) for four 15 min washes, and then rinsed three times for 1 min in deionized water before re-blocking and re-probing with the indicated antibodies.
GST (glutathione S-transferase)–Csk-SH2 pull-down assay
Creation of the GST fusion protein containing the SH2 domain of Csk (GST–Csk-SH2), which was used to pull down and identify Csk-binding proteins in cells, has been described elsewhere [41,42]. In the present study, platelet lysates were prepared as described above and incubated with 30 μg of GST protein for 2 h at 4 °C, followed by two incubations for 1 h each with 50 μl of a 50% slurry of glutathione–Sepharose beads (Amersham) to remove GST and any platelet proteins that might adhere to GST. Precleared lysates were incubated on a rocker with 30 μg of GST–Csk-SH2 protein overnight at 4 °C, after which 50 μl of a 50% slurry of glutathione–Sepharose beads was added and the suspensions were allowed to rotate for a further 1 h at 4 °C. The beads were washed three times with 1× lysis buffer and resuspended in SDS/PAGE sample buffer, before being resolved by SDS/PAGE (7.5% gels) under reducing conditions and then electroblotted on to PVDF membranes. The membranes were blocked overnight with 3% (w/v) BSA in Tris-buffered saline containing 0.1% Tween-20 and probed with anti-phosphotyrosine–HRP (PY20), anti-paxillin or anti-Hic-5 antibody and the binding was detected by chemiluminescence as described above. The molecular masses of the tyrosine-phosphorylated proteins detected in the PY20 immunoblot were estimated using Kodak 1D imaging software (Scientific Imaging Systems).
RESULTS
Hic-5 is the only paxillin family member in human platelets, whereas murine platelets express both Hic-5 and paxillin
Hic-5 has been reported to be the only member of the paxillin family that is expressed in mature human platelets [37,38]. Murine platelets are often used to provide mechanistic insights into signal transduction pathways that operate during adhesion, secretion, aggregation and formation of thrombi by human platelets. Therefore we sought to determine whether murine platelets express the same complement of paxillin family members as human platelets. Western blot analysis of human and murine platelet lysates using an antibody specific for human Hic-5 (Figure 1A) confirmed the presence in human platelet lysates of a single band with an apparent molecular mass of 53 kDa, which is the expected molecular mass of Hic-5. This band was not detected in murine platelets, indicating either that the anti-(human Hic-5) antibody does not cross-react with murine Hic-5 or that Hic-5 is not expressed in murine platelets. Western blot analysis of human and murine platelet lysates using an anti-paxillin antibody revealed the presence of a single band that migrated with an apparent molecular mass of 53 kDa in human platelets, and of three bands that migrated with apparent molecular masses of 68, 53 and 45 kDa in murine platelets (Figure 1B), which are the predicted molecular masses of paxillin, Hic-5 and leupaxin respectively. These results demonstrate that murine and human platelets express a different complement of paxillin family members. Specifically, murine platelets express three members of the paxillin family, including paxillin, Hic-5 and leupaxin, whereas human platelets express only Hic-5.
Figure 1. Murine and human platelets express different members of the paxillin family.
(A) Hic-5 in human platelet lysates was detected by immunoblotting (IB) with an anti-human Hic-5 antibody that does not cross-react with Hic-5 in murine platelet lysates. (B) Hic-5 (55 kDa) in human platelet lysates, and paxillin (68 kDa), Hic-5 (53 kDa) and leupaxin (45 kDa) in murine platelet lysates, were detected with an anti-paxillin antibody that cross-reacts with Hic-5 and leupaxin.
Hic-5 in human platelets and paxillin, predominantly, in murine platelets function as aggregation-dependent Csk-binding proteins
After confirming the results of a previous report [38] that Hic-5 becomes tyrosine phosphorylated in human platelets activated by CRP (Figure 2A), which activates platelets specifically via the GPVI–FcRγ-chain collagen receptor complex, we sought to determine which of the complement of paxillin family members expressed in murine platelets become tyrosine phosphorylated upon activation. As shown in Figure 2(B), both paxillin and Hic-5, but not leupaxin, became tyrosine phosphorylated following CRP-induced platelet aggregation.
Figure 2. Paxillin family members in platelets were tyrosine phosphorylated in aggregated platelets.
(A) Hic-5 was tyrosine phosphorylated in aggregated human platelets. (B) Paxillin and Hic-5, but not leupaxin, become tyrosine phosphorylated in aggregated murine platelets. Washed human and murine platelets were stirred without or with CRP for 3 min. Lysates were prepared and subjected to immunoprecipitation with an antibody raised against paxillin that also cross reacts well with Hic-5 and leupaxin. As a control, lysates from platelets stirred without CRP were subjected to immunoprecipitation with normal mouse immnunoglobulin (NMIg). Immunoprecipitates (IP) were immunoblotted (IB) with a phosphotyrosine-specific antibody (PY) as described in the Materials and methods section.
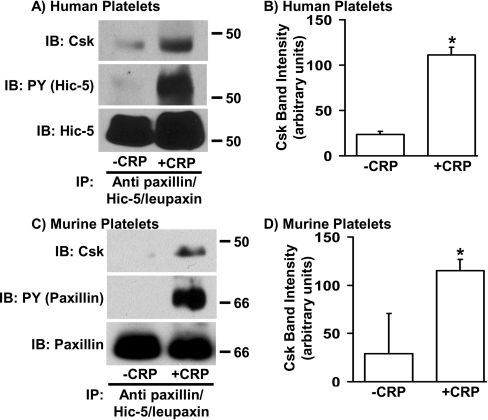
Previous studies in cells other than platelets have shown that tyrosine phosphorylation of paxillin [29–34] and Hic-5 [39,43] supports binding of Csk via its SH2 domain. To determine the ability of paxillin and/or Hic-5 to bind to Csk in platelets, the proteins were immunoprecipitated from resting and CRP-aggregated human and murine platelets. The presence of co-immunoprecipitated Csk and the tyrosine phosphorylation state of the immunoprecipitated paxillin family members were assessed by Western blot analysis using anti-Csk- and anti-phosphotyrosine-specific antibodies respectively. As shown in Figure 3, increased amounts of Csk co-immunoprecipitated with Hic-5 from aggregated compared with the resting human platelets, which was coincident with increased Hic-5 tyrosine phosphorylation (Figures 3A and 3B). Similarly, increased amounts of Csk co-precipitated with paxillin/Hic-5 from aggregated compared with resting murine platelets, consistent with increased levels of paxillin tyrosine phosphorylation (Figures 3C and 3D). These results indicate that Hic-5 in human platelets, and either paxillin or Hic-5 in murine platelets, function as Csk-binding proteins during platelet aggregation.
Figure 3. Csk co-immunoprecipitates with tyrosine-phosphorylated Hic-5 from CRP-stimulated human platelet lysates and with tyrosine-phosphorylated paxillin/Hic-5 from murine platelet lysates.
Washed human (A and B) and murine (C and D) platelets were stirred without or with CRP for 3 min. Lysates were prepared and subjected to immunoprecipitation (IP) with an anti-paxillin antibody that cross-reacts with Hic-5 and leupaxin. Immunoprecipitates were immunoblotted (IB) with anti-Csk (A and C, top panels), anti-phosphotyrosine (PY; A and C, middle panels) and either anti-Hic-5 (A, bottom panel) or anti-paxillin/Hic-5/leupaxin (C, bottom panel). The amount of Csk that co-immunoprecipitated with paxillin and/or Hic-5 was determined by densitometry and is reported as the mean band intensity±S.D. observed in three and two independent experiments performed from human (B) and murine (D) platelets respectively. *, A paired t test was used to determine statistical significance. Note that CRP-induced aggregation significantly enhanced association of Csk with Hic-5 in human platelets (P<0.03) and with paxillin/Hic-5 in murine platelets (P<0.04).
Because the anti-paxillin antibody used for these studies recognizes murine paxillin and Hic-5 equally well in murine platelets, and because the human Hic-5-specific antibody fails to recognize murine Hic-5, it was not possible to use immunoprecipitation to determine the extent to which Csk binds to paxillin compared with Hic-5 in murine platelets. Therefore, we made use of a GST fusion protein containing the SH2 domain of Csk (GST–Csk-SH2) [41,42] to pull down Csk-binding proteins from lysates of resting and CRP-aggregated human and murine platelets. Paxillin family members that co-precipitated with the GST–Csk-SH2 fusion protein were identified by Western blot analysis using the anti-paxillin antibody that cross-reacts with human and murine Hic-5 and leupaxin. As shown in Figure 4(A), GST–Csk-SH2 pulled down Hic-5 from lysates of CRP-aggregated, but not resting, human platelets. In contrast, although paxillin and Hic-5 were both abundantly expressed (Figure 4B, right-hand lane), the GST–Csk-SH2 domain construct strongly precipitated paxillin, but only weakly precipitated Hic-5, from lysates of aggregated murine platelets (Figure 4B, middle lane). These results suggest that Hic-5 functions as a Csk-binding protein in aggregated human platelets, where it is the only member of the paxillin family member present, but that Csk binds preferentially to paxillin in aggregated murine platelets, which contain both paxillin and Hic-5.
Figure 4. Hic-5 in human platelets and paxillin in murine platelets function as Csk-binding proteins.
(A) Hic-5 co-immunoprecipitates with the SH2-domain of Csk from CRP-aggregated but not resting human platelets. (B) Paxillin predominantly, and Hic-5 weakly, co-immunoprecipitate with the SH2-domain of Csk from CRP-aggregated but not resting murine platelets. Washed platelets were stirred without or with CRP for 3 min, after which platelet lysates were prepared. Lysates were precleared by incubating with GST conjugated to glutathione–Sepharose beads to remove non-specific binding proteins and the GST–Csk-SH2 pull-down was performed as described in the Materials and methods section. Paxillin family members associated with GST–Csk-SH2 fusion protein (left-hand panels) and those present in platelet lysates (right-hand panels) were analysed by immunoblotting (IB) with anti-human Hic-5 (A) or with an anti-paxillin antibody that cross-reacts with Hic-5 and leupaxin (B).
Lyn, constitutively associated with paxillin and Hic-5, is regulated by Csk recruitment
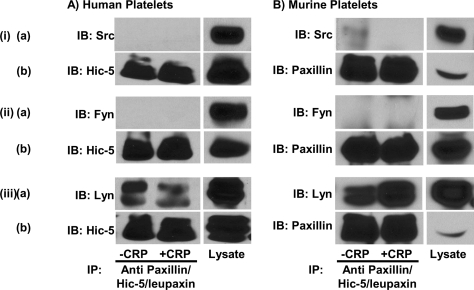
The SH3 domain of Src has been shown to interact constitutively with proline-rich regions of paxillin [44]. To determine whether any of the SFKs that are involved in signal transduction pathways that control platelet activation and aggregation are associated with Hic-5 in human platelets, or with paxillin and Hic-5 in murine platelets, Hic-5 and paxillin/Hic-5 were immunoprecipitated from resting or CRP-aggregated human and murine platelets respectively, and Western blot analysis of co-immunoprecipitated proteins was performed using specific antibodies against the SFKs: p60Src, p59Fyn and p53/56Lyn. As shown in Figure 5, all three of these SFKs were present in lysates of human (Figure 5A) and murine (Figure 5B) platelets. However, only p53/56Lyn co-immunoprecipitated with Hic-5 from human platelets (Figure 5A), and with paxillin and Hic-5 from murine platelets (Figure 5B), whereas Lyn failed to co-immunoprecipitate with a non-specific control antibody from the same lysates (results not shown). Interestingly, the interaction of Lyn with Hic-5 in human platelets and with paxillin/Hic-5 in murine platelets was not affected by the state of platelet activation.
Figure 5. SFK Lyn, but not Src or Fyn, is constitutively associated with paxillin family members in platelets.
Washed human (A) and murine (B) platelets were stirred without or with CRP for 3 min. Lysates were prepared and subjected to immunoprecipitation with an anti-paxillin antibody that cross-reacts with Hic-5 and leupaxin. Lysates and immunoprecipitates (IP) were immunoblotted (IB) with antibodies specific for p60Src (i, a), p59Fyn (ii, a) and p53/56Lyn (iii, a). Parallel immunoprecipitates were immunoblotted with anti-human Hic-5 (A: i,b; ii,b; and iii,b) or with an anti-paxillin antibody that cross-reacts with Hic-5 and leupaxin (B: i,b; ii,b; and iii,b) to show equal antigen loading.
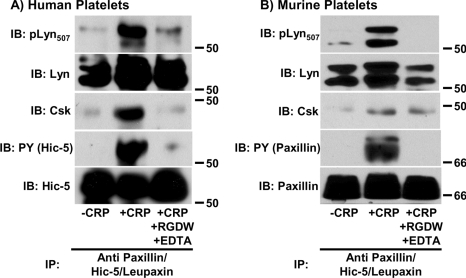
In other cell systems, recruitment of Csk to tyrosine-phosphorylated paxillin results in feedback inhibition of nearby SFKs via Csk-mediated phosphorylation of the SFK C-terminal inhibitory tyrosine residue [29–34]. To determine whether recruitment of Csk to tyrosine-phosphorylated paxillin and Hic-5 in aggregated murine and human platelets respectively, results in feedback inhibition of associated p53/56Lyn, Hic-5 or paxillin/Hic-5 immunoprecipitates from human and murine platelets were subjected to Western blot analysis using an antibody that specifically reacts with the phosphorylated form of the C-terminal inhibitory tyrosine residue (Tyr507) of p53/56Lyn (pLyn507). We found that phosphorylation of Tyr507 on paxillin/Hic-5-associated p53/56Lyn molecules coincided with recruitment of Csk to tyrosine phosphorylated Hic-5 in human platelets (Figure 6A) and paxillin in murine platelets (Figure 6B). These results demonstrate that Hic-5 in human platelets, and paxillin in murine platelets, function as Csk-binding proteins that negatively regulate that activity of the associated SFK, p53/56Lyn, during the platelet aggregation process. Finally, because previous studies [38] have shown that tyrosine phosphorylation of Hic-5 is dependent on activation of outside-in signalling by the platelet integrin αIIbβ3, we repeated these experiments in the presence of the RGDW peptide and the calcium chelator EDTA, which together effectively block binding of soluble fibrinogen to the αIIbβ3 complex. As shown in the right-hand lanes of Figures 6(A) and 6(B), both Csk recruitment to Hic-5 (in human platelets) or paxillin (in murine platelets) and Csk-mediated inactivation of Lyn require prior integrin engagement. These results demonstrate that the paxillin family members Hic-5 in human platelets and paxillin and Hic-5 in murine platelets become tyrosine phosphorylated and function as Csk-binding proteins that regulate the activity of Lyn in an aggregation-dependent manner. Additional studies performed using unstirred murine platelets stimulated with fibrinogen in the presence of MnCl2 to directly activate αIIbβ3 revealed that fibrinogen binding to activated αIIbβ3 does not induce tyrosine phosphorylation of Hic-5 in human platelets (Figure 7A), but is sufficient to induce paxillin and Hic-5 tyrosine phosphorylation and Csk association in murine platelets (Figures 7B and 7C). These findings indicate that the mechanisms by which paxillin family members become activated and function as Csk-binding proteins in human and murine platelets may have fundamental differences.
Figure 6. Csk-mediated inhibition of paxillin/Hic-5-associated Lyn requires αIIbβ3 engagement in human (A) and murine (B) platelets.
Platelets were stimulated with CRP in the presence (activated platelets) or absence (aggregated platelets) of RGDW and EDTA as described in the Materials and methods section. Lysates were prepared and immunoprecipitated with an anti-paxillin antibody that cross-reacts with Hic-5 and leupaxin. Immunoprecipitates (IP) were immunoblotted (IB) with antibodies specific for the phosphorylated form of the C-terminal inhibitory tyrosine residue of Lyn (pLyn507), Lyn, Csk, phosphotyrosine (PY) and either human Hic-5 (A) or paxillin/Hic-5/leupaxin (B).
Figure 7. Csk-binding functions of paxillin family members are differentially activated in human and murine platelets.
Binding of fibrinogen to activated αIIbβ3 does not induce tyrosine phosphorylation of, or recruitment of Csk to, Hic-5 in human platelets (A), but is sufficient to induce tyrosine phosphorylation of, and recruitment of Csk to, paxillin and Hic-5 in murine platelets (B) and (C). Platelets were incubated under resting conditions (R), in the presence of 250 μg/ml fibrinogen alone (F), 0.5 mM MnCl2 alone (M), or fibrinogen and MnCl2 together (F + M). The latter condition supports binding of added fibrinogen to αIIbβ3 integrins that are directly activated by MnCl2. Platelet lysates were prepared and subjected to immunoprecipitation (IP) with an anti-paxillin antibody that cross-reacts with Hic-5 and leupaxin. Immunoprecipitates were separated by SDS/PAGE and immunoblotted (IB) with antibodies specific for phosphotyrosine (PY), Csk and either human Hic-5 (A) or paxillin/Hic-5/leupaxin (B).
DISCUSSION
The haemostatic function of platelets requires the co-ordination and integration of multiple signalling pathways. Platelet responses are initiated by the integrin-independent binding of soluble and insoluble agonists to their receptors, and are amplified by subsequent integrin-dependent aggregation following binding of fibrinogen to activated αIIbβ3. SFKs play pivotal roles in signal transduction by certain agonist receptors, and in αIIbβ3-mediated signal transduction. Therefore the pathways that contribute to regulation of SFK activity determine the extent and outcome of platelet activation.
Csk is an important regulator of SFK activity [27]; however, the Csk-binding proteins that recruit Csk to sites of SFK activity in platelets have not yet been characterized. Whereas paxillin has been shown to function as an important Csk-binding protein that regulates SFK activity in other cells [29–34], the ability of the paxillin family member Hic-5, which substitutes for paxillin in human platelets [37,38], to function as a regulator of SFK activity has not been characterized previously. The purpose of the present study was therefore to identify paxillin family members in human and murine platelets and to characterize their potential to function as Csk-binding proteins that regulate SFK activity during platelet activation. We found that, whereas human platelets express only Hic-5, murine platelets express three members of the paxillin family, including paxillin, Hic-5 and leupaxin. We established that Hic-5 in human platelets, and both Hic-5 and paxillin in murine platelets, become tyrosine phosphorylated upon induction of platelet aggregation. However, whereas Hic-5 functions as a Csk-binding protein in human platelets, paxillin is the predominant Csk-binding protein in murine platelets. The SFK Lyn, but not Src or Fyn, is constitutively associated with these paxillin family members in resting and GPVI-activated human and murine platelets; however, phosphorylation of Hic-5/paxillin-associated Lyn on its C-terminal inhibitory tyrosine residue was observed only upon recruitment of Csk to tyrosine-phosphorylated paxillin family members, all of which required integrin engagement.
Our finding that murine platelets express paxillin, Hic-5 and leupaxin, whereas human platelets express only Hic-5, reveals a fundamental difference between the platelets of these often-studied species. Previous studies have shown that, in humans, megakaryocytes and megakaryocyte-derived cell lines express paxillin and trace amounts of Hic-5, but the pattern of expression of paxillin family members switches such that mature human platelets express only Hic-5 [37,38]. To our knowledge, ours is the first study to report that the switch from paxillin to Hic-5 does not occur in murine platelets. Furthermore, it has been shown previously that Hic-5 becomes tyrosine phosphorylated following stimulation of human platelets with PMA, GPCR agonists such as thrombin and TXA2 analogues, or the GPVI receptor agonist, collagen, and that RGD peptides, which block binding of fibrinogen to activated αIIbβ3, block GPCR-induced tyrosine phosphorylation of Hic-5 [38]. Our results expand upon this previous study by showing that phosphorylated Hic-5 is able to recruit Csk and support phosphorylation of Lyn on its inhibitory C-terminal tyrosine residue. Furthermore, we show that a different paxillin family member, paxillin itself, serves this function in murine platelets. The finding that Hic-5 is not as effective a Csk-binding protein as is paxillin in murine platelets, even though it is expressed as abundantly, suggests that paxillin and Hic-5 have different affinities for Csk. Finally, the finding that paxillin and Hic-5 in murine platelets became tyrosine phosphorylated and bound Csk upon direct binding of fibrinogen to activated αIIbβ3, whereas tyrosine phosphorylation of and Csk binding to Hic-5 in human platelets required platelet aggregation, indicates that aggregation-dependent, post-ligand-binding events [45] are required to activate the Csk-binding function of paxillin family members in human but not murine platelets, lending support to the notion that human and murine platelets might activate pathways to regulate SFKs in fundamentally different ways.
The SFKs associated with paxillin family members are in the best position to be regulated by Csk recruited to tyrosine phosphorylated paxillin and Hic-5. We found that, in human and murine platelets, Lyn, but not Src or Fyn, is associated with members of the paxillin family in a manner that is independent of their tyrosine phosphorylation state, suggesting that this interaction may be mediated by the SH3-domain of Lyn and a proline-rich region within these paxillin family members. The N-terminus of paxillin contains a proline-rich region that has characteristics of an SH3-binding motif [46], and Hic-5 also contains proline-rich segments, which may serve as potential SH3-domain-binding sites [36]. Paxillin has been shown to bind to a GST fusion protein containing the SH3 domain of c-Src [44] and to support constitutive binding of Src in a breast carcinoma cell line [47]. The present study is the first to show that the SFK associated with paxillin and Hic-5 in platelets is Lyn. We also observed that the fraction of Lyn that is constitutively associated with these paxillin family members in platelets becomes phosphorylated on its C-terminal inhibitory tyrosine residue, coincident with Hic-5 and paxillin tyrosine phosphorylation and Csk recruitment. Nevertheless, the effect of Csk-mediated inhibition of Hic-5/paxillin-associated Lyn has yet to be determined. Platelets express several SFK members, and it has been proposed that certain SFKs regulate different aspects of platelet signalling [16]. The role played by Lyn in platelet activation is particularly complex, since Lyn deficiency was found to initially delay signalling, but subsequently potentiate responses such as spreading and aggregation, in platelets activated via GPVI, suggesting that Lyn both facilitates GPVI signal transduction and participates in a novel inhibitory pathway in platelets [10]. In support of a negative regulatory role for Lyn in platelets, Maxwell et al. [48], have shown previously that platelets deficient in either Lyn or the inositol phosphatase, SHIP (SH2-containing inositol phosphatase-1), exhibit enhanced spreading on fibrinogen and calcium mobilization, suggesting that both Lyn and SHIP act as negative regulators of αIIbβ3-mediated outside-in signalling. Because of the pleiomorphic effects of Lyn deficiency, examining the function of platelets from genetically engineered mice in which the ability of Lyn to associate with paxillin family members has been impaired will be required to determine the specific function mediated by paxillin/Hic-5-associated Lyn.
In conclusion, the results of the present study suggest a model in which integrin engagement-dependent Hic-5 tyrosine phosphorylation in human platelets and paxillin tyrosine phosphorylation in murine platelets contributes to negative regulation of Lyn activity (Scheme 1). Platelet aggregation results in tyrosine phosphorylation of Hic-5 and paxillin, with which Lyn is constitutively associated via its SH3 domain (Scheme 1A). Tyrosine-phosphorylated Hic-5 and paxillin provide docking sites for the SH2 domain of Csk, resulting in the juxtaposition of the kinase domain of Csk with the C-terminal inhibitory tyrosine residue (Tyr507) of paxillin- or Hic-5-associated Lyn (Scheme 1B). Csk phosphorylates Tyr507 of Lyn, enabling this phosphotyrosine residue (pTyr507) to engage in an intramolecular interaction with the SH2 domain of Lyn that inhibits Lyn activity (Scheme 1C). The specific SFK-dependent pathways in platelets, e.g. those involved in signalling by the GPVI collagen receptor, the GPIb/V/IX receptor for VWF, or integrins, that are modulated by Csk binding to paxillin family members, and the extent to which other Csk binding proteins regulate these pathways, remain the subjects of interesting and important future investigations.
Scheme 1. A model of regulation of Lyn activity by Csk binding to members of the paxillin family in aggregated human and murine platelets.
(A) Lyn, via its SH3 domain, is bound constitutively to the polyproline region of paxillin or Hic-5, both of which are unphosphorylated in resting platelets. (B) Paxillin and Hic-5 become tyrosine phosphorylated during platelet aggregation, leading to recruitment of Csk from the cytosol and juxtaposition of the kinase domain of Csk with the C-terminal inhibitory tyrosine (Y507) of Lyn. (C) Csk phosphorylates of Tyr507 of Lyn resulting in an intramolecular interaction between phospho-Tyr507 and the SH2 domain of Lyn that inhibits Lyn activity.
Acknowledgments
The present study was supported by grants from the National Institutes of Health (#P01 HL44 and #R01 HL90926) awarded to P. J. N. and D. K. N.
References
- 1.Wagner D. D., Burger P. C. Platelets in inflammation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2003;23:2131–2137. doi: 10.1161/01.ATV.0000095974.95122.EC. [DOI] [PubMed] [Google Scholar]
- 2.Murugappan S., Shankar H., Kunapuli S. P. Platelet receptors for adenine nucleotides and thromboxane A2. Semin. Thromb. Hemost. 2004;30:411–418. doi: 10.1055/s-2004-833476. [DOI] [PubMed] [Google Scholar]
- 3.Gibbins J. M. Platelet adhesion signalling and the regulation of thrombus formation. J. Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 4.Andrews R. K., Berndt M. C. Platelet physiology and thrombosis. Thromb. Res. 2004;114:447–453. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Kahn M. L. Platelet-collagen responses: molecular basis and therapeutic promise. Semin. Thromb. Hemost. 2004;30:419–425. doi: 10.1055/s-2004-833477. [DOI] [PubMed] [Google Scholar]
- 6.Nieswandt B., Watson S. P. Platelet collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 7.Shattil S. J., Newman P. J. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 8.Ezumi Y., Shindoh K., Tsuji M., Takayama H. Physical and functional association of the Src family kinases Fyn and Lyn with the collagen receptor glycoprotein VI-Fc receptor γ chain complex on human platelets. J. Exp. Med. 1998;188:267–276. doi: 10.1084/jem.188.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross B. S., Lee J. R., Clements J. L., Turner M., Tybulewicz V. L., Findell P. R., Koretzky G. A., Watson S. P. Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor in platelets. J. Biol. Chem. 1999;274:5963–5971. doi: 10.1074/jbc.274.9.5963. [DOI] [PubMed] [Google Scholar]
- 10.Quek L. S., Pasquet J. M., Hers I., Cornall R., Knight G., Barnes M., Hibbs M. L., Dunn A. R., Lowell C. A., Watson S. P. Fyn and Lyn phosphorylate the Fc receptor γ chain downstream of glycoprotein VI in murine platelets, and Lyn regulates a novel feedback pathway. Blood. 2000;96:4246–4253. [PubMed] [Google Scholar]
- 11.Obergfell A., Eto K., Mocsai A., Buensuceso C., Moores S. L., Brugge J. S., Lowell C. A., Shattil S. J. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J. Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc. Natl. Acad. Sci. U.S.A. 1986;83:852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rendu F., Lebret M., Danielian S., Fagard R., Levy-Toledano S., Fischer S. High pp60c-src level in human platelet dense bodies. Blood. 1989;73:1545–1551. [PubMed] [Google Scholar]
- 14.Horak I. D., Corcoran M. L., Thompson P. A., Wahl L. M., Bolen J. B. Expression of p60fyn in human platelets. Oncogene. 1990;5:597–602. [PubMed] [Google Scholar]
- 15.Pestina T. I., Stenberg P. E., Druker B. J., Steward S. A., Hutson N. K., Barrie R. J., Jackson C. W. Identification of the Src family kinases, Lck and Fgr in platelets: their tyrosine phosphorylation status and subcellular distribution compared with other Src family members. Arterioscler. Thromb. Vasc. Biol. 1997;17:3278–3285. doi: 10.1161/01.atv.17.11.3278. [DOI] [PubMed] [Google Scholar]
- 16.Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenberg P. E., Pestina T. I., Barrie R. J., Jackson C. W. The Src family kinases, Fgr, Fyn, Lck, and Lyn, co-localize with coated membranes in platelets. Blood. 1997;89:2384–2393. [PubMed] [Google Scholar]
- 18.Zhao Y. H., Krueger J. G., Sudol M. Expression of cellular yes protein in mammalian tissues. Oncogene. 1990;5:1629–1635. [PubMed] [Google Scholar]
- 19.Xu W., Harrison S. C., Eck M. J. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 20.Williams J. C., Weijland A., Gonfloni S., Thompson A., Courtneidge S. A., Superti-Furga G., Wierenga R. K. The 2.35 Å crystal structure of the inactivated form of chicken Src: a dynamic molecule with multiple regulatory interactions. J. Mol. Biol. 1997;274:757–775. doi: 10.1006/jmbi.1997.1426. [DOI] [PubMed] [Google Scholar]
- 21.Piwnica-Worms H., Saunders K. B., Roberts T. M., Smith A. E., Cheng S. H. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987;49:75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- 22.Cartwright C. A., Eckhart W., Simon S., Kaplan P. L. Cell transformation by pp60c-src mutated in the carboxy-terminal regulatory domain. Cell. 1987;49:83–91. doi: 10.1016/0092-8674(87)90758-6. [DOI] [PubMed] [Google Scholar]
- 23.Irby R. B., Mao W., Coppola D., Kang J., Loubeau J. M., Trudeau W., Karl R., Fujita D. J., Jove R., Yeatman T. J. Activating SRC mutation in a subset of advanced human colon cancers. Nat. Genet. 1999;21:187–190. doi: 10.1038/5971. [DOI] [PubMed] [Google Scholar]
- 24.Thomas S. M., Brugge J. S. Cellular functions regulated by Src family kinases. Annu. Rev. Cell Dev. Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 25.Ponniah S., Wang D. Z., Lim K. L., Pallen C. J. Targeted disruption of the tyrosine phosphatase PTPα leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- 26.Su J., Muranjan M., Sap J. Receptor protein tyrosine phosphatase α activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- 27.Okada M., Nada S., Yamanashi Y., Yamamoto T., Nakagawa H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J. Biol. Chem. 1991;266:24249–24252. [PubMed] [Google Scholar]
- 28.Hirao A., Hamaguchi I., Suda T., Yamaguchi N. Translocation of the Csk homologous kinase (Chk/Hyl) controls activity of CD36-anchored Lyn tyrosine kinase in thrombin-stimulated platelets. EMBO J. 1997;16:2342–2351. doi: 10.1093/emboj/16.9.2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabe H., Hata A., Okada M., Nakagawa H., Hanafusa H. Analysis of the binding of the Src homology 2 domain of Csk to tyrosine-phosphorylated proteins in the suppression and mitotic activation of c-Src. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3984–3988. doi: 10.1073/pnas.91.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergman M., Joukov V., Virtanen I., Alitalo K. Overexpressed Csk tyrosine kinase is localized in focal adhesions, causes reorganization of αvβ5 integrin, and interferes with HeLa cell spreading. Mol. Cell Biol. 1995;15:711–722. doi: 10.1128/mcb.15.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaller M. D., Parsons J. T. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for Crk. Mol. Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabe H., Shoelson S. E., Hanafusa H. Possible v-Crk-induced transformation through activation of Src kinases. J. Biol. Chem. 1995;270:31219–31224. doi: 10.1074/jbc.270.52.31219. [DOI] [PubMed] [Google Scholar]
- 33.Cao H., Sanguinetti A. R., Mastick C. C. Oxidative stress activates both Src-kinases and their negative regulator Csk and induces phosphorylation of two targeting proteins for Csk: caveolin-1 and paxillin. Exp. Cell Res. 2004;294:159–171. doi: 10.1016/j.yexcr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Radel C., Rizzo V. Integrin mechanotransduction stimulates caveolin-1 phosphorylation and recruitment of Csk to mediate actin reorganization. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H936–H945. doi: 10.1152/ajpheart.00519.2004. [DOI] [PubMed] [Google Scholar]
- 35.Schaller M. D. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–6472. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- 36.Brown M. C., Turner C. E. Paxillin: adapting to change. Physiol. Rev. 2004;84:1315–1339. doi: 10.1152/physrev.00002.2004. [DOI] [PubMed] [Google Scholar]
- 37.Hagmann J., Grob M., Welman A., van Willigen G., Burger M. M. Recruitment of the LIM protein hic-5 to focal contacts of human platelets. J. Cell Sci. 1998;111:2181–2188. doi: 10.1242/jcs.111.15.2181. [DOI] [PubMed] [Google Scholar]
- 38.Osada M., Ohmori T., Yatomi Y., Satoh K., Hosogaya S., Ozaki Y. Involvement of Hic-5 in platelet activation: integrin αIIbβ3-dependent tyrosine phosphorylation and association with proline-rich tyrosine kinase 2. Biochem. J. 2001;355:691–697. doi: 10.1042/bj3550691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishino M., Aoto H., Sasaki H., Suzuki R., Sasaki T. Phosphorylation of Hic-5 at tyrosine 60 by CAKβ and Fyn. FEBS Lett. 2000;474:179–183. doi: 10.1016/s0014-5793(00)01597-0. [DOI] [PubMed] [Google Scholar]
- 40.Rathore V., Stapleton M. A., Hillery C. A., Montgomery R. R., Nichols T. C., Merricks E. P., Newman D. K., Newman P. J. PECAM-1 negatively regulates GPIb/V/IX signaling in murine platelets. Blood. 2003;102:3658–3664. doi: 10.1182/blood-2003-06-1888. [DOI] [PubMed] [Google Scholar]
- 41.Kawabuchi M., Satomi Y., Takao T., Shimonishi Y., Nada S., Nagai K., Tarakhovsky A., Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi S., Takayama Y., Ogawa A., Tamura K., Okada M. Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J. Biol. Chem. 2000;275:29183–29186. doi: 10.1074/jbc.C000326200. [DOI] [PubMed] [Google Scholar]
- 43.Thomas S. M., Hagel M., Turner C. E. Characterization of a focal adhesion protein, Hic-5, that shares extensive homology with paxillin. J. Cell Sci. 1999;112:181–190. doi: 10.1242/jcs.112.2.181. [DOI] [PubMed] [Google Scholar]
- 44.Weng Z., Taylor J. A., Turner C. E., Brugge J. S., Seidel-Dugan C. Detection of Src homology 3-binding proteins, including paxillin, in normal and v-Src-transformed Balb/c 3T3 cells. J. Biol. Chem. 1993;268:14956–14963. [PubMed] [Google Scholar]
- 45.Brass L. F., Zhu L., Stalker T. J. Minding the gaps to promote thrombus growth and stability. J. Clin. Invest. 2005;115:3385–3392. doi: 10.1172/JCI26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner C. E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 47.Ren Y., Meng S., Mei L., Zhao Z. J., Jove R., Wu J. Roles of Gab1 and SHP2 in paxillin tyrosine dephosphorylation and Src activation in response to epidermal growth factor. J. Biol. Chem. 2004;279:8497–8505. doi: 10.1074/jbc.M312575200. [DOI] [PubMed] [Google Scholar]
- 48.Maxwell M. J., Yuan Y., Anderson K. E., Hibbs M. L., Salem H. H., Jackson S. P. SHIP1 and Lyn kinase negatively regulate integrin αIIbβ3 signaling in platelets. J. Biol. Chem. 2004;279:32196–32204. doi: 10.1074/jbc.M400746200. [DOI] [PubMed] [Google Scholar]