Abstract
Aims
To examine the effect of concomitant cimetidine or antacid administration on the pharmacokinetic profile of sildenafil citrate in healthy male volunteers in two open-label, randomized studies.
Methods
The first study was a parallel-group design in which 22 healthy male volunteers received sildenafil (50 mg) on days 1 and 5 and cimetidine (800 mg) or placebo on days 3, 4, 5, and 6. Blood samples were collected predose and at specified times up to 48 h postdose on days 1 and 5 to determine plasma levels of sildenafil and its metabolite, UK-103,320. The second study was a two-way crossover design in which 12 volunteers received sildenafil with or without a 30-ml dose of a magnesium hydroxide/aluminium hydroxide antacid. Blood samples were collected and analysed as in the first study. The two study periods were separated by at least 14 days.
Results
Coadministration of cimetidine had no statistically significant effect on the tmax or kel of sildenafil but caused a statistically significant increase in sildenafil AUCt and Cmax of 56% and 54%, respectively (P<0.01). Differences between the two treatment groups were smaller for the metabolite than for sildenafil, although cimetidine treatment did significantly (P<0.05) increase the AUCt for UK-103,320 by 30%. Antacid coadministration had no statistically significant effect on any pharmacokinetic parameter of sildenafil or UK-103,320. Whether taken alone, with cimetidine, or with an antacid, sildenafil was well tolerated. Most adverse events were mild in nature, and no subject withdrew from either study for any reason related to the drug.
Conclusions
Cimetidine co-administration produced an increase in sildenafil plasma levels; however, this increase is not sufficient to warrant dosage adjustment of either drug. Antacid coadministration had no effect on the pharmacokinetic profile of sildenafil.
Keywords: sildenafil citrate; UK-103,320; pharmacokinetics; gastric hyperacidity; peptic ulcer; cimetidine; antacid; safety
Introduction
Peptic ulcers, gastritis, dyspepsia, and other hypersecretory conditions are common in older adults. Approximately 11% of adults are affected by one or more of these disorders, although the prevalence may be as high as 26% among chronic users of nonsteroidal anti-inflammatory agents, many of whom are elderly [1, 2]. Erectile dysfunction (ED) is also widespread, particularly among older individuals: it has been estimated to affect up to 40% of 40-year-old men and 67% of 70-year-old men [3]. Thus, ED is likely to occur in a substantial proportion of men with ulcers or hypersecretory disorders, and medications for each condition are likely to be taken concomitantly, raising the potential for drug interactions.
Sildenafil citrate (Viagra®, Pfizer) is a selective inhibitor of cyclic guanosine monophosphate (cGMP)–specific phosphodiesterase type 5 and is an effective treatment for ED of various aetiologies [4, 5]. In vitro studies have shown that sildenafil is metabolized via two cytochrome P450 (CYP) enzymes, CYP3A4, the major route, and CYP2C9, the minor route [6–8], although the consequences of nonspecific inhibition of this system on sildenafil pharmacokinetics are unknown. Cimetidine is a histamine H2 antagonist commonly prescribed for duodenal ulcer disease, benign gastric ulcers, and hypersecretory states [9, 10]. Cimetidine is also a nonspecific inhibitor of the cytochrome P450 system that has been reported to alter the pharmacokinetics, and possibly the pharmacodynamics, of drugs whose metabolic clearance depends on this enzyme system [11, 12]. Other drug interactions associated with cimetidine are related to its effects on gastric pH, which can influence the absorption of some compounds [10].
Antacids may interact with other drugs by altering their absorption or chelation. Aluminium-, calcium-, and magnesium-based antacids, which are the most widely used remedies for specific and nonspecific gastrointestinal complaints, are therefore also associated with a variety of drug interactions [13]. Absorption of sildenafil from the gastrointestinal tract is thought to be pH-dependent and increasing under acidic conditions, suggesting that antacids could have the potential to affect its pharmacokinetic and/or pharmacodynamic behaviour.
Because sildenafil is likely to be taken by men with ED being treated for conditions related to gastric hyperacidity, the possibility of drug interactions merits exploration. The two open-label, randomized phase I studies reported here were conducted to determine the effects of cimetidine and antacids on the pharmacokinetics of sildenafil and its major metabolite, UK-103,320.
Methods
Subjects
Healthy male volunteers, aged 18–45 years with a body weight of 61–91 kg, were eligible for inclusion in each study. Subjects were excluded if they had evidence of any clinically significant disease or laboratory test abnormality or if they smoked. No prescription/over-the-counter medications or any experimental drugs were to be taken 2 and 4 weeks before the study, respectively. In addition, subjects with a supine blood pressure >140/90 mmHg or <90/60 mmHg and a pulse rate >100 bpm or <45 bpm were ineligible. General medical examination and laboratory safety tests were performed before the study to exclude significant illness, allergies, drug or alcohol dependence, or conditions that may affect absorption or metabolism of the study drugs. Both trials were reviewed and approved by the local institutional review boards, and all subjects gave their informed written consent.
Protocol
In the first study, after an overnight fast, the two groups of volunteers received a single dose of 50 mg sildenafil on day 1, followed by a 1-day washout. An 800-mg dose of cimetidine (group I) or placebo (group II) was administered on days 3, 4, 5, and 6, again after an overnight fast. On day 5 subjects received another 50-mg oral dose of sildenafil approximately 2 h post cimetidine/placebo dosing. Subjects continued to fast for an additional 4 h after sildenafil dosing on days 1 and 5.
In the second study, fasted subjects received 50 mg sildenafil alone or 50 mg sildenafil in combination with an antacid (30 ml of a suspension containing 90-mg ml−1 magnesium hydroxide and 100-mg ml−1 aluminium hydroxide). The postdose protocol was identical to that of the first study. A minimum of 14 days later, the entire protocol was repeated, and all subjects who had previously received sildenafil alone now received sildenafil in combination with an antacid, and vice versa.
On days 1 and 5 in the first study, and during the first and second periods in the second study, vital signs were checked before sildenafil administration and at 0.5, 1, 2, 4, 6, and 24 h thereafter. An ECG was performed before and at 1 and 24 h after each sildenafil dose. Observed or volunteered adverse events were monitored at each study visit, and laboratory safety tests were performed before and 24 h after each dose of sildenafil.
Pharmacokinetic measurements
On days 1 and 5 in the first study and during the first and second periods of the second study, blood samples were collected before sildenafil dosing and 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 16, 20, 24, 32, 40, and 48 h postdose to determine plasma concentrations of sildenafil and its main metabolite, UK-103,320.
The simultaneous determination of parent drug and metabolite was achieved using automated sequential trace enrichment, followed by high-performance liquid chromatography (HPLC) [14]. The limits of quantification for the assay were 1 ng ml−1 for both analytes. The overall imprecision (CV) for concentrations of 3, 125, and 200 ng ml−1 was 5.1%, 3.2%, and 3% for sildenafil and 3.4%, 3.1%, and 2.9% for UK-103,320, respectively. The inaccuracy (bias) of the assay ranged from −2.3% to 3.5% for sildenafil and from −7% to 4.8% for UK-103,320.
The maximum plasma concentration (Cmax) was estimated directly from the experimental data, and tmax was defined as the first occurrence of Cmax. The terminal elimination phase rate constant (kel) was estimated by least squares regression analysis of the plasma concentration-time data obtained during the terminal log-linear elimination phase. The mean half-life (t1/2) was calculated as 0.693/mean kel. The area under the plasma concentration–time curve from 0 to the time of the last detectable concentration time point (AUCt) was estimated using a linear trapezoidal approximation. The area from time t to infinity (AUCt–∞) was estimated as Ct/kel, where Ct represents the plasma concentration at time t. The total area under the curve (AUC) was estimated as the sum of AUCt plus AUCt–∞.
Statistical evaluation
In the first study, a sample size of 20 subjects (10 in each group) was deemed sufficient to provide at least an 80% power of detecting a 50% difference in the change between days 1 and 5 in the mean AUC of sildenafil using a 5% significance level. In the second study, a sample size of 12 subjects (six in each treatment sequence group) was considered adequate to have at least an 80% power of detecting a 50% difference in the mean AUC and Cmax of sildenafil with and without antacid, using a 5% significance level, and to ensure sufficient residual degrees of freedom to allow useful conclusions to be drawn from the data analysis.
In the first study, AUCt and Cmax (log transformed) and kel and tmax (untransformed) were subjected to analysis of variance (anova) appropriate for a parallel-group study, and comparisons were made between the two treatment groups with respect to changes from day 1 to day 5. Similar analyses were performed on the pharmacokinetic data from the second study, except that the anovas were appropriate for a crossover study, and the comparisons were between periods.
Adverse events were considered treatment-related if the investigator noted them as such, if an uncertain relationship was noted, or if no record of possible relationship was made. Laboratory test data were evaluated against the investigator's abnormality criteria and assessed by the sponsor for causality.
Results
Subjects
Twenty-two subjects, aged 18–39 years (mean body weight 60–80 kg), enrolled in the first study and 20 completed it. Two subjects withdrew on day 1 for reasons unrelated to the study. All 22 subjects were assessed for safety and tolerability, and 20 provided pharmacokinetic results (10 in each group). Twelve subjects entered and completed the second study. They ranged in age from 20 to 43 years (body weight 64–83 kg).
Effects of cimetidine
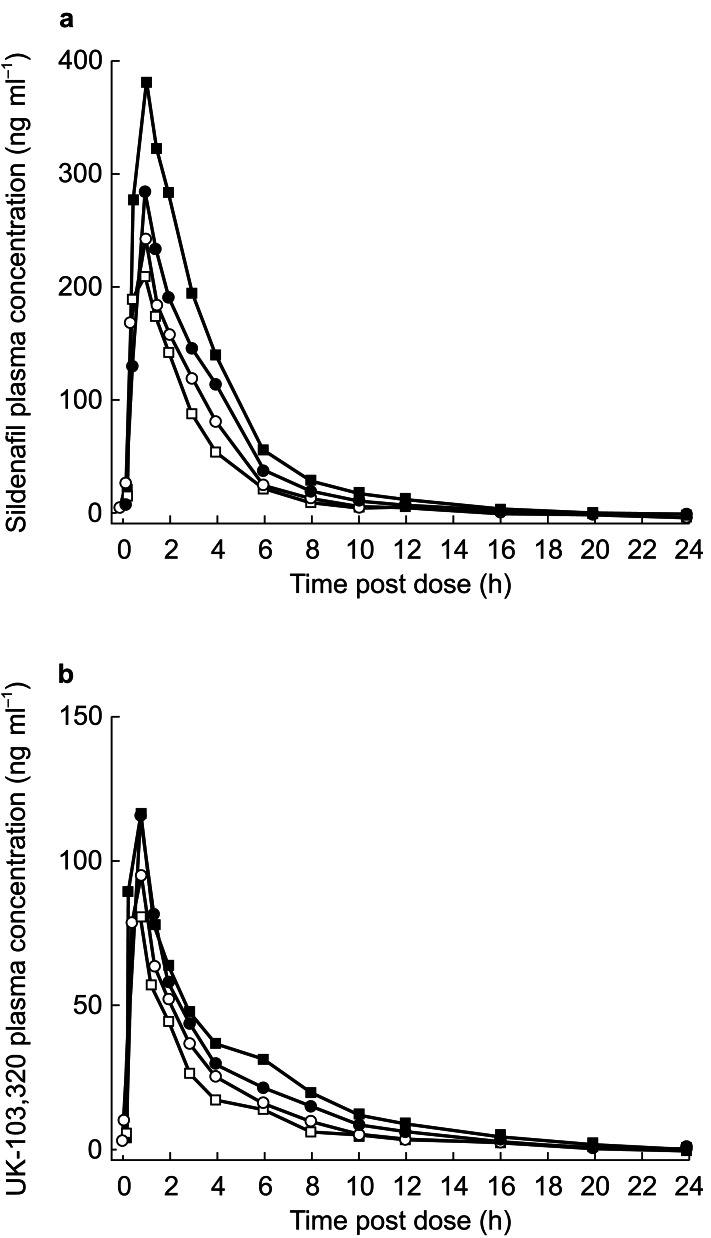
Pharmacokinetic parameters for sildenafil and UK-103,320 are shown in Table 1, and plasma concentration profiles are shown in Figure 1. After sildenafil administration on days 1 and 5, Cmax of both parent and metabolite was reached at approximately 1 h postdose in both the cimetidine and placebo groups. However, the co-administration of cimetidine did cause significant increases in both the AUCt (P<0.01) and Cmax (P<0.01) of sildenafil compared with co-administration of placebo.
Table 1.
Pharmacokinetic parameters of sildenafil and UK-103,320 following administration of sildenafil (S) alone or in combination with cimetidine (C) or placebo (P). Geometric means are presented for AUC and Cmax. Arithmetic means are presented for tmax and kel. P-value for comparison between treatment groups; n=10.
| Day | S+C | Day 5/1 | S+P | Day 5/1 | Ratio* or difference† | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|
| Sildenafil | ||||||||
| AUC (ng ml−1 h) | 1 | 883 | 666 | |||||
| 5 | 1241 | 1.41 | 598 | 0.9 | 1.56 | 1.21, 2.03 | 0.002 | |
| Cmax (ng ml−1) | 1 | 283 | 250 | |||||
| 5 | 383 | 1.35 | 220 | 0.88 | 1.54 | 1.14, 2.09 | 0.0076 | |
| tmax (h) | 1 | 1.4 | 1.1 | |||||
| 5 | 0.9 | −0.5 | 1.1 | 0 | −0.5 | −1.13, 0.13 | 0.111 | |
| kel (h−1) | 1 | 0.228 | 0.270 | |||||
| 5 | 0.197 | −0.03 | 0.266 | −0.00 | −0.03 | −0.09, 0.04 | 0.38 | |
| UK-103,320 | ||||||||
| AUC (ng ml−1 h) | 1 | 346 | 261 | |||||
| 5 | 407 | 1.18 | 236 | 0.9 | 1.30 | 1.01, 1.69 | 0.045 | |
| Cmax (ng ml−1) | 1 | 111 | 98 | |||||
| 5 | 115 | 1.04 | 88 | 0.90 | 1.16 | 0.97, 1.39 | 0.090 | |
| tmax (h) | 1 | 1.2 | 1.1 | |||||
| 5 | 1.1 | −0.05 | 0.9 | −0.15 | 0.1 | −0.64, 0.84 | 0.780 | |
| kel (h−1) | 1 | 0.209 | 0.176 | |||||
| 5 | 0.178 | −0.03 | 0.216 | 0.04 | −0.07 | −0.13, −0.02 | 0.014 | |
ratio of geometric means.
difference in arithmetic means.
Figure 1.
Plasma concentration over time for sildenafil (a) and UK-103,320 (b) following administration of a 50-mg dose of sildenafil alone (○ day 1; □ day 5) or in combination with cimetidine (• day 1; ▪ day 5).
In groups I (sildenafil+cimetidine) and II (sildenafil+placebo), the ratio of the mean AUCt between day 5 and day 1 was 1.41 and 0.9, respectively; thus, the difference between treatment groups was statistically significant (P=0.002), with cimetidine increasing exposure to sildenafil by approximately 56%. Similarly, the respective Cmax ratios between days 5 and 1 were 1.35 and 0.88 for groups I and II, a significant difference between treatment groups (P=0.0076). Cimetidine had no effect on the kel of sildenafil and therefore did not alter its terminal half-life.
Overall, the differences between treatment groups were smaller for UK-103,320 than for sildenafil. Although cimetidine did not statistically significantly affect the Cmax of UK-103,320, it did significantly increase exposure (AUCt) to the metabolite by approximately 30%. Cimetidine caused a small (0.07 h−1) reduction in UK-103,320 kel with a consequent 0.7-h increase in the terminal half-life.
Effects of antacids
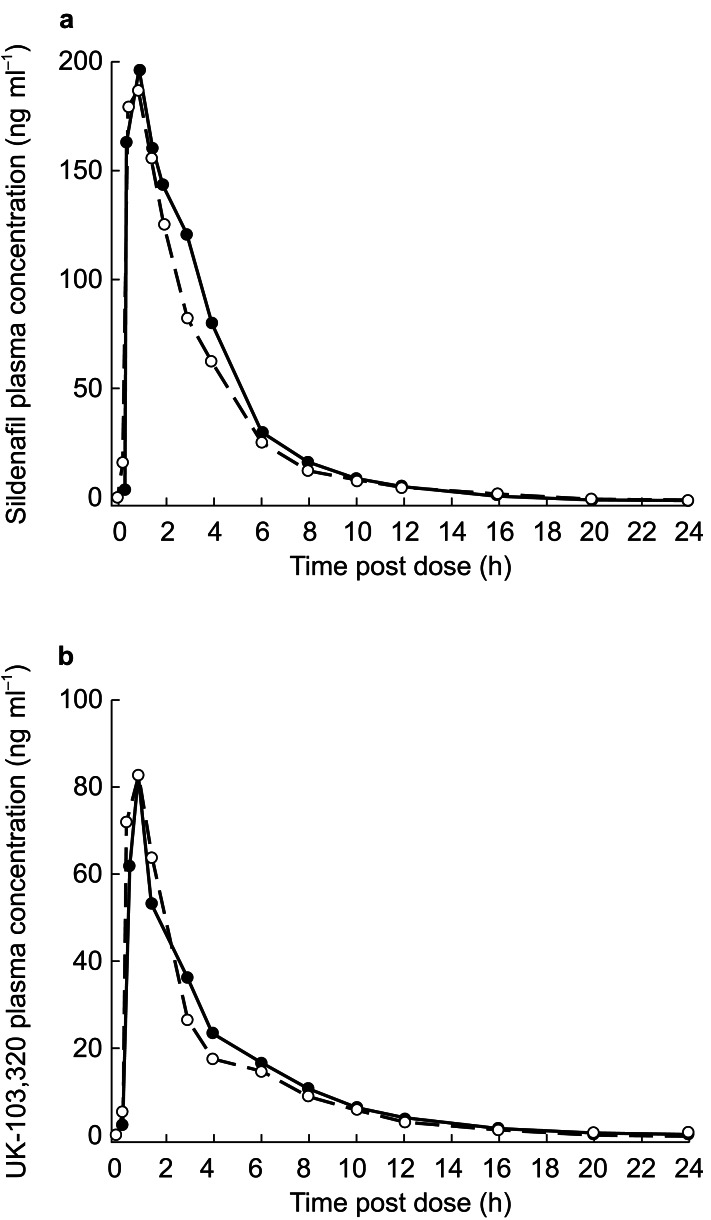
Pharmacokinetic parameters for sildenafil and UK-103,320 are shown in Table 2. Plasma concentration profiles (Figure 2) showed that the Cmax of both parent and metabolite were reached at approximately 1 h postdose, whether sildenafil was administered with or without an antacid. No statistically significant treatment differences were observed for any pharmacokinetic parameter of sildenafil or UK-103,320.
Table 2.
Pharmacokinetic parameters for sildenafil and UK-103,320 in the presence and absence of an antacid. Geometric means are presented for AUC and Cmax. Arithmetic means are presented for tmax and kel. P-value for comparison between treatment groups; n=12.
| Parameter | Sildenafil+antacid | Sildenafil+placebo | Ratio* or difference† | 95% CI | P-value |
|---|---|---|---|---|---|
| Sildenafil | |||||
| AUC (ng ml−1 h) | 627 | 700 | 89.5% | 79.8, 100.5 | 0.112 |
| Cmax (ng ml−1) | 238 | 238 | 99.9% | 80.3, 124.3 | 0.995 |
| tmax (h) | 1.21 | 0.79 | −0.42 | −0.79, −0.04 | 0.070 |
| kel (h−1) | 0.22 | 0.23 | 0.007 | −0.021, 0.035 | 0.668 |
| UK-103,320 | |||||
| AUC (ng ml−1 h) | 271.5 | 258.9 | 95.4% | 88.6, 102.6 | 0.269 |
| Cmax (ng ml−1) | 95.5 | 99.5 | 104.2% | 86.7, 125.2 | 0.694 |
| tmax (h) | 1.08 | 0.79 | −0.29 | −0.70, 0.12 | 0.229 |
| kel (h−1) | 0.22 | 0.21 | −0.005 | −0.033, 0.023 | 0.757 |
ratio of geometric means.
difference in arithmetic means.
Figure 2.
Plasma concentration over time for sildenafil (a) and UK-103,320 (b) following administration of a 50-mg dose of sildenafil alone (•) or in combination with an antacid (○).
Safety and tolerability
Whether taken alone or in combination with cimetidine or antacids, sildenafil was well tolerated. No subject withdrew from either study for any reason attributable to the drug, and no treatment-related laboratory abnormalities or serious adverse events were observed. In the first study, the incidence of adverse events was similar across treatment groups (group I: sildenafil 3/11, sildenafil+cimetidine 4/10; group II: sildenafil 6/11, sildenafil+cimetidine 2/10), as well as on days 1 and 5, indicating that neither cimetidine nor placebo affected the safety or tolerability of sildenafil. Most treatment-related adverse events were mild and resolved within 1 day, with headache being the most common adverse event. In the second study, all treatment-related adverse events were mild in severity, and their incidence was similar between the two periods.
No clinically significant changes in blood pressure and pulse rate or abnormalities in ECG were reported; furthermore, no subject discontinued because of laboratory test result abnormalities.
Discussion
The first study reported here was conducted to evaluate the effects of multiple-dose cimetidine co-administration on the pharmacokinetic characteristics of sildenafil and UK-103,320. The potential for interactions with this agent were of particular interest because cimetidine is an inhibitor of the hepatic cytochrome P450 CYP3A4 isoform [11, 12], which is the major P450 enzyme responsible for metabolizing sildenafil [7]. Moreover, cimetidine may also influence the absorption of some compounds through its effect on gastric pH [10]. It should be noted that although sildenafil is a P450 substrate, it is not an inhibitor of CYP3A4 at clinically relevant doses [7, 15] and would thus not be expected to alter the pharmacokinetic profile of CYP3A4 inhibitors, such as cimetidine. Cimetidine had no significant effect on the tmax or kel of sildenafil, although it did cause a significant increase in sildenafil AUCt and Cmax, 56% and 54%, respectively. Differences in pharmacokinetic parameters between the two treatment groups were smaller for UK-103,320 than for the parent drug. These data suggest that cimetidine inhibited a proportion of the gut wall and hepatic first pass metabolism of sildenafil, increasing Cmax and AUC, whilst not changing its systemic clearance or kel.
Given the efficacy and safety profile of sildenafil, this increase in exposure is of no clinical significance and does not require dosage adjustment of either drug when taken concomitantly. This was supported by the fact that there were no serious adverse events or treatment-related laboratory test abnormalities, no subject withdrew from the study for any study-related reason, and most adverse events were mild. In contrast, co-administration of more potent CYP3A4 inhibitors, such as the antibiotic erythromycin [15] and the protease inhibitors ritonavir and saquinavir [16], can lead to much greater increases in sildenafil plasma levels (300%, 140%, and 160% increases in Cmax, respectively). This has resulted in recommendations for a lower, 25-mg starting dose of sildenafil when co-administered with these agents [17, 18].
The second study reported here was conducted to assess the effect of antacid co-administration on the pharmacokinetics of sildenafil and UK-103,320. Antacids are known to interact with a number of drug classes, such as tetracyclines, nonsteroidal anti-inflammatory agents, and β-blockers, affecting drug absorption through gastric pH changes, adsorption, or chelation [13, 19]. In the current study, no significant effects were noted on any pharmacokinetic parameter of either the parent drug or its metabolite. Thus, pH changes induced by antacid co-administration do not significantly affect sildenafil absorption and consequent plasma concentration profile.
In conclusion, multiple doses of cimetidine (800 mg d−1) caused statistically significant increases in sildenafil systemic exposure, but these changes were thought to be clinically insignificant. Co-administration with an antacid did not alter the pharmacokinetic profile of sildenafil.
Acknowledgments
This study was funded by Pfizer Global Research and Development.
References
- 1.Nandurkar S, Talley NJ, Xia H, Mitchell H, Hazel S, Jones M. Dyspepsia in the community is linked to smoking and aspirin use but not to Helicobacter pylori infection. Arch Intern Med. 1998;158:1427–1433. doi: 10.1001/archinte.158.13.1427. [DOI] [PubMed] [Google Scholar]
- 2.Griffin MR. Epidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injury. Am J Med. 1998;104:23S–29S. doi: 10.1016/s0002-9343(97)00207-6. [DOI] [PubMed] [Google Scholar]
- 3.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts male aging study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 4.Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 5.Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 6.Warrington JS, Shader RI, Von Moltke LL, Greenblatt DJ. In vitro biotransformation of sildenafil (Viagra®): identification of human cytochromes and potential drug interactions. Drug Metabol Dispos. 2000;28:392–397. [PubMed] [Google Scholar]
- 7.Hyland R, Roe EGH, Jones BC. Smith DA. Identification of the cytochrome P450 enzymes involved in the N-demethylation of sildenafil. Br J Clin Pharmacol. 2001;51:239–248. doi: 10.1046/j.1365-2125.2001.00318.x. 10.1046/j.1365-2125.2001.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merry C, Barry MG, Ryan M, et al. Interaction of sildenafil and indinavir when co-administered to HIV-positive patients. AIDS. 1999;13:F101–F107. doi: 10.1097/00002030-199910220-00001. [DOI] [PubMed] [Google Scholar]
- 9.Binder HJ, Donaldson RM., Jr Effect of cimetidine on intrinsic factor and pepsin secretion in man. Gastroenterol. 1978;74:371–375. [PubMed] [Google Scholar]
- 10.Sedman AJ. Cimetidine–drug interactions. Am J Med. 1984;76:109–114. doi: 10.1016/0002-9343(84)90758-7. [DOI] [PubMed] [Google Scholar]
- 11.Deakin M, Williams JG. Histamine H2-receptor antagonists in peptic ulcer disease. Drugs. 1992;44:709–719. doi: 10.2165/00003495-199244050-00003. [DOI] [PubMed] [Google Scholar]
- 12.Wormsley KG. Safety profile of ranitidine: a review. Drugs. 1993;46:976–985. doi: 10.2165/00003495-199346060-00004. [DOI] [PubMed] [Google Scholar]
- 13.D'Arcy PF, McElnay JC. Drug–antacid interactions: Assessment of clinical importance. Drug Intell Clin Pharm. 1987;21:607–617. doi: 10.1177/1060028087021007-806. [DOI] [PubMed] [Google Scholar]
- 14.Cooper JDH, Muirhead DC, Taylor JE, Baker PR. Development of an assay for the simultaneous determination of sildenafil (Viagra®) and its metabolite (UK-103,320) using automated sequential trace enrichment of dialysates and high performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;701:87–95. doi: 10.1016/s0378-4347(97)00339-3. [DOI] [PubMed] [Google Scholar]
- 15.Muirhead GJ, Faulkner S, Harness JA, McEwen J, Taubel J. The effects of steady-state erythromycin and azithromycin on the pharmacokinetics of sildenafil in healthy volunteers. Br J Clin Pharmacol. 2002;53(Suppl. 1):37–43. doi: 10.1046/j.0306-5251.2001.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muirhead GJ, Wulff MB, Fielding A, Kleinermans D, Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000;50:99–107. doi: 10.1046/j.1365-2125.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfizer Laboratories, Division of Pfizer Inc. New York: Pfizer Inc.; 2000. Viagra® (sildenafil citrate) prescribing information. [Google Scholar]
- 18.Cheitlin MD, Hutter AM, Brindis RG, et al. ACC/AHA Expert Consensus Document. Use of sildenafil (Viagra®) in patients with cardiovascular disease. J Am Coll Cardiol. 1999;33:273–282. doi: 10.1016/s0735-1097(98)00656-1. [DOI] [PubMed] [Google Scholar]
- 19.Brunton LL. Agents for control of gastric acidity and treatment of peptic ulcers. In: Gilman AG, Rail TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. 9. New York: Pergamon Press; 1990. pp. 901–915. [Google Scholar]