Abstract
Aims
To investigate the effects of age and renal and hepatic impairment on the pharmacokinetics, tolerability and safety of sildenafil (single 50-mg oral dose) and its major circulating N-desmethyl metabolite, UK-103,320.
Methods
Three open-label, parallel-group studies were conducted. The first study compared sildenafil pharmacokinetics, safety and toleration in 15 healthy young male subjects (mean age 30 years; range 19–45 years) to 15 healthy elderly male subjects (mean age 70 years; range 65–81 years). The second study included eight male volunteers with normal renal function and 16 male volunteers with varying degrees of renal impairment as assessed by measurement of creatinine clearance (CLcr). The third study included 12 male volunteers with normal hepatic function and 12 male volunteers with chronic stable hepatic cirrhosis (Child-Pugh A and B). For all three studies, blood and urine samples were collected predose and at specified intervals up to 48 h postdose for assays of sildenafil and UK-103,320, and measurements of protein binding.
Results
Significant differences in Cmax and AUC were observed between the young and the elderly subjects for both the parent drug and the metabolite. In the elderly, AUC values were approximately twice as high and Cmax values 60–70% higher than those for young men, while t1/2 values were approximately 1 h longer for sildenafil and 2 h longer for UK-103,320. Due to a significantly smaller unbound fraction of drug in the elderly, free drug concentrations were only approximately 40% higher in the elderly group compared to the young group. In the renal impairment study, significant correlations with CLcr were demonstrated for sildenafil oral clearance (CL/F) and Cmax and UK-103,320 Cmax and AUC. Pairwise comparisons between subjects with normal renal function and those with severe renal impairment (CLcr<30 ml min−1) supported these findings, showing significant increases in Cmax and AUC for both the parent drug and the metabolite in the severely impaired subjects. The hepatic impairment study demonstrated that the pharmacokinetics of sildenafil were altered in subjects with chronic stable cirrhosis, as shown by a 46% reduction in CL/F and a 47% increase in Cmax compared with subjects with normal hepatic function, suggesting a reduction in first-pass metabolism as well as systemic clearance. The increase in systemic exposure for UK-103,320 was approximately twice that seen for the parent drug. In all three studies, sildenafil was well tolerated, most adverse events were mild and no subjects discontinued treatment.
Conclusions
Sildenafil pharmacokinetics were affected by age and by renal and hepatic impairment, suggesting that a lower starting dose of 25 mg should be considered for patients with severely compromised renal or hepatic function.
Keywords: sildenafil; UK-103,320; pharmacokinetics; elderly; renal impairment; hepatic impairment; safety
Introduction
Sildenafil citrate (Viagra®, Pfizer) is an orally active inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE 5) [1–3], and has been shown to be an effective treatment for male erectile dysfunction [1, 4]. When administered orally to healthy volunteers as single doses of 1.25–200 mg, sildenafil is rapidly absorbed and metabolized, with a maximum plasma concentration achieved within 1 h and a terminal half-life of between 3 and 6 h [5, 6]. Its absolute oral bioavailability is approximately 40% [5, 6]. Sildenafil is cleared almost exclusively by hepatic metabolism [7]. The primary metabolite of sildenafil is the N-desmethyl metabolite, UK-103,320, which has a similar PDE specificity profile, but is approximately half as potent as the parent drug [5]. Its plasma concentrations reach about 40–50% of those of the parent drug following oral dosing [5, 6].
Early pharmacokinetic studies were conducted in healthy young volunteers, in whom the drug was generally well tolerated, with most adverse effects reported as mild, ranging from headaches and flushing to transient visual disturbances [1, 5, 6]. However, since erectile dysfunction is common among older men, and is strongly associated with other chronic disease states [8, 9], it is of particular importance to investigate the effects of age and hepatic and renal impairment on sildenafil pharmacokinetics, and to further assess the safety and tolerability profile of the drug in these populations. Since age, hepatic and renal impairment may be associated with reductions in drug clearance, dose adjustments may be necessary in these patient populations.
Ageing is associated with reductions in hepatic and renal function and alterations in plasma protein concentrations [10–12]. Reductions in hepatic blood flow and a decline in the activity of hepatic microsomal P450 enzymes may contribute to reduced clearance of hepatically metabolized drugs in the elderly [10], while the characteristic age-associated decline in renal function may reduce the clearance of drugs that are metabolized/excreted by the kidney [11]. In addition, serum concentrations of albumin decrease (∼0.5 g l−1 per decade) and α1-acid glycoprotein concentrations may increase slightly with age; however, age-related effects on protein binding are generally not believed to be clinically significant [10].
Although renal metabolism and excretion are not believed to contribute substantially to sildenafil clearance, secondary effects of renal impairment may affect the clearance of sildenafil. These include alterations in protein binding subsequent to renal albumin loss or increased synthesis of inflammatory proteins such as α1-acid glycoprotein and inhibition of hepatic metabolizing enzymes by endogenous substances that accumulate in the blood of patients with renal dysfunction [12]. For extensively bound drugs that are acidic, an increase in the unbound fraction may be expected, while for those that are basic in nature, a decrease in the unbound fraction may be predicted. Since sildenafil is highly protein bound (96%), but weakly basic (pKa = 6.5) [7], it is uncertain whether protein binding would be substantially altered in patients with impaired renal function. However, significant inhibition of hepatic microsomal enzyme activity secondary to renal impairment might be expected to produce elevated sildenafil plasma concentrations as a result of the reduction in sildenafil clearance.
Proposed mechanisms to explain the effect of chronic liver disease on drug metabolism include a reduction in liver size with a corresponding reduction in hepatic blood flow, a global decline in hepatocyte function, impaired uptake of drugs and a selective loss of phase I metabolism (oxidation, reduction, hydrolysis and demethylation) as a result of impaired diffusion of oxygen [10]. Since the five principal pathways of sildenafil metabolism require demethylation, oxidation and hydroxylation [7], an effect of hepatic impairment on sildenafil clearance appears likely; however, evaluation of sildenafil pharmacokinetics in patients with hepatic impairment should provide specific information on the magnitude of the effect and whether dose adjustments are advisable.
We report findings from three open-label, parallel-group single-dose trials that specifically investigated the pharmacokinetics, safety and tolerability of sildenafil 50 mg in elderly men (≥65 years of age), those with renal impairment (creatinine clearance [CLcr] ≤80 ml min−1) and those with hepatic dysfunction (chronic stable cirrhosis of Child-Pugh classification A or B).
Methods
Subjects
Healthy men between the ages of 18 and 45 years (young group) or ≥65 years (elderly group) were eligible for inclusion in the effect of age study, provided that they were within 10% (young subjects) or 20% (elderly subjects) of the ideal weight for age and height. Men with normal renal function (CLcr >80 ml min−1) and those with mild (CLcr = 50–80 ml min−1), moderate (CLcr = 30–49 ml min−1) or severe (CLcr<30 ml min−1) renal impairment were eligible for inclusion in the second study. They were required to be 18–70 years of age and to be within 30% of the ideal weight for height and frame size. Subjects with renal dysfunction due to insulin-dependent diabetes mellitus or nephrotic syndrome were excluded, and those with severe renal dysfunction were not permitted to be on dialysis. Recipients of renal transplants were also not eligible for inclusion. Concurrent use of chronically administered drugs was permitted during the trial for subjects with renal dysfunction. For the hepatic impairment study, men with normal hepatic function and those with biopsy-proven chronic stable hepatic cirrhosis (Child-Pugh classification A or B) were eligible for inclusion. Candidates were excluded from this study if they had undergone portacaval shunt surgery, if they had signs of hepatic encephalopathy more than grade II portal systemic encephalopathy (PSE) score, if they had coronary artery disease or if they were currently taking neuroleptic or antidepressant drugs.
Consensus exclusion factors for all three studies included evidence of any clinically significant disease or laboratory test abnormality, any evidence of drug or alcohol dependence or cigarette smoking. Nonsteroidal anti-inflammatory drugs and anticoagulant drugs were not permitted in the 2 weeks prior to the study and during it, and subjects taking antihypertensive vasodilators (e.g. nifedipine and hydralazine) were excluded from the studies. Other medications were permitted on a case-by-case basis if taken at least 4 h after sildenafil administration. Antacids were not permitted within 2 h of sildenafil dosing. All three trials were reviewed and approved by the local institutional review board or ethics committee, and all subjects provided written informed consent.
Study designs
Effect of age
In the 2 weeks prior to the study, all subjects underwent a screening procedure that included a 12-lead electrocardiogram (ECG), a range of laboratory tests, urinalysis and a urine drug screen. A negative alcohol breath test was required at the time of admission. Each subject received a single oral dose of sildenafil administered as two 25-mg capsules in the morning following an overnight fast. Subjects were not permitted to eat or drink caffeinated beverages for 4 h following dosing, after which a standard meal was served.
Effect of renal impairment
In the 3 weeks prior to the study, all subjects underwent a screening process, including measurements of CLcr, laboratory safety tests, physical examination, measurements of blood pressure and pulse rate and 12-lead ECG. Caffeine, methylxanthines, alcohol and unaccustomed exercise were discouraged in the 2 days prior to dosing. On the evening before dosing, subjects entered the study centre and underwent a physical examination, including a breath alcohol test, and urine samples were collected for a drug screen and urinalysis. After an overnight fast, sildenafil was administered as two 25-mg capsules on the following morning. Standard meals were provided after the blood samples were collected at 4 and 10 h, and noncaffeinated drinks were freely provided 4 h after dosing. Concomitant medications were permitted only after the 4-h postdose sampling.
Effect of hepatic impairment
Standing and supine blood pressure and pulse rate, laboratory safety tests, physical examination and 12-lead ECG were performed in the 3 weeks prior to the study. Caffeine, methylxanthines, alcohol and unaccustomed exercise were discouraged in the 2 days prior to dosing. Subjects entered the study centre on the evening before dosing and underwent another physical examination. Urine samples were collected for drug screening and urinalysis. An evening meal was provided and sildenafil (two 25-mg capsules) was administered the following morning at least 2 h after a light breakfast. Noncaffeinated drinks were freely provided and concomitant medications were permitted 4 h after dosing; antacids were permitted 2 h after dosing.
Pharmacokinetic assessments
In the effects of age study, 7 ml blood samples were collected predose and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 20, 24, 32, 40 and 48 h postdose for assays of sildenafil and UK-103,320. Blood samples sufficient to provide at least 10 ml of plasma were also collected at 1 h postdose for analysis of sildenafil and UK-103,320 protein binding.
In the renal impairment study, 7 ml blood samples were collected for analysis of sildenafil and UK-103,320 before drug administration and at 0.5, 1, 1.5, 2, 4, 6, 8, 10, 12, 18, 24, 36 and 48 h after drug administration. An additional 10 ml blood sample was drawn immediately prior to dosing for determination of protein binding. Protein electrophoresis was also performed on the predose blood sample. Creatinine clearance was measured based on urine samples collected predose and from 0 to 24 h postdose. A plasma sample from the 24-h blood collection was assayed for creatinine concentrations.
In the hepatic impairment study, blood samples were collected for determination of sildenafil and UK-103,320 concentrations predose and at 0.25, 0.5, 1, 1.5, 2, 6, 10, 18, 24, 36 and 48 h postdose. A blood sample was also collected at 1 h postdose for determination of protein binding.
In all three studies, the simultaneous determination of plasma concentrations of sildenafil and its major metabolite were analysed using automated sequential trace enrichment of dialysates and high-performance liquid chromatography [13]. The limits of quantification were 1 ng ml−1 for both analytes. The overall imprecision (CV) was 5.1%, 3.2% and 3.0% for sildenafil and 3.4%, 3.1% and 2.9% for UK-103,320 concentrations of 3, 125 and 200 ng ml−1, respectively. The inaccuracy (bias) of the assay at all concentrations ranged from −2.3 to 3.5% for sildenafil, and from −7.0 to 4.8% for UK-103,320.
Plasma protein binding of sildenafil and UK-103,320 was determined by an equilibrium dialysis technique across a Spectropor membrane in the effects of age and hepatic impairment studies using automated sequential trace enrichment of dialysates. For the renal impairment study, predose plasma samples were spiked with [14C]-sildenafil and then subjected to equilibrium dialysis across a Spectropor membrane. After equilibrium dialysis the analytical methodology and performance were the same as the plasma assay.
The pharmacokinetic parameters calculated for sildenafil and its primary metabolite were maximum observed plasma concentration (Cmax), area under the plasma concentration–time curve to infinity (AUC), time to the first occurrence of Cmax (Tmax), terminal phase rate constant (kel), terminal phase half life (t1/2), fraction of unbound drug, maximum observed plasma concentration of unbound drug (free Cmax), area under the plasma concentration–time curve of unbound drug to infinity (free AUC), oral clearance of sildenafil (CL/F) and the creatinine clearance (CLcr).
Safety and tolerability
Laboratory safety tests, vital signs and ECGs were performed at regular intervals during the studies. The nature and severity of observed or volunteered adverse events were recorded throughout the study periods.
Statistical analysis
Effect of age
Based on data from a previous trial, a sample size of 15 subjects in each group was considered sufficient to have at least 80% power of detecting a 50% difference between the two groups in AUC and Cmax at the 5% significance level. Analysis of variance (anova) was used to compare sildenafil and UK-103,320 AUC and Cmax (natural log transformed), Tmax, kel and fraction unbound in the young vs the elderly groups.
Effect of renal impairment
A sample size of 24 subjects (normal function = eight, mild impairment = five, moderate = four, severe = seven) was selected as a compromise between the need to minimize exposure of renally impaired subjects to sildenafil and the need to maintain residual degrees of freedom to support firm conclusions. Oral clearance (CL/F) and Cmax for sildenafil and Cmax and AUC for UK-103,320 were analysed using regression techniques to determine their relationship to CLcr and age. CL/F, Cmax, kel and protein binding (fraction unbound) for sildenafil and Cmax, AUC, kel and protein binding for UK-103,320 were subjected to anova and pairwise comparisons were made between the subject groups.
Effect of hepatic impairment
Based on data from a previous trial, a sample size of 24 subjects (12 per group) was considered sufficient to detect a 65% increase in the mean AUC of sildenafil, with 80% power at the 5% significance level. Pharmacokinetic parameters for sildenafil and UK-103,320 were compared for subjects with normal vs cirrhotic livers using paired t-tests (AUC, Cmax, kel and fraction unbound), or Wilcoxon signed ranks test (Tmax). AUC and Cmax were subjected to natural logarithmic transformation prior to analysis.
Results
Effects of age
Fifteen young men (mean age 30 years; range 19–45 years) and 15 elderly men (mean age 70 years; range 65–81 years) completed the study. All subjects were white, except for one young subject who was Hispanic. Height and weight ranges were similar for both groups.
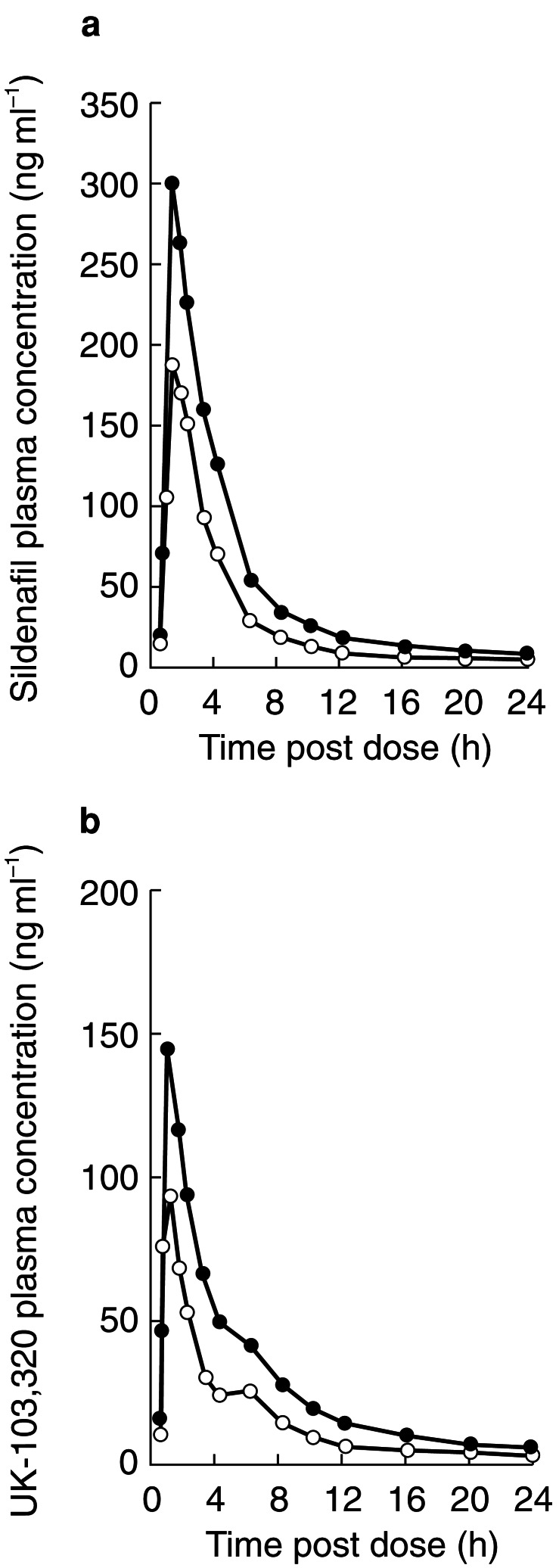
Pharmacokinetic results for young and elderly subjects are summarized in Table 1. Plasma concentrations of sildenafil and UK-103,320 rose rapidly in the young and elderly subjects, with a Tmax of approximately 1 h in both groups (Figure 1). In the young men, plasma sildenafil concentrations decreased to nondetectable concentrations (<1 ng ml−1) by approximately 16 h postdose, while concentrations remained detectable for up to 36 h in the elderly men. Concentrations of UK-103,320 were detectable for up to 24 h in the young subjects and 36 h for the elderly subjects.
Table 1.
Pharmacokinetic parameters of sildenafil and UK-103,320 in young and elderly subjects after administration of single 50-mg sildenafil dose. Geometric mean±SDs are presented for Cmax and AUC; arithmetic mean±SDs are presented for kel, fraction unbound and CL/F; median plus range are presented for Tmax; and harmonic means are presented for t1/2.
| Parameter | Young (n = 15) | Elderly (n = 15) |
|---|---|---|
| Sildenafil | ||
| Cmax (ng ml−1) | 178±70 | 303±102 |
| Ratio (elderly/young) | – | 1.70 |
| 95% CI | – | 1.29, 2.24 |
| P-value | – | 0.0005 |
| AUC (ng ml−1 h) | 586±205 | 1077±283 |
| Ratio (elderly/young) | – | 1.84 |
| 95% CI | – | 1.43, 2.35 |
| P-value | – | <0.0001 |
| Tmax (h) | 1.0 [0.5–2.0] | 1.0 [1.0–2.0] |
| kel (h−1) | 0.268±0.064 | 0.183±0.091 |
| Fraction unbound (%) | 4.3±1.1 | 3.4±1.1 |
| CL/F (ml min−1) | 1537±722 | 800±220 |
| t1/2 (h) | 2.6 | 3.8 |
| UK-103,320 | ||
| Cmax (ng ml−1) | 90±38 | 146±52 |
| Ratio (elderly/young) | – | 1.62 |
| 95% CI | – | 1.22, 2.16 |
| P-value | – | 0.0016 |
| AUC (ng ml−1 h) | 282±144 | 582±232 |
| Ratio (elderly/young) | – | 2.07 |
| 95% CI | – | 1.48, 2.88 |
| P-value | – | 0.0001 |
| Tmax (h) | 1.0 [0.5–1.0] | 1.0 [0.5–1.5] |
| kel (h−1) | 0.222±0.078 | 0.132±0.045 |
| Fraction unbound (%) | 4.9±0.9 | 3.8±1.2 |
| t1/2 (h) | 3.1 | 5.2 |
Figure 1.
(a) Mean plasma concentration profiles of sildenafil (ng ml−1) in young (○) and elderly (•) subjects following administration of a 50-mg dose of sildenafil. (b) Mean plasma concentration profiles of UK-103,320 (ng ml−1) in young (○) and elderly (•) subjects following administration of a 50-mg dose of sildenafil.
Although sildenafil AUC and Cmax values overlapped considerably between groups, mean values were significantly greater for elderly subjects, with mean AUC values about twice as high (P <0.0001) and mean Cmax values about 60–70% higher (P <0.001) in the elderly men than in the young men. Mean sildenafil kel was significantly lower in the elderly group (0.183 h−1) than in the young group (0.268 h−1; P <0.01), corresponding to a difference in half-life of 1.2 h. Similar patterns for these parameters were observed for UK-103,320.
Plasma protein binding was greater in the elderly than the young, leading to a statistically significant (P = 0.04) reduction in the unbound fraction of drug (3.4% in the elderly vs 4.3% in the young). This led to free concentrations of unbound drug that were approximately 40% higher in elderly subjects. Mean (±s.d.) values for free Cmax were 7.4±2.8 and 9.9±2.6 ng ml−1 and free AUC were 24.3±8.4 and 35.3±16.9 ng ml−1 for the elderly and young, respectively.
Effects of renal impairment
A total of 24 men (aged 22–72 years) enrolled in and completed the renal impairment study. Eight of these had normal renal function (CLcr >80 ml min−1), five had mild impairment (CLcr = 50–80 ml min−1), four had moderate impairment (CLcr = 30–49 ml min−1) and seven had severe impairment (CLcr<30 ml min−1). All subjects were white, and weighed 57–90 kg. Hypertension was present in five subjects with mild renal impairment, three with moderate impairment and six with severe impairment. Most were taking antihypertensive drugs. One subject in the normal function group had hypertension and Henoch-Schönlein purpura.
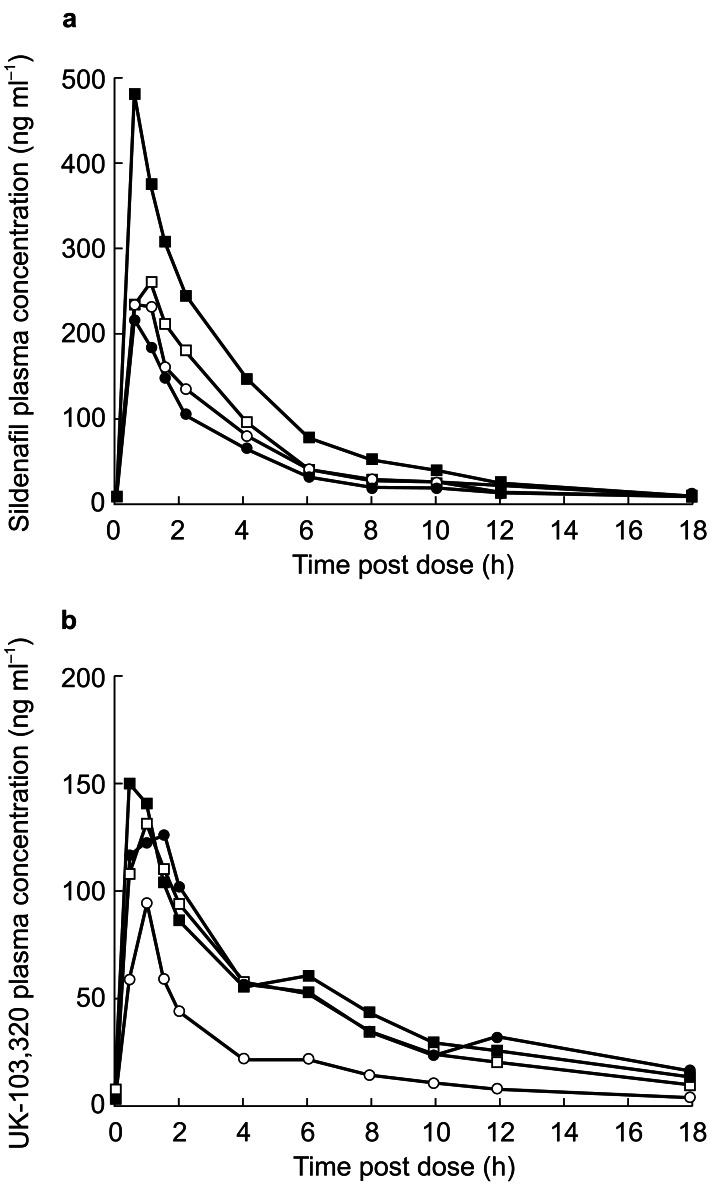
The pharmacokinetic parameters for sildenafil and UK-103,320 in men with normal and impaired renal function are summarized in Table 2. Plasma concentrations of sildenafil and UK-103,320 overlapped considerably among the four groups (Figure 2). Absorption of sildenafil was rapid in all groups, with Tmax occurring within 1.5 h postdose in all subjects. The only significant between-group differences in sildenafil pharmacokinetics were in the subjects with severe renal impairment vs those with normal renal function. In those with severe impairment, CL/F was significantly decreased compared with those with normal function (P <0.01), where the ratio of means was 0.50 (95% confidence interval [CI]: 0.32, 0.76). A significant increase in Cmax was also observed in these subjects compared with the normal group (P <0.01), where the ratio of means was 1.88 (95% CI: 1.24, 2.87).
Table 2.
Pharmacokinetic parameters of sildenafil and UK-103,320 in healthy volunteers and those with mild, moderate or severe renal impairment after administration of single 50-mg sildenafil dose. Geometric mean±SDs are presented for Cmax, AUC and CL/F; arithmetic mean±SDs are presented for kel (UK-103,320) and fraction unbound; median plus range are presented for Tmax; and harmonic means are presented for kel (sildenafil) and t1/2.
| Renal impairment | ||||
|---|---|---|---|---|
| Parameter | Normal (n = 8) | Mild (n = 5) | Moderate (n = 4) | Severe (n = 7) |
| Sildenafil | ||||
| Cmax (ng ml−1) | 246±120 | 256±77 | 288±188 | 464±162 |
| Ratio (/normal) | – | 1.04 | 1.17 | 1.88 |
| 95% CI | – | 0.65, 1.65 | 0.71, 1.92 | 1.24, 2.87 |
| P-value | – | 0.8677 | 0.5231 | 0.0051 |
| AUC (ng ml−1 h) | 756±373 | 683±164 | 882±415 | 1519±639 |
| Tmax (h) | 0.8 [0.5–1.0] | 0.5 [0.5–1.5] | 1.0 [0.5–1.5] | 0.5 [0.5–0.5] |
| kel (h−1) | 0.204±0.056 | 0.164±0.051 | 0.230±0.053 | 0.176±0.032 |
| Fraction unbound (%) | 2.7±0.8 | 2.4±0.7 | 2.0±0.5 | 2.2±0.5 |
| CL/F (ml min−1) | 1102±594 | 1220±313 | 945±317 | 549±193 |
| Ratio (/normal) | – | 1.11 | 0.86 | 0.50 |
| 95% CI | – | 0.69, 1.77 | 0.52, 1.42 | 0.32, 0.76 |
| P-value | – | 0.6583 | 0.5329 | 0.0028 |
| t1/2 (h) | 3.4 | 4.2 | 3.0 | 3.9 |
| UK-103,320 | ||||
| Cmax (ng ml−1) | 87±36 | 151±93 | 103±122 | 156±49 |
| Ratio (/normal) | – | 1.73 | 1.17 | 1.79 |
| 95% CI | – | 0.96, 3.09 | 0.63, 2.20 | 1.05, 3.04 |
| P-value | – | 0.0656 | 0.6031 | 0.0335 |
| AUC (ng ml−1 h) | 302±237 | 684±1043 | 525±1196 | 907±411 |
| Ratio (/normal) | – | 2.26 | 1.74 | 3.00 |
| 95% CI | – | 0.93, 5.54 | 0.66, 4.54 | 1.33, 6.76 |
| P-value | – | 0.0714 | 0.2446 | 0.0106 |
| Tmax (h) | 1.0 [1.0–1.0] | 1.0 [0.5–1.5] | 1.0 [0.5–1.5] | 1.0 [0.5–1.0] |
| kel (h−1) | 0.129±0.064 | 0.090±0.029 | 0.118±0.062 | 0.090±0.042 |
| Fraction unbound (%) | 3.4±0.9 | 2.9±0.8 | 2.5±0.8 | 2.6±0.5 |
| t1/2 (h) | 5.4 | 7.7 | 5.9 | 7.7 |
Figure 2.
(a) Mean plasma concentration profiles of sildenafil (ng ml−1) in men with normal renal function (○) and those with mild (•), moderate (□) or severe (▪) impairment following administration of a 50-mg dose of sildenafil. (b) Mean plasma concentration profiles of UK-103,320 (ng ml−1) in men with normal renal function (○) and those with mild (•), moderate (□) or severe (▪) impairment following administration of a 50-mg dose of sildenafil.
Formal statistical analysis of sildenafil AUC values was not performed, although systemic exposure to the drug was similar across the normal, mild and moderate impairment groups. Mean exposure in the subjects with severe renal impairment was approximately twice that observed in the other groups. No significant differences were observed between groups in sildenafil kel and consequently half-life or protein binding.
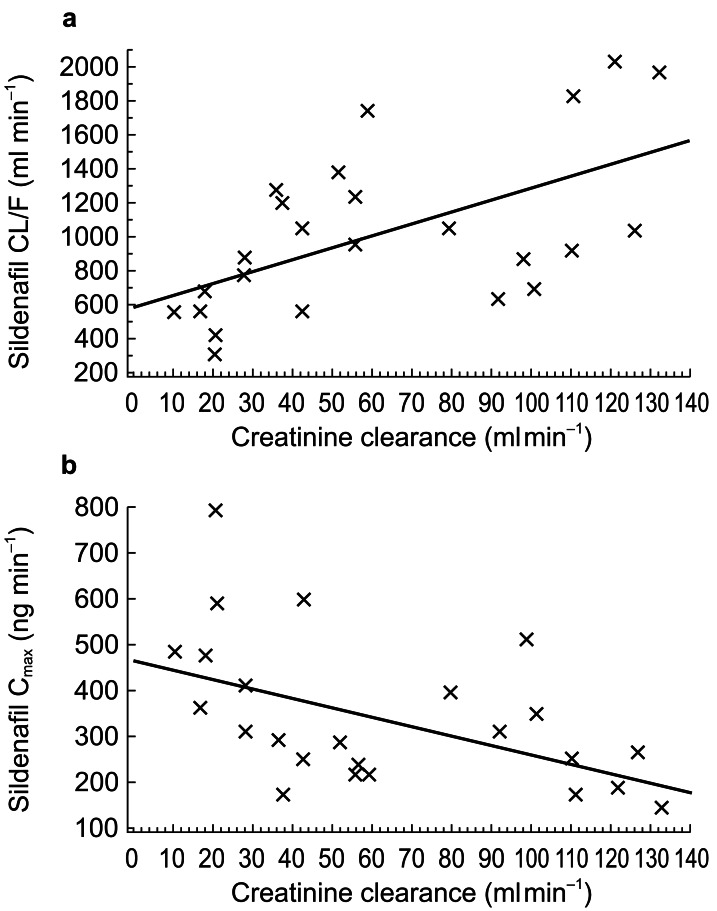
A significant correlation was demonstrated between CLcr and CL/F for sildenafil (P <0.01): CL/F decreased with CLcr. CLcr was also significantly correlated with Cmax for sildenafil (P <0.05), which increased with decreasing CLcr (Figure 3).
Figure 3.
Regression analysis showing correlation of creatinine clearance with (a) sildenafil CL/F, and (b) sildenafil Cmax.
There were no overall significant differences between groups in any UK-103,320 pharmacokinetic parameters. However, pairwise tests indicated a significant difference between the subjects with normal renal function and those with severe impairment for UK-103,320 Cmax (P <0.05), where the ratio of means was 1.79 (95% CI: 1.05, 3.04), and for AUC (P <0.05), where the ratio of means was 3.00 (95% CI: 1.33, 6.76). In addition, pairwise comparisons showed that UK-103,320 protein binding was significantly increased (P <0.05) in the men with severe renal impairment compared with those with normal function, where the mean treatment difference for fraction unbound was −0.84 (95% CI: −1.66, −0.02). CLcr was significantly correlated with Cmax for UK-103,320 (P <0.05) and AUC for UK-103,320 (P <0.05), which increased with decreasing CLcr.
Effects of hepatic impairment
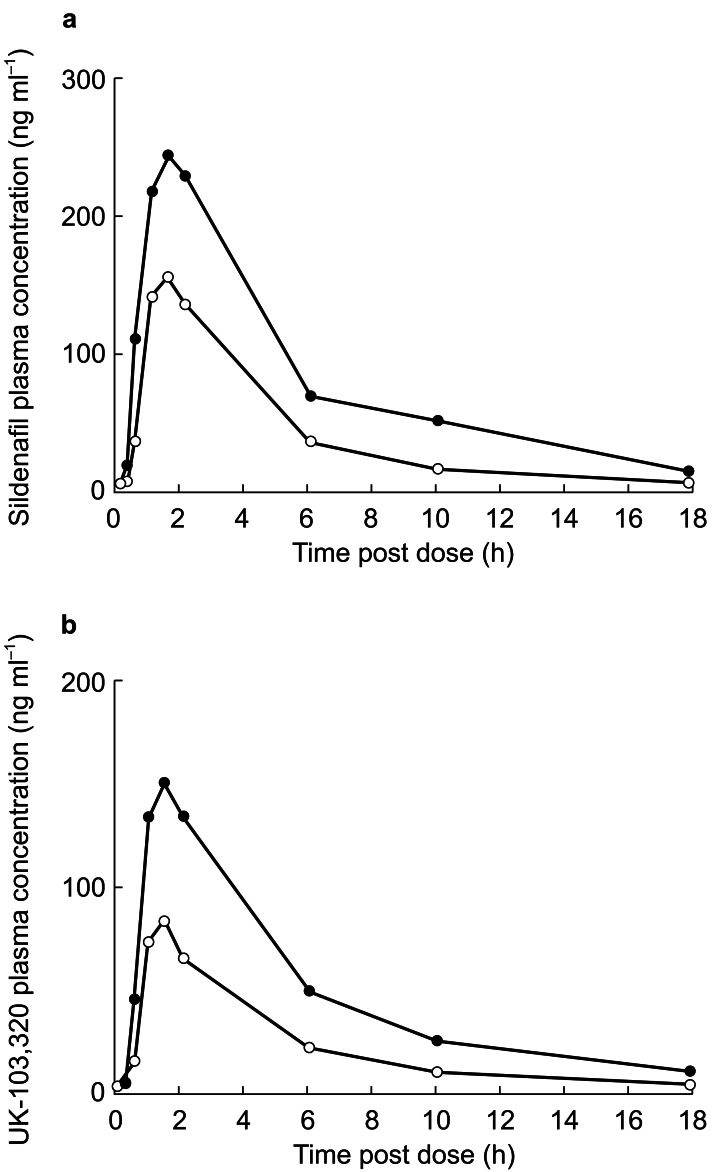
A total of 24 men (aged 32–63 years) enrolled in and completed the study. Twelve of these had normal hepatic function and 12 had biopsy-confirmed hepatic cirrhosis (seven were Child-Pugh class A and five were Child-Pugh class B). Plasma concentration profiles of sildenafil and UK-103,320 are shown in Figure 4.
Figure 4.
(a) Mean plasma concentration profiles of sildenafil (ng ml−1) in men with normal hepatic function (○) and those with chronic stable hepatic cirrhosis (•) following administration of a 50-mg dose of sildenafil. (b) Mean plasma concentration profiles of UK-103,320 (ng ml−1) in men with normal hepatic function (○) and those with chronic stable hepatic cirrhosis (•) following administration of a 50-mg dose of sildenafil.
Absorption of sildenafil was rapid in both the men with normal hepatic function and those with hepatic dysfunction, with Tmax values of 1.4 h and 1.6 h, respectively. The decline in plasma concentrations after this peak was biphasic in both groups, with an initial rapid elimination phase followed by a second slower phase that became apparent at low concentrations. Plasma concentrations of sildenafil and UK-103,320 were increased in the hepatically impaired subjects (Table 3). The increase in systemic exposure to sildenafil in the cirrhotic subjects was statistically significant (P <0.05), with a ratio of the mean AUC values of 1.85 (95% CI: 1.11, 3.07) and a 46% reduction in CL/F. An increase in sildenafil Cmax was also observed (ratio of means = 1.47, 95% CI: 0.97, 2.22), although it was not statistically significant (probably due to the small sample size). The kel decreased from 0.215 in normal subjects to 0.163 in cirrhotic subjects with consequent increase in half-life of 34% (3.2–4.3 h, respectively). No significant differences were shown between groups in sildenafil protein binding (Table 3). No clear relationship was evident between Child-Pugh score and pharmacokinetic parameters, although sildenafil AUC values in the range of the normal subjects were observed in four of seven subjects with class A impairment but only one of five with class B impairment.
Table 3.
Pharmacokinetic parameters of sildenafil and UK-103,320 in normal subjects and those with hepatic impairment after administration of single 50-mg sildenafil dose. Geometric mean±SDs are presented for Cmax, AUC and CL/F; arithmetic mean±SDs are presented for kel and fraction unbound; median plus range are presented for Tmax; and harmonic means are presented for t1/2.
| Parameter | Normal | Cirrhotic |
|---|---|---|
| Sildenafil | ||
| Cmax (ng ml−1) | 155±56 | 228±131 |
| Ratio (/normal) | – | 1.47 |
| 95% CI | – | 0.97, 2.22 |
| P-value | – | 0.0667 |
| AUC (ng ml−1 h) | 664±254 | 1225±296 |
| Ratio (/normal) | – | 1.85 |
| 95% CI | – | 1.11, 3.07 |
| P-value | – | 0.0227 |
| Tmax (h) | 1.5 [1.0–2.0] | 1.5 [1.0–2.0] |
| kel (h−1) | 0.215±0.059 | 0.163±0.051 |
| Fraction unbound (%) | 3.46±0.61 | 3.70±1.34 |
| CL/F (ml min−1) | 1255±533 | 680±712 |
| t1/2 (h) | 3.2 | 4.3 |
| UK-103,320 | ||
| Cmax (ng ml−1) | 83±38 | 155±51 |
| Ratio (/normal) | – | 1.87 |
| 95% CI | – | 1.31, 2.68 |
| P-value | – | 0.0026 |
| AUC (ng ml−1 h) | 343±162 | 873±343 |
| Ratio (/normal) | – | 2.54 |
| 95% CI | – | 1.68, 3.85 |
| P-value | – | 0.0004 |
| Tmax (h) | 1.0 [1.0–2.0] | 1.5 [1.0–2.0] |
| kel (h−1) | 0.223±0.101 | 0.120±0.048 |
| Fraction unbound (%) | 4.86±1.01 | 5.55±1.45 |
| t1/2 (h) | 3.1 | 5.8 |
The concentration–time profiles for UK-103,320 were very similar to those of the parent compound, indicating rapid conversion of the drug during first-pass metabolism. In addition, Cmax in subjects with cirrhosis was significantly increased for UK-103,320 (ratio of means = 1.87, 95% CI: 1.31, 2.68; P <0.01). The systemic exposure to UK-103,320 was more than double that seen for the patients with normal liver function (ratio of UK-103,320 AUC means = 2.54, 95% CI: 1.68, 3.85; P <0.001). The higher ratio of UK-103,320 AUC to sildenafil AUC in subjects with cirrhosis (71%) compared with those with normal function (52%) suggests that liver dysfunction may affect elimination of the metabolite to a greater degree than that of the parent compound. No significant differences were shown between groups in protein binding of the metabolite (Table 3).
Safety and tolerability
In all three studies, sildenafil was well tolerated and most adverse events were mild in severity (Table 4). No relationship was demonstrated between sildenafil and laboratory abnormalities. There were no severe or serious adverse events, and no subject discontinued treatment for any reason.
Table 4.
Incidence of adverse events and laboratory abnormalities for all studies.
| Effect of age | Renal impairment | Hepatic impairment | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of Subjects with: | Young (n = 15) | Elderly (n = 15) | Normal (n = 8) | Mild (n = 5) | Mod (n = 4) | Severe (n = 7) | Normal (n = 12) | Cirrhotic (n = 12) |
| Adverse events (all-cause) | 2 | 2 | 0 | 0 | 0 | 0 | 3 | 2 |
| Adverse events (treatment-related)* | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Laboratory abnormalities (all-cause) | 0 | 5† | 4 | 4 | 4 | 7 | 1 | 12 |
| Laboratory abnormalities (treatment-related) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Mild dizziness and moderate headache in one subject in the young group, mild tachycardia and moderate dizziness in one subject in the elderly group and mild headache in one subject with hepatic impairment.
Includes four elderly subjects with abnormalities at baseline and 48 h postdose.
Discussion
Three open-label, parallel-group studies were conducted to determine the effects of age, renal and hepatic dysfunction on the pharmacokinetics and safety profile of sildenafil. In the study comparing a single oral 50-mg dose of sildenafil in young and elderly subjects, significant differences were observed between the two groups in AUC and Cmax for both the parent drug and the metabolite, although there was considerable overlap between groups. The higher mean AUC (1077 vs 586 ng ml−1 h) and Cmax (303 vs 178 ng ml−1) values in the elderly men were probably attributable to differences in oral clearance and volume of distribution. The differences could be partially accounted for by the observed differences in plasma protein binding between the two groups with the reduced free fraction in the elderly leading to a reduced clearance. The consequence of this is that differences in free sildenafil Cmax, 9.9 vs 7.4 ng ml−1 h, and free sildenafil AUC, 35.3 vs 24.3 ng ml−1, for elderly vs young were smaller than those for total plasma sildenafil concentrations.
Since hepatic metabolism accounts for more than 85% of sildenafil metabolism, age-related effects on hepatic function are likely to play a large role in the observed increases in sildenafil concentrations in the elderly [7]. The main changes noted with ageing, a reduction in hepatic blood flow of approximately 40% and a reduction in the activity of oxygen requiring enzymes [10], would both be expected to affect sildenafil clearance.
In the second study investigating the pharmacokinetics of sildenafil and UK-103,320 in men with normal renal function and varying degrees of renal impairment, the pharmacokinetics of sildenafil and UK-103,320 in men with mild or moderate renal impairment were not significantly different from those in normal men. In contrast, the pharmacokinetics of the drug and metabolite were significantly altered in men with severely impaired renal function, resulting in drug exposure that was approximately double that observed in men with normal renal function. The correlation between the degree of renal impairment (CLcr) and parameters such as Cmax, AUC and CL/F suggests that the reduction in oral clearance of sildenafil is related either directly to renal function or indirectly to the effects of renal impairment on hepatic function. Given the limited excretion of sildenafil and UK-103,320 in the urine, the latter explanation appears more likely.
The presence of circulating endogenous substances that inhibit hepatic metabolic clearance has been suggested as the mechanism for reduced oral clearance of drugs in patients with chronic renal failure [12]. This proposed mechanism is based on the findings from the single pass perfusion study in rat liver of Terao and Shen [14]. In their study, the metabolic activity of normal livers perfused with normal blood and uremic livers perfused with normal blood were found to be similar, while that of normal livers perfused with uremic blood was shown to be lower. The existence of endogenous inhibitory substances in uremic blood was postulated as the likely explanation for this difference. In addition in a study of the pharmacokinetics of bopindolol, the drug was found to accumulate in patients with chronic renal failure but the disposition in patients on regular haemodialysis did not differ from that of subjects with normal renal function [15]. The mechanism underlying these effects is thought to depend on the accumulation of endogenous metabolic inhibitors that are subsequently removed by regular haemodialysis [15].
As would be predicted for a drug with a relatively high CL/F and first-pass metabolism [5, 6], the pharmacokinetics of sildenafil were altered in subjects with chronic stable cirrhosis, as shown by a 46% reduction in CL/F and a 47% increase in Cmax compared with normal subjects. This suggests a reduction in hepatic first-pass metabolism as well as systemic clearance in these individuals, resulting in a systemic exposure (AUC) to sildenafil approximately 85% greater than that observed in age- and weight-matched healthy subjects. However, it should be noted that in five men with cirrhosis, sildenafil plasma concentrations were in the range of those seen in healthy volunteers. These findings are similar to those reported for other drugs whose metabolism is predominantly hepatic. The effects are probably due to one of a number of consequences stemming from decreased permeability of the sinusoid in the cirrhotic liver, namely hepatocellular insufficiency, portacaval shunting and alterations in hepatic blood flow [16].
Despite the reduced oral clearance of the drug in men with hepatic impairment, the increase in systemic exposure to UK-103,320 was approximately twice that seen for the parent drug. The ratio of UK-103,320 AUC to sildenafil AUC was 71% in cirrhotic subjects and 52% in normal subjects, suggesting that liver dysfunction affects elimination of the metabolite to a greater degree than that of the parent compound.
In each of the three studies, sildenafil was well tolerated and most adverse events were mild in severity. No relationship was demonstrated between sildenafil and laboratory abnormalities. There were no serious or severe adverse events, and no subject withdrew for any reason.
In conclusion, the studies described here have shown that sildenafil plasma concentrations are increased in the elderly (≥65 years) and subjects with severe renal impairment (CLcr<30 ml min−1) and hepatic impairment. Given that higher plasma concentrations may increase both efficacy and the incidence of adverse events, a starting dose of 25 mg should be considered in patients with severely compromised renal or heaptic function.
References
- 1.Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 2.Jeremy JY, Ballard SA, Naylor AM, Miller MAW, Angelini GD. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 3.Moreland RB, Goldstein I, Traish A. Sildenafil, a novel inhibitor of phosphodiesterase type 5 in human corpus cavernosum smooth muscle cells. Life Sci. 1998;62:PI 309–PI 318. doi: 10.1016/s0024-3205(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 5.Muirhead GJ, Rance DJ, Walker DK, Wastall P. Comparative human pharmacokinetics and metabolism of single dose oral and intravenous sildenafil. Br J Clin Pharmacol. 2002;53(Suppl. 1):13–20. doi: 10.1046/j.0306-5251.2001.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects. Br J Clin Pharmacol. 2002;53(Suppl. 1):5–12. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog, and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 8.NIH Consensus Development Panel on Impotence. Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 9.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 10.Le Couteur DG, McLean AJ. The aging liver. Drug clearance and an oxygen diffusion barrier hypothesis. Clin Pharmacokinet. 1998;34:359–373. doi: 10.2165/00003088-199834050-00003. [DOI] [PubMed] [Google Scholar]
- 11.Mühlberg W, Platt D. Age-dependent changes of the kidneys: pharmacological implications. Gerontology. 1999;45:243–253. doi: 10.1159/000022097. [DOI] [PubMed] [Google Scholar]
- 12.Yuan R, Venitz J. Effect of chronic renal failure on the disposition of highly hepatically metabolized drugs. Int J Clin Pharmacol Ther. 2000;38:243–253. doi: 10.5414/cpp38245. [DOI] [PubMed] [Google Scholar]
- 13.Cooper JDH, Muirhead DC, Taylor JE, Baker PR. Development of an assay for the simultaneous determination of sildenafil (Viagra®) and its metabolite (UK-103,320) using automated sequential trace enrichment of dialysates and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;701:87–95. doi: 10.1016/s0378-4347(97)00339-3. [DOI] [PubMed] [Google Scholar]
- 14.Terao N, Shen DD. Reduced extraction of I-propranolol by perfused rat liver in the presence of uremic blood. J Pharmacol Exp Ther. 1985;233:277–284. [PubMed] [Google Scholar]
- 15.MacDonald NJ, Grant AC, Rodger RS, Meredith PA, Elliot HL. The effect of renal impairment on the pharmacokinetics and metabolism of bopindolol. Br J Clin Pharmacol. 1991;31:697–700. doi: 10.1111/j.1365-2125.1991.tb05596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan DJ, McLean AJ. Clinical pharmacokinetic and pharmacodynamic considerations in patients with liver disease. An update. Clin Pharmacokinet. 1995;29:370–391. doi: 10.2165/00003088-199529050-00005. [DOI] [PubMed] [Google Scholar]