Abstract
Aims
To analyse the pharmacokinetics of sildenafil citrate in patients with erectile dysfunction in order to characterize covariate relationships and assist in the development of rational dosage strategies.
Methods
A population pharmacokinetic sampling strategy was incorporated into five phase III clinical study protocols. Overall, 2077 patients, 1335 of whom received sildenafil, were asked to take an additional dose of study drug before their scheduled clinic visits on four or five occasions throughout the study duration. A single plasma sample was obtained at random times postdose (range 1–7 h), and a total of 4582 samples were assayed (average 3.4 samples per individual).
Results
For the population average patient (age 58 years; aspartate transaminase [AST], 24 IU l−1; weight, 87 kg; not receiving CYP3A4 potential inhibitors), typical values for sildenafil (mean±SE) were 58.5±1.4 l h−1 for apparent clearance (CL/F), 310±6.92 l for volume of distribution (V/F), and 2.6±0.176 h−1 for first-order absorption constant (ka). The value for ka is associated with meal consumption within 2 h predose, at all other times ka was equivalent to an instantaneous bolus administration. The interindividual variabilities were 29% for CL/F, 20% for V/F, and 210% for ka. Over a dose range of 25–100 mg sildenafil, the pharmacokinetics exhibited dose proportionality. There was evidence of nonproportionality (40% increase on average) in relative bioavailability with respect to the 200-mg dose (P < 0.001) relative to the other doses. Age, AST concentration, and co-administration with CYP3A4 potential inhibitors significantly influenced CL/F of sildenafil (P < 0.001, for each relationship). For age and AST, the extent of the linear relationships (extrapolated from population average values) included a 4% decrease in CL/F for every decade increase and a 6% decrease in CL/F for every 10-unit increase, respectively. Following co-administration of CYP3A4 potential inhibitors, a 14% decrease in CL/F was estimated. Only body weight was found to significantly (P < 0.001) influence V/F (a 6% increase in V/F for every 10-kg increase).
Conclusions
The pharmacokinetics of, and covariate influences on, sildenafil in patients with erectile dysfunction were shown to be consistent with those demonstrated in phase I volunteer studies.
Keywords: Sildenafil citrate, erectile dysfunction, population pharmacokinetics, CYP3A4 inhibitors
Introduction
Erectile dysfunction (ED) is a condition that affects an estimated 10% of the adult male population [1]. Treatment options for men with ED have advanced significantly during the past 10–15 years and include intracavernous prostaglandin injections [2], vacuum constriction therapy [3] and transurethral alprostadil pellets [4]. However, efficacy and/or long-term satisfaction with these treatment options have been suboptimal.
Sildenafil citrate (Viagra®, Pfizer), the first oral therapeutic agent for the treatment of ED [5], is a potent and selective inhibitor of cyclic guanosine monophosphate (cGMP)–specific phosphodiesterase type 5, the predominant isozyme metabolizing cGMP in the corpus cavernosum [6]. Penile erection depends on relaxation of corpora cavernosa smooth muscle. In response to sexual stimuli, cavernous nerves and endothelial cells release nitric oxide (NO), which stimulates formation of cGMP via guanylate cyclase. By selectively inhibiting cGMP catabolism in cavernosal smooth muscle cells, sildenafil restores the erectile response to sexual stimulation without causing erections in the absence of such stimulation [5].
Phase I studies in volunteers have shown that the pharmacokinetics of sildenafil are influenced by a number of factors, such as age, renal function and hepatic status [7]. To investigate the impact of these findings, a population pharmacokinetic sampling strategy was incorporated into a number of phase III clinical studies. Analysis of these data would discern the nature and extent of the a priori covariate relationships in patients not subject to the limited inclusion and exclusion criteria of a phase I study, in addition to quantifying any additional relationships a posteriori. The aim of this analysis was to use the resultant pharmacokinetic data to aid interpretation of efficacy and safety data.
Methods
Studies incorporated into analysis
A pharmacokinetic sampling schedule was incorporated into five phase III studies. Combination of the data enabled analysis over a wide range of sildenafil doses (25, 50, 100 and 200 mg) following fixed and individualized dosing regimens. In each of the studies, patients were asked to take an additional dose of study medication before specific clinic visits and record the date and time of administration, the date and time of the previous dose, and the date and time of their last meal. The time of food intake relative to dosing was subsequently categorized into six groups (0–1 h, 1–2 h, >2 h pre and postdose).
Population analyses
Formal population pharmacokinetic analysis of plasma concentration data was performed using the nonlinear mixed effects modelling approach. The software package NONMEM, version IV, level 2.2 (or later) was used to derive the population mean (and variance) values for specific pharmacokinetic parameters, for example, apparent clearance (CL/F), which was subsequently used to derive estimates of exposure. One- and two-compartment structural pharmacokinetic models incorporating first-order absorption (with and without lag time) were fitted to sildenafil concentration time data using standard population pharmacokinetic methodology. The appropriateness of these models was assessed using standard goodness of fit criteria and deletion diagnostics [8]. Beal and Sheiner provide a full description of population pharmacokinetic/pharmacodynamic theory and the estimation methods used in packages such as NONMEM [9].
The first-order estimation method was used to derive population pharmacokinetic parameters, the interindividual variability in these parameters, and the residual variability between observed and predicted plasma concentration values. Using CL/F as an example, interindividual variability was modelled as:
where ηiCL/F is a zero mean, normally distributed, random variable with a variance of ω2 and denotes the proportional difference between the typical parameter value in the population (CL/F)p and the parameter value for subject i (CL/F)i. Interindividual variability was modelled in the same way for all parameters. The ω2 are the diagonal elements of the interindividual variance-covariance matrix, Ω. The off-diagonal elements of this matrix were also considered to assess possible correlations between parameters. Residual variability was modelled as proportional, additive, or combined proportional and additive. The proportional model was parameterized as:
where Cijk and Ĉijk are the kth measured and model predicted concentrations, respectively, and the εijk denotes the residual intraindividual random error, which is distributed with a mean of zero and variance σ2. The elements of Ω and σ2 were estimated as components of the population model.
Covariate identification
Linear and nonlinear relationships between the individual parameter estimates and the various covariate indices for demography, biochemistry and concomitant medication were explored and, where indicated, used to refine the population model and characterize sources of interindividual and intra-individual variability. To facilitate this process, generalized additive models (GAM) were developed for parameter covariate relationship elucidation, as previously described [10]. The GAM used in this analysis was implemented in the software, Xpose v.1.1 [8] developed for an S-plus environment (v.3.3 for Windows, Statistical Sciences Inc.).
Covariate model finalization
Covariates parameter relationships based on the regression models found in the GAM step (or from univariate testing in a NONMEM model) were included into a population model via a stepwise procedure. The statistical significance of the covariate relationship was tested by comparing the NONMEM objective function values (−2 log likelihood) before and after covariate inclusion. The difference in objective function value between the two models follows an asymptotic chi-square distribution with degrees of freedom equal to the difference in the number of parameters between contending models. Covariates were added to the model if they significantly decreased the objective function value (for this analysis, a value of 11 was chosen for the inclusion of each additional parameter, corresponding to the 0.1% level of significance). The significance of each of the covariates in the fully developed model was further tested by fixing each structural model parameter used to characterize the covariate relationship to a null value and performing reduced-full model pair comparisons. The resultant final model only contained covariates that met the predefined statistical criteria and were evident in at least 15 patients.
The validity of the derived population model was assessed via deletion diagnostics (the population model parameter values were re-estimated following sequential removal and replacement of individual study data). All models and simulations were run on either a DEC AlphaStation operating under a VMS environment or a Hewlett Packard workstation operating under UNIX. S-Plus (Xpose v.1.1) and Excel v.5.0 were run on a PC platform under Windows 3.1.
Results
Demographic data
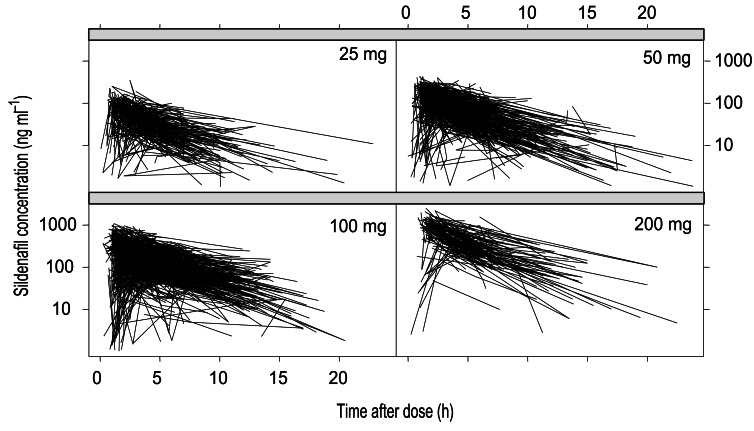
Table 1 gives a description of patient demography, biochemistry and concomitant medications at baseline for sildenafil-treated patients. Investigators were encouraged to obtain each plasma sample at a different time relative to dose on each occasion, and the use of recommended ‘sample windows’ was encouraged (1–3 h, 3–5 h, 5–7 h, and >7 h postdosing). Figure 1 shows the observed sildenafil concentrations analysed across all five studies.
Table 1.
Demographics and biochemistry at baseline.
| Mean±s.d./Count | Range | Count (%) | |
|---|---|---|---|
| n | 1335 | ||
| Age (y) | 57.7±10.2 | 19–87 | |
| Weight (kg) | 86.6±14.1 | 49–159 | |
| Race | |||
| White | 1240 | 92.9 | |
| Black | 53 | 3.9 | |
| Asian | 14 | 1.0 | |
| Other | 29 | 2.2 | |
| ED aetiology | |||
| Organic | 786 | 58.9 | |
| Psychogenic | 211 | 15.8 | |
| Mixed | 338 | 25.3 | |
| Biochemical parameters | |||
| Serum creatinine (mg dl−1) | 0.98±0.19 | 0.20–2.23 | |
| Albumin (g dl−1) | 4.37±0.27 | 3.40–5.38 | |
| Bilirubin (µmol l−1) | 0.66±0.27 | 0.20–2.39 | |
| AST (IU l−1) | 24.1±8.0 | 9–91 | |
| ALT (IU l−1) | 27.7±13.0 | 6–124 | |
| Alkaline phosphatase (IU l−1) | 81.0±24.7 | 18–315 | |
| Alcohol consumption (units per week)* | |||
| none | 400 | 29.9 | |
| 1–15 | 542 | 40.6 | |
| 16–30 | 174 | 13.0 | |
| >30 | 220 | 16.5 | |
| Smokers | 297 | 22.2 | |
| Concomitant medications | |||
| Thiazide and related diuretics | 36 | 2.7 | |
| Loop and potassium-sparing diuretics | 31 | 2.3 | |
| ACE-I and angiotensin II antagonists | 177 | 13.3 | |
| Calcium channel blockers | 151 | 11.3 | |
| ‘Cardioselective’ β-blockers† | 64 | 4.8 | |
| ‘Noncardioselective’ β-blockers‡ | 35 | 2.6 | |
| CYP2C9 potential inhibitors§ | 115 | 8.6 | |
| CYP3A4 potential inhibitors∥ | 214 | 16.0 | |
| CYP2D6 potential inhibitors¶ | 136 | 10.2 | |
| CYP450 potential inducers# | 31 | 2.3 | |
ED=erectile dysfunction; AST=aspartate transaminase; ALT=alanine transaminase; ACE-I=angiotensin-converting enzyme inhibitor
one unit is equivalent to one glass of wine
classified as atenolol, betaxolol, bisoprolol, metoprolol, acebutolol
classified as propranolol, carteolol, carvedilol, celiprolol, esmolol, labetolol, levobunolol, metipranolol, nadolol, oxprenolol, pindolol, sotalol, timolol
classified as nonsteroidal anti-inflammatory drugs, tolbutamide, warfarin
classified as verapamil, diltiazem, nicardipine, chloramphenicol, erythromycin, azithromycin, clarithromycin, allopurinol, sodium valproate, cimetidine, metronidazole, dextropropoxyphene, nitrendipine, dextromethorphan, nifedipine, ergotamine, felodipine, tamoxifen, cyclosporin, ketoconazole, fluconazole, itraconazole, miconazole, danazol, sulindac, acetazolamide, colchicine, metoclopraide, glucocorticoids, hypnotics, anxiolytics, methadone, isoniazid, amiodarone, terfenadine, quinidine
classified as antimalarials, selective serotonin reuptake inhibitors, tricyclic and related antidepressants, antipsychotics
-blockers, quinidine, timolol
classified as barbiturates, carbamazepine, phenytoin, griseofulvin, rifampicin, phenylbutazone, omeprazole, sulphadimidine, trimethoprim.
Figure 1.
Sildenafil Concentration vs time after dose.
Sildenafil pharmacokinetics
The plasma concentration time data was appropriately described by a one-compartment disposition model with first-order input. There was no evidence that a more complicated structural model was required. A proportional model adequately described residual variability.
Full covariate model
All the significant covariates identified in the GAM were included in a NONMEM model and subjected to sequential removal and replacement. At the end of this process, the significant covariates of CL/F were age, aspartate transaminase (AST) concentration, co-administration of CYP3A4 potential inhibitors and co-administration of ‘noncardioselective’ β-blockers. Both AST and alanine transaminase (ALT) resulted in a significant reduction in objective function value, but because AST reduced the objective function value the greatest following linear and log-linear incorporation into the model, it was deemed to provide the best representation of hepatic status. The only significant covariate of V/F was body weight.
For the first-order absorption constant (ka), information on the time of dosing relative to the patients last meal was incorporated as a covariate. The most parsimonious model, with the smallest objective function value, estimated ka under two conditions. One parameter encapsulated food intake ≤2 h predose, with another parameter encapsulating all other times relative to the sildenafil dose.
A factor was introduced to account for an observed nondose proportional increase in exposure at the 200-mg dose relative to all other doses. The most parsimonious model, with the smallest objective function value, simply expressed the nonproportionality as an alteration of relative bioavailability (Frel) following this dose. This model predicts a supra-increase in exposure, with no alteration in elimination half-life following a 200-mg dose. Furthermore, this model suggests that over the range of 25–100 mg the pharmacokinetics of sildenafil could be regarded as dose proportional.
Using the ‘full’ covariate model, each study was sequentially removed and replaced and the model rerun with four of the five available studies (Table 2). The parameters characterizing age, AST, body weight and dose relationships were sensitive to individual study deletions. There was no obvious explanation for this finding with respect to the age relationship because there were no differences in the age range of patients within each of the five studies. For the remainder of these relationships it was apparent that dissimilar distributions within the five studies accounted for the degree of study sensitivity evident.
Table 2.
Structural and covariate parameter estimates for the ‘full’ population pharmacokinetic model after removal of component studies.
| Parameter Estimates (ratios of parameter estimates [n =1] studies/[n =5] studies) | ||||||
|---|---|---|---|---|---|---|
| Parameter | None | Study I | Study II | Study III | Study IV | Study V |
| CL/F (l h−1) | 59.0 (1.00) | 58.0 (0.98) | 58.1 (0.98) | 58.1 (0.98) | 61.1 (1.04) | 60.2 (1.02) |
| V/F (l) | 309 (1.00) | 322 (1.04) | 305 (0.99) | 295 (0.95) | 319 (1.03) | 310 (1.00) |
| ka (h−1) | 2.56 (1.00) | 2.73 (1.07) | 2.67 (1.04) | 2.56 (1.00) | 2.50 (0.98) | 2.40 (0.94) |
| Age (% change in CL/F | 3.6 (1.00) | 1.0 (0.27) | 3.4 (0.95) | 3.5 (0.96) | 5.0 (1.38) | 5.5 (1.52) |
| per 10 years) | ||||||
| AST (% change in CL/F | 6.2 (1.00) | 5.9 (0.95) | 5.3 (0.85) | 4.2 (1.16) | 8.0 (1.29) | 3.9 (0.59) |
| per 10 IU l−1) | ||||||
| CYP3A4 (θ*CL/F) | 0.88 (1.00) | 0.89 (1.01) | 0.90 (1.02) | 0.85 (0.97) | 0.89 (1.01) | 0.89 (1.01) |
| ‘Noncardioselective’ | 0.84 (1.00) | 0.81 (0.96) | 0.85 (1.01) | 0.83 (0.99) | 0.77 (0.92) | 0.98 (1.16) |
| β-blockers (θ*CL/F) | ||||||
| Weight (% change in | 5.8 (1.00) | 6.6 (1.13) | 5.7 (0.97) | 4.1 (0.7) | 7.0 (1.21) | 7.0 (1.19) |
| V/F per 10 kg) | ||||||
| Frel (200 mg vs≤100 mg) | 0.42 (1.00) | 0.44 (1.06) | 0.42 (0.99) | 0.40 (0.95) | NA | 0.42 (1.00) |
CL/F=apparent clearance; V/F=volume of distribution; ka =first-order absorption constant; AST=aspartate transaminase; θ =multiplicative factor for population typical value; Frel =relative bioavailability.
Finally, interactions between covariate relationships were ascertained. Estimates of CL/F for patients receiving only ‘noncardioselective’ β-blockers, only CYP3A4 potential inhibitors or both were obtained. The effect of ‘noncardioselective’ β-blockers was mediated via a small group of patients (n =6) who were also receiving CYP3A4 potential inhibitors. Patients receiving only ‘noncardioselective’ β-blockers showed no reduction in overall CL/F; consequently, any covariate influence is likely to be artefactual. This relationship was therefore not progressed into the final covariate model.
Final covariate model
Three covariates were considered to influence the CL/F of sildenafil to a meaningful extent: age, AST concentration and co-administration of CYP3A4 potential inhibitors. Body weight remained a covariate of V/F, as did differential absorption rates on ka. Nonproportionality with dose was best described by an increase in Frel associated with the 200-mg dose group. All relationships with continuous covariates were adequately described by a linear function.
The covariates for CL/F (extrapolated from population average values) were predicted as follows: with age, a 4% decrease in CL/F for every decade increase, for AST, a 6% decrease in CL/F for every 10-unit increase, and for CYP3A4 potential inhibitors, a 14% decrease in CL/F following co-administration (P < 0.001). Tables 3 and 4 show the interaction between the significant covariate effects on CL/F across the range of covariate values seen in the five studies. In the extreme case, relative to a 20-year-old-patient with normal AST levels, an 80-year-old patient with AST elevation (90 IU l−1) would be predicted to exhibit up to a 60% decrease in CL/F, a reduction that would be greater if CYP3A4 potential inhibitors were simultaneously being prescribed. However, as illustrated in Table 4, most patients studied were within the 50- to 60-year age group, with normal AST levels. Consequently, there seems to be little scope for a reduction of clearance of this magnitude in most of the 1335 patients studied.
Table 3.
Interaction between covariates in the final population pharmacokinetic model and the influence of covariate combinations on CL/F.
| 10 | 30 | 50 | 70 | 90 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AST (IU l−1) CYP3A4 (yes/no) | no | yes | no | yes | no | yes | no | yes | no | yes |
| Age (y) | CL/F (l h−1) | |||||||||
| 20 | 69 | 60 | 61 | 53 | 53 | 46 | 45 | 39 | 37 | 32 |
| 40 | 65 | 56 | 57 | 49 | 50 | 43 | 42 | 36 | 34 | 30 |
| 60 | 60 | 52 | 53 | 46 | 46 | 40 | 39 | 34 | 32 | 28 |
| 80 | 56 | 48 | 49 | 42 | 43 | 37 | 36 | 31 | 30 | 26 |
CL/F=apparent clearance; AST=aspartate transaminase.
Table 4.
Interaction between covariates in the final population pharmacokinetic model and the prevalence of covariate combinations.
| 0–20 | 20–40 | 40–60 | 60–80 | 80–100 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AST (IU l−1) CYP3A4 (yes/no) | no | yes | no | yes | no | yes | no | yes | no | yes |
| Age (y) | Number of Patients | |||||||||
| 10–30 | 2 | 0 | 13 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30–50 | 64 | 3 | 148 | 19 | 13 | 3 | 2 | 0 | 0 | 1 |
| 50–60 | 210 | 50 | 532 | 89 | 25 | 5 | 2 | 1 | 0 | 1 |
| 70–90 | 29 | 9 | 80 | 31 | 1 | 2 | 1 | 0 | 0 | 0 |
The relationship between body weight and V/F (extrapolated from population average values) predicted a 6% increase in V/F for every 10-kg increase (P < 0.001). The nonproportionality with the 200-mg dose predicted a 40% increase in bioavailability relative to all other doses (P < 0.001).
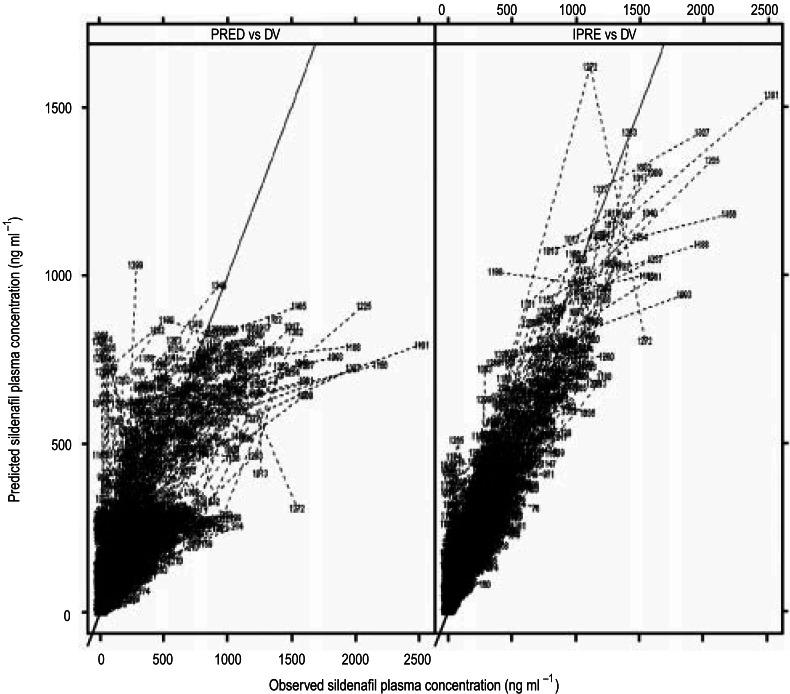
The typical population parameter values for sildenafil are shown in Table 5, and the observed and predicted concentrations for the population model are shown in Figure 2. Following inclusion of explanatory covariates, the level of interindividual variability on CL/F and V/F was reduced, a decrease from 51% to 29% for CL/F and from 46% to 20% for V/F. The estimate of interindividual variability on ka was large and imprecise, possibly a consequence of insufficient data for adequate characterization of this parameter (few data were obtained at times <1 h post dose). The value of ka reported is that associated with meal consumption within 2 h predose; input rates at all other times relative to food intake were equivalent to an instantaneous bolus administration.
Table 5.
Population parameter estimates for the final population pharmacokinetic model.
| Parameter | Estimate |
|---|---|
| CL/F (l h−1) for typical patient* | 58.5±1.40† |
| Age (% change in CL/F per 10 years) | 3.65±1.90† |
| AST (% change in CL/F per 10 IU l−1) | 6.25±2.17† |
| CYP3A4 potential inhibitor co-administration (θ*CL/F) | 0.862±0.0509† |
| V/F (l) for typical patient (87 kg) | 310±6.92† |
| Weight (% change in V/F per 10 kg) | 6.13±1.42† |
| ka (h−1) | 2.60±0.176† |
| Frel (200 mg vs≤100 mg) | 0.408±0.0779† |
| ωCL/F | 29±20%‡ |
| ωV/F | 20±50%‡ |
| ωka | 210±25%‡ |
| ωFrel | 34±11%‡ |
| σ | 47.6±11.5%§ |
CL/F=apparent clearance; V/F=volume of distribution; ka =first-order absorption constant; AST=aspartate transaminase; θ=multiplicative factor for population typical value; Frel =relative bioavailability
58 years old, AST 24 IU l−1, not receiving CYP3A4 potential inhibitor
mean±standard error of the estimate
mean±relative standard error of the variance
coefficient of variation±standard error of estimate ka =this value is associated with meal consumption within 2 h predose, at all other times input rate is equivalent to an instantaneous bolus administration.
Figure 2.
Observed (DV) vs model-predicted sildenafil plasma concentrations. The left-hand panel shows the population-predicted (PRED) and the right-hand panel the individually predicted (IPRE) sildenafil plasma concentrations.
The level of residual variability is likely to be a consequence of a number of factors. These include sampling and dosage history inaccuracies and occasion to occasion changes in one or more of the pharmacokinetic parameters that could not be formally captured in this model (one sample was obtained per individual on each occasion).
Using individual Bayesian estimates derived from the 1335 sildenafil-treated patients, estimates of pharmacokinetic parameters following administration of 25–200 mg sildenafil are shown in Table 6. Because studies II and III had flexible dosing regimens, it was possible for individuals in these studies to receive more than one dose; therefore, the number of individuals who actually received each dose is illustrated. In general, the parameter values obtained from this group of patients were in close agreement with those obtained following administration of sildenafil to volunteers [11].
Table 6.
Summary of individual and derived parameter estimates for the final population pharmacokinetic model for each dose of sildenafil studied (mean±s.d.).
| Sildenafil Dose | ||||
|---|---|---|---|---|
| 25 mg (n =224) | 50 mg (n =450) | 100 mg (n =591) | 200 mg (n =115) | |
| AUC (ng·h ml−1) | 464±175 | 950±346 | 1963±860 | 5486±1965 |
| Cmax (ng ml−1) | 84.4±80.5 | 157±49.2 | 328±237 | 903±287 |
| Tmax (h) | 1.09±0.89 | 1.03±0.76 | 1.16±0.99 | 0.99±0.82 |
| t1/2 (h) | 3.60±0.72 | 3.67±0.69 | 3.82±0.84 | 3.70±0.73 |
| CL/F (l h−1) | 58.2±8.31 | 57.4±8.29 | 56.5±8.60 | 56.9±8.51 |
| V/F (l) | 295±29.7 | 297±29.4 | 303±36.4 | 296±29.6 |
| Frel | 1.04±0.27 | 1.05±0.25 | 1.05±0.29 | 1.50±0.36 |
| ka (h−1) | 12.9±26.1 | 14.9±27.8 | 14.1±25.2 | 20.8±27.4 |
| ke (h−1) | 0.200±0.038 | 0.195±0.035 | 0.188±0.038 | 0.194±0.035 |
AUC=area under the curve; CL/F=apparent clearance; V/F=volume of distribution; ka =first-order absorption constant; Cmax =maximum observed concentration; Tmax =time to Cmax; t1/2 =half-life; Frel =relative bioavailability.
Conclusions
The result of these analyses suggests that the pharmacokinetics of sildenafil in patients with ED are consistent with many of the findings obtained from volunteer studies. Features that are common to patient and volunteer data include dose proportionality in sildenafil pharmacokinetics over the range of 25–100 mg with evidence of nonproportionality at higher doses, food-related alteration of sildenafil absorption rate, comparable values for AUC, Cmax, Tmax and half-life between patients and volunteers, evidence of reduced sildenafil CL/F with hepatic status (transaminase elevations), increasing age and co-administration of CYP3A4 potential inhibitors.
Because hepatic transaminase elevation, ageing, and concomitant medications have been shown to reduce sildenafil CL/F in this population of 1335 patients and because there is additional clinical evidence that increasing exposure levels may be associated with an increased incidence of adverse events, doses as high as 200 mg may have limited scope for clinical usage. In phase II/III studies the 200-mg dose did not confer significant additional clinical benefit in terms of efficacy [12]. Therefore, on the basis of patient pharmacokinetics and efficacy and tolerability data, the clinically effective dose range for sildenafil treatment appears to be 25–100 mg.
References
- 1.Feldman HA, Goldstein I, Hatzichristou D, Krane R, McKinlay J. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 2.Linet O, Ogrine F. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. N Eng J Med. 1996;334:873–877. doi: 10.1056/NEJM199604043341401. [DOI] [PubMed] [Google Scholar]
- 3.Aloni R, Heller L, Keren O, Mendelson E, Davidoff G. Noninvasive treatment for erectile dysfunction in the neurogenically disabled population. J Sex Marital Ther. 1992;18:243–249. doi: 10.1080/00926239208403410. [DOI] [PubMed] [Google Scholar]
- 4.Padma-Nathan H, Hellstrom W, Kaiser F. Treatment of men with erectile dysfunction with transurethral alprostadil. N Engl J Med. 1997;336:1–7. doi: 10.1056/NEJM199701023360101. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein I, Lue T, Padma-Nathan H, Rosen R, Steers W, Wicker P. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 6.Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–2171. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- 7.Muirhead G, Wilner K, Colburn W, Haug-Pihale X, Rovieux B. The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil citrate. Br J Clin Pharmacol. 2002;53(Suppl 1):21–30. doi: 10.1046/j.0306-5251.2001.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 9.Beal SL, Sheiner LB. San Francisco: NONMEN Project Group, University of California at San Francisco; 1998. NONMEM User's Guide. [Google Scholar]
- 10.Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–528. doi: 10.1007/BF01061469. [DOI] [PubMed] [Google Scholar]
- 11.Nichols DJ, Muirhead G, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53(Suppl 1):5–12. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales A. Oral sildenafil (Viagra®) in the treatment of erectile dysfunction: efficacy and safety of 50-mg, 100-mg, and 200-mg doses. Int J Impot Res. 1998;10(Suppl 3):S40. doi: 10.1038/sj.ijir.3900354. [DOI] [PubMed] [Google Scholar]