Abstract
Aims
To determine the absolute bioavailability, dose proportionality and the effects of food on the pharmacokinetics of single oral doses of sildenafil citrate.
Methods
Three open-label, randomized crossover studies were conducted in healthy male subjects. Absolute bioavailability was determined by comparing pharmacokinetic data after administration of single oral and intravenous 50-mg doses of sildenafil (n = 12 subjects). Food effects were examined by comparing pharmacokinetic data for sildenafil and its primary circulating metabolite, UK-103,320, after administration of a single oral 100-mg dose in the fasted and fed states (n = 34 subjects). Dose proportionality was assessed from pharmacokinetic data obtained after administration of four single oral doses of sildenafil (25, 50, 100 and 200 mg) to 32 subjects. The safety and tolerability of sildenafil were also assessed in all of these studies.
Results
The calculated absolute oral bioavailability of sildenafil was 41% (90% CI: 36–47). Food slowed the rate of absorption, delaying mean tmax by approximately 1 h and reducing Cmax by 29% (90% CI: 19–38). Systemic exposure, as assessed by the mean area under the plasma concentration–time curve (AUC), was reduced by 11% (90% CI: 6–16). These food effects were not considered to be of clinical significance. There was statistical evidence of nonproportionality in Cmax and AUC over the dose range 25–200 mg. However the degree of nonproportionality was small, with predicted increases in Cmax and AUC of 2.2- and 2.1-fold, respectively, for a doubling in dose, and was thought to be clinically nonsignificant. Sildenafil was well tolerated in the three studies; the majority of adverse events were mild and transient.
Conclusions
Sildenafil had a mean absolute bioavailability of 41%. Food caused small reductions in the rate and extent of systemic exposure; these reductions are unlikely to be of clinical significance. Across the dose range of 25–200 mg, systemic exposure increased in a slightly greater than dose-proportional manner.
Keywords: sildenafil; UK-103,320; pharmacokinetics; bioavailability; food effects; safety
Introduction
Erectile dysfunction (ED) is a widespread condition with a markedly negative impact on quality of life [1]. Until recently, no effective oral therapy existed; the only treatments available were highly cumbersome or invasive, and many men found them unacceptable as solutions to the problem of achieving or maintaining a satisfactory erection [2].
The ideal oral treatment would have the following attributes: easy and discreet administration within a reasonably short time prior to sexual activity, reliable efficacy, good tolerability across the therapeutic range, high selectivity for the site of action, lack of central nervous system effects, rapid absorption that yields prompt onset of action and a plasma half-life that produces an appropriate duration of action while avoiding accumulation upon repeated once-daily use.
Sildenafil citrate (Viagra®, Pfizer), the first oral agent to be introduced for the treatment of ED, meets these criteria. It is rapidly absorbed and acts within 30 min to 1 h; it has a short plasma half-life of approximately 4 h; and it is well tolerated in the dosage range studied, with no clinically appreciable effects on heart rate or blood pressure [3–5]. In clinical trials, single oral doses of sildenafil between 25 and 100 mg have been shown to be effective in the treatment of erectile dysfunction of organic, psychogenic or mixed aetiologies [4, 6, 7].
Three open-label, randomized studies were conducted to investigate the pharmacokinetics of sildenafil following single oral doses in healthy volunteers. The objectives were to estimate absolute bioavailability by comparing pharmacokinetic data after oral (PO) and intravenous (IV) dosing; to determine the effect of food on the drug's absorption, bioavailability and elimination; and to assess dose proportionality of the pharmacokinetics over the dose range of 25–200 mg. The studies also included assessments of safety and tolerability.
Methods
Subjects and study design
Healthy male volunteers, aged 18–45 years, were eligible for inclusion in each study if they weighed between 60 and 90 kg, and were within acceptable weight ranges for their height and frame size. Subjects were excluded from this study if they had evidence of any clinically significant disease or abnormality, including asthma, eczema, drug hypersensitivity and/or a personal or family history of bleeding disorder, migraine or peptic ulceration; if they had taken any prescription or over-the-counter medication in the 2 weeks prior to the study (except paracetamol) or any experimental drug in the 6 months prior to the study; if they abused drugs or consumed excessive amounts of alcohol per week or smoked more than five cigarettes per day; or if the results of hepatitis B or HIV tests were positive.
For each study, power calculations were performed a priori. For the absolute bioavailability study, a sample size of 12 subjects was considered sufficient to estimate the difference in bioavailability between PO and IV sildenafil with reasonable confidence. For the food effects study, a sample size of 34 (17 per sequence group) was considered sufficient to detect a 20% difference in AUC and Cmax of sildenafil with a probability of 0.80 when testing at the 5% level. Using the same testing parameters, a sample size of 32 (eight per sequence group) was chosen for the dose-proportionality study.
All subjects provided written informed consent before entering, and the protocols were reviewed and approved by the local ethics committee.
The absolute bioavailability study was a two-way crossover study in which 12 subjects received one 50-mg dose of PO sildenafil and one 50-mg dose of IV sildenafil. The dosing sequence was randomized, with six subjects allocated to each of the two sequences. Each dose was separated by a washout period of at least 10 days.
Before participating in the study, the subjects underwent routine laboratory safety tests, a urine screen for drugs of abuse, a 12-lead electrocardiogram (ECG), physical examination and measurement of supine blood pressure and heart rate. They were not permitted to consume alcohol or caffeine-containing food or drinks for 36 h before dosing and throughout their stay in the study centre. They also were not permitted to use products containing salicylates from 2 weeks prior to the study until after the follow-up visit. Strenuous exercise was also discouraged.
On the evening before dosing, the subjects entered the study centre and abstained from food and drink (except water) from 22:00 onward. Dosing occurred between 8:00 and 9:00 the following morning. Oral sildenafil was administered as two 25-mg capsules taken with 240 ml water, and IV sildenafil was administered as 50 ml of a 1-mg ml−1 solution infused at 1 ml min−1. Standardized meals were provided at about 4 and 10 h after drug administration, with a snack at 12–14 h postdose. Paracetamol (up to 1 g per day) was permitted for pain relief during the study.
The food-effect study was also a two-way crossover design, in which 34 subjects received 1 PO dose of sildenafil (100 mg) while fasting and a second dose after consuming a high-fat meal. The dosing sequence was randomized, with 17 subjects assigned to each sequence. Each dose was separated by a washout period of 7 days.
The subjects underwent a screening examination similar to that of the bioavailability study before participation. During the 48 h prior to each study period, the subjects were asked to refrain from unaccustomed exercise and consumption of caffeine, methylxanthines or alcohol. On the evening prior to each dose, subjects entered the study unit and abstained from all food and drink (except water) from 22:00 onward. Dosing took place between 7:00 and 9:00. Sildenafil was supplied as one 100-mg tablet for each study period. Subjects who received the drug after food consumed a high-fat breakfast (fat 67.9 g, protein 49.9 g, carbohydrate 44.8 g) with a total caloric value of 4092 kjoules, and took the sildenafil tablet within 5 min of completing it. Fasting subjects took the drug with 240 ml of water, and fed subjects took it with 50 ml of water. A standard lunch and dinner were served after the 4-h and 10-h postdose blood samples had been collected.
The dose-proportionality study was designed as a four-way crossover study, in which 32 subjects received four single doses of sildenafil (25, 50, 100 and 200 mg), each separated by washout periods of at least 7 days. The dosing sequences were randomized, with eight subjects assigned to each treatment sequence. The protocol for each period was identical to that of the food-effect study, except that sildenafil doses were administered as a 25-mg, 50-mg or 100-mg tablet, or as two 100-mg tablets, taken with 240 ml of water.
Pharmacokinetic assessments
In the absolute bioavailability study, blood samples sufficient to provide 3 ml of plasma for pharmacokinetic analyses were collected in the morning prior to PO and IV dosing of sildenafil. After each dose, samples were taken at appropriate time points up to 120 h. In the food-effect and dose-proportionality studies, blood samples were collected predose and at appropriate intervals up to 24 h after dosing.
Plasma concentrations of sildenafil in the absolute bioavailability study were determined using high-performance liquid chromatography (HPLC) and atmospheric pressure chemical ionization mass spectroscopy. The calibration range of the assay was 0.1–2.0 ng ml−1. The limit of quantification for sildenafil was 0.3 ng ml−1 and the interassay variation ranged from −35 to 8.6%. Total plasma concentrations of sildenafil and its main active metabolite, UK-103,320, in the food-effect and dose-proportionality studies were determined using automated sequential trace enrichment of dialysates and HPLC as described by Cooper et al.[8]. The calibration range of the assay was 0.1–2.0 ng ml−1. The limits of quantification were 1 ng ml−1 for both sildenafil and UK-103,320. The overall imprecision (CV) was 5.1%, 3.2% and 3.0% for sildenafil and 3.4%, 3.1% and 2.9% for UK-103,320 concentrations of 3.00, 125 and 200 ng ml−1, respectively. The inaccuracy (bias) of the assay at all concentrations ranged from −2.3% to 3.5% for sildenafil and −7.0% to 4.8% for UK-103,320.
All pharmacokinetic parameters were calculated by noncompartmental analysis. The following pharmacokinetic parameters were calculated for sildenafil and its primary circulating metabolite UK-103,320: maximum observed plasma concentration (Cmax), time to reach maximum observed plasma concentration (tmax), apparent terminal elimination rate constant (kel), apparent terminal half-life (t1/2), area under the plasma concentration-time curve from time zero to the time of the last measurable concentration (AUCt), and area under the plasma concentration-time curve extrapolated to infinity (AUC). In addition, in the absolute bioavailability study, clearance (CL) and steady state volume of distribution (Vss) were calculated after IV dosing, together with an estimate of absolute bioavailability (F).
Safety and tolerability
In all studies, laboratory safety tests and physical examinations were performed prior to dosing and at regular intervals postdose. Supine blood pressure, heart rate and 12-lead ECG were also monitored throughout the absolute bioavailability study. These evaluations were repeated 7–14 days after the final dose.
The nature and severity of all observed or volunteered adverse events and the investigator's assessment of the relationship of each adverse event to study treatment were recorded throughout the studies.
Statistical evaluation
For the absolute bioavailability study, natural log-transformed AUC and Cmax and untransformed tmax and kel data were subjected to analyses of variance (anova) appropriate to the study design. The 90% confidence intervals (CI) for the ratio between geometric means for AUC and Cmax for PO/IV administration and differences between arithmetic means for tmax and kel after PO and IV administration were calculated.
For the food-effect study, AUC, Cmax, tmax and kel data for sildenafil administered in the fed and fasted state were compared using anova appropriate to the study design. For Cmax and AUC data, the means and 90% CI were computed on the logarithmic scale and then back transformed to give the ratio and CI on a nominal scale. For kel and tmax, means and 90% CI were calculated without transformations and compared between treatments.
For the dose-proportionality study, an anova appropriate to the study design was performed on natural log-transformed dose-normalized AUC, Cmax, tmax and kel values for sildenafil. Ninety percent CIs for the differences between treatment means (25 mg vs 50, 100 and 200 mg; 50 mg vs 100 mg, and 100 mg vs 200 mg) and standard errors associated with these differences were calculated. For normalized AUC and Cmax, the mean difference and 90% CI were back transformed to obtain the mean ratio of treatments and 90% CI for the ratio. Using a linear model, data were considered to be dose proportional if the normalized AUC and Cmax CI for the ratios were within the interval of 80–125%, and if there were no significant differences between treatments for mean tmax and kel.
Results
Subjects
All subjects were between the ages of 18 and 45 years, and weighed between 59 and 97 kg. One subject was withdrawn from the dose-proportionality trial after the first treatment period (200-mg dose of sildenafil) due to abnormal results of liver function tests taken at screening. Data from this subject were included in the safety evaluations but not the pharmacokinetic evaluations. All of the other subjects completed their respective studies. No other subjects from the other studies had medical histories or findings that would adversely affect interpretation of the results, and no other serious protocol violations were identified.
Pharmacokinetics
Absolute bioavailability
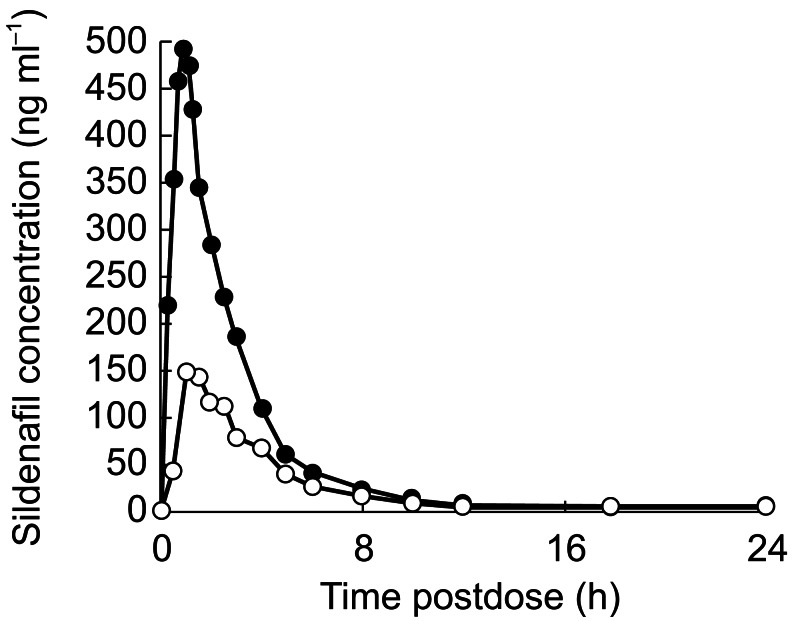
Figure 1 shows the total plasma concentration profiles of sildenafil after IV and PO administration. Table 1 shows the mean pharmacokinetic parameters after PO and IV dosing. During IV infusion, sildenafil plasma levels increased rapidly; peak plasma concentrations were achieved in seven of the 12 subjects before the end of the 1-h infusion. At the end of the infusion, plasma concentrations declined in a biphasic manner, with a mean terminal phase half-life of 3.9 h. All subjects had very low, albeit detectable plasma concentrations of sildenafil at 24 h (1 ng ml−1) and 32 h (0.3 ng ml−1) after receiving the drug, compared with a peak plasma concentration of 531 ng ml−1. After PO administration, plasma concentrations of sildenafil increased rapidly, with Cmax occurring between 0.5 and 2.5 h postdose. Plasma concentrations then declined in a biphasic manner with a mean terminal half-life of 4.1 h. Ten of 12 subjects had barely detectable plasma concentrations of sildenafil at 24 h (0.8 ng ml−1) and 32 h (0.3 ng ml−1) after receiving the drug, compared with a peak plasma concentration of 159 ng ml−1. After both PO and IV administration, AUCt accounted for more than 99% of the total extrapolated AUC (where t = 32 h in most cases). The absolute oral bioavailability, calculated from the ratio of AUC geometric means (PO/IV) was 41% (90% CI: 35.6–47.3). The Cmax after oral administration was 30.1% of the Cmax after IV infusion (90% CI: 26.8–33.8). The terminal phase rate constant and consequently terminal half-lives were not significantly different between the two routes of administration.
Figure 1.
Mean plasma concentration profiles of sildenafil following oral (○) and intravenous (•) administration of 50-mg sildenafil; values are least squares mean, n = 12 per group.
Table 1.
Pharmacokinetic parameters of sildenafil following intravenous (IV) or oral (PO) administration of 50-mg sildenafil. Geometric mean data are presented for AUC, AUCt and Cmax, arithmetic means are presented for tmax, kel, CL, V and Vss, and harmonic means are presented for t1/2. Absolute oral bioavailability (F) was calculated from the ratio of AUC geometric means following oral/intravenous administration. n = 12 per group.
| Parameter | IV | PO |
|---|---|---|
| AUC (ng ml−1 h) | 1291 | 530 |
| AUCt (ng ml−1 h) | 1289 | 528 |
| Cmax (ng ml−1) | 531 | 159 |
| tmax (h) | 0.73 | 1.46 |
| kel (h−1) | 0.18 | 0.17 |
| t1/2 (h) | 3.92 | 4.07 |
| CL (l h−1) | 40.8 | – |
| V (l) | 234 | – |
| Vss (l) | 105 | – |
| F (90% CI) | 41% (35.6–47.3) | |
Food effects
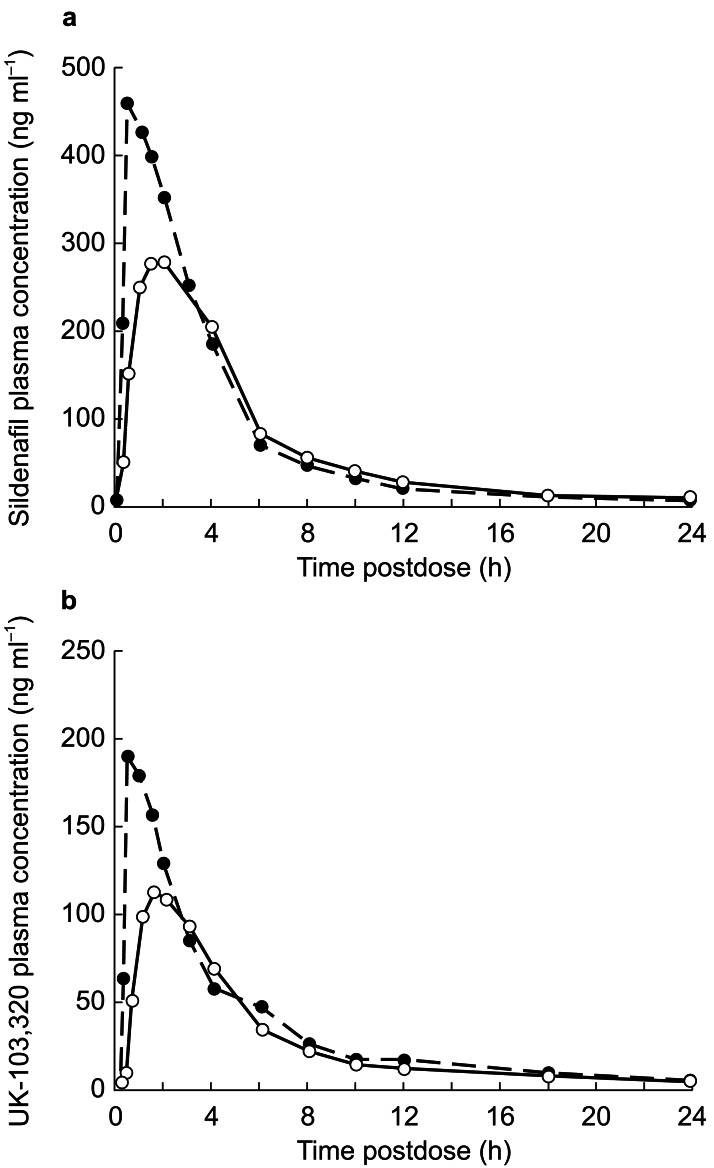
The pharmacokinetic parameters of sildenafil and UK-103,320 are summarized in Table 2. In the food-effect study, statistically significant differences were observed between the fasted and fed states for sildenafil Cmax and tmax (P <0.001). In fed subjects, the Cmax of sildenafil was 71% (90% CI: 62–81) of that in fasting subjects. Food also slowed the rate of absorption, delaying the mean tmax by 1.1 h (90% CI: 0.7–1.5). The relative bioavailability of sildenafil after the high-fat meal was 89% (90% CI: 84–94) of that after fasting (P <0.01). In addition, food produced a small reduction of 0.014 h−1 in kel and a consequent increase of 0.35 h in t1/2; these changes were statistically significant but unlikely to be of clinical significance. The data for UK-103,320 were similar to those of sildenafil; in fasting subjects, AUC and Cmax were both higher and tmax was shorter than in fed subjects. Plasma concentration profiles for sildenafil and UK-103,320 are shown in Figure 2.
Table 2.
Pharmacokinetic parameters of sildenafil and UK-103,320 following administration of 100-mg sildenafil in the fed or fasted state. Geometric means are presented for AUC, AUCt and Cmax, arithmetic means are presented for tmax and kel and harmonic means are presented for t1/2. Fed:fasted ratios for AUC and Cmax were derived from comparing the geometric means for these parameters. n = 34 per group.
| Sildenafil | UK-103,320 | |||
|---|---|---|---|---|
| Parameter | Fed | Fasted | Fed | Fasted |
| AUC (ng ml−1 h) | 1489 | 1670 | 571 | 729 |
| Fed:fasted AUC ratio (90% CI) | 0.89 (84–94) | |||
| AUCt (ng ml−1 h) | 1465 | 1651 | 547 | 700 |
| Cmax (ng ml−1) | 364* | 514 | 137 | 215 |
| Fed:fasted Cmax ratio (90% CI) | 0.71 (62–81) | |||
| tmax (h) | 2.04* | 0.95 | 1.94 | 0.90 |
| kel (h−1) | 0.16† | 0.17 | 0.11 | 0.12 |
| t1/2 (h) | 4.33† | 3.98 | 6.1 | 5.94 |
P <0.001
P <0.05 compared to fasted group.
Figure 2.
Plasma concentration profiles of sildenafil (a) and UK-103,320 (b) following oral administration of 100-mg sildenafil in nonfasting (fed) (○) and fasting (•) subjects; values are least squares mean, n = 34 per group.
Dose proportionality
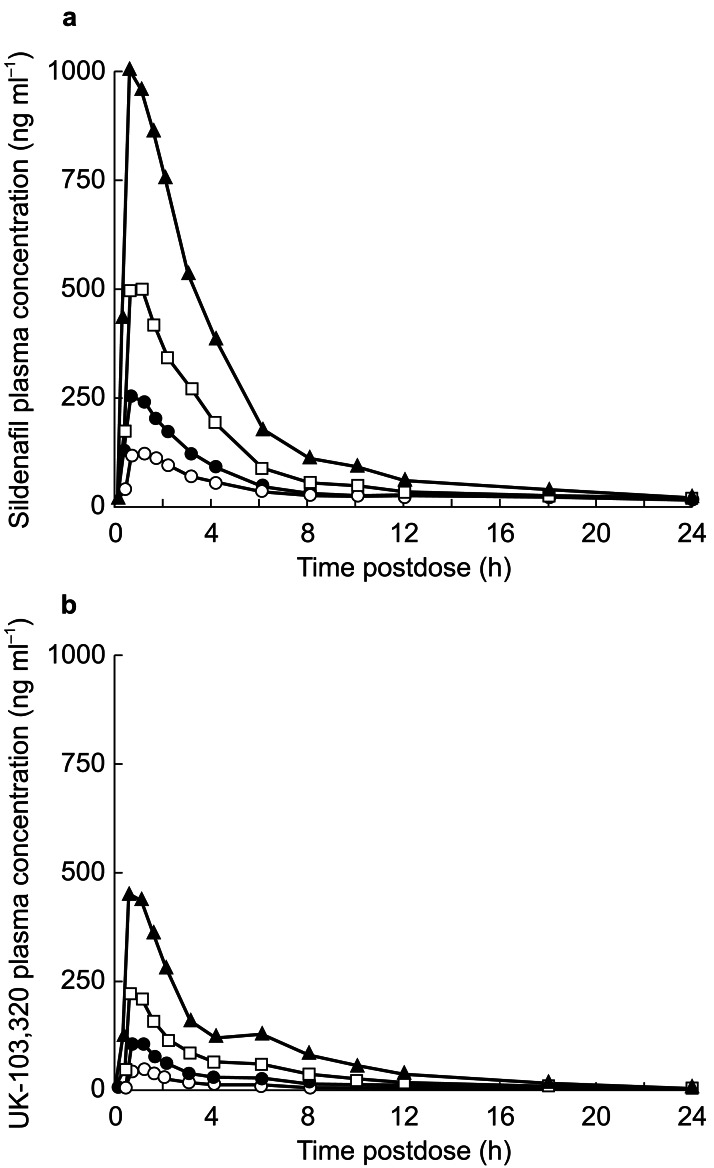
Absorption of sildenafil was rapid after all doses, with mean tmax values of approximately 1 h (Figure 3). After all doses, plasma concentrations declined after peaking in a bi-exponential manner with mean terminal half-life values of between 2.6 and 3.8 h (Table 3). Increases in Cmax and AUC were proportional to dose when consecutive dose increments were compared, but there was evidence of nonproportionality over the whole dose range studied for both parameters. Cmax values were dose-normalized by dividing the Cmax by the dose of drug (Table 3). The respective adjusted geometric means were 5.1, 5.4, 5.6 and 5.8 ng ml−1 for the 25-, 50-, 100- and 200-mg doses, respectively. Likewise, the adjusted geometric means for dose-normalized AUC were 13.7, 14.8, 16.9 and 18.78 ng ml−1 h. Regression analysis provided estimates of the nonproportionality by fitting a power function to the data. The power functions were 1.16 for AUC and 1.06 for Cmax, resulting in a predicted 2.23-fold increase in AUC and a 2.08-fold increase in Cmax for every twofold increase in dose. The extent of this nonproportionality is unlikely to be pharmacokinetically and clinically significant. No significant differences were observed between doses in tmax. For the four doses tested, the adjusted arithmetic mean kel values declined slightly with dose, and the corresponding harmonic mean t1/2 increased slightly with increasing dose (Table 3). These apparent changes were attributed to improved definition of the terminal phase at the higher doses of sildenafil.
Figure 3.
Plasma concentration profiles of sildenafil (a) and UK-103,320 (b) following PO doses of 25(○), 50(•), 100(□), or 200 mg(▴); values are least squares mean, n = 32 per group.
Table 3.
Pharmacokinetic parameters of sildenafil and UK-103,320 following administration of single oral doses of sildenafil ranging from 25 to 200 mg (dose-proportionality study). Geometric means are presented for AUC, AUCt and Cmax, arithmetic means are presented for tmax and kel and harmonic means are presented for t1/2. n = 32 per group.
| 25 mg | 50 mg | 100 mg | 200 mg | |
|---|---|---|---|---|
| Sildenafil | ||||
| AUC (ng ml−1 h) | 361* | 738 | 1685 | 3755 |
| AUCt (ng ml−1 h) | 334 | 727 | 1667 | 3702 |
| Cmax (ng ml−1) | 127 | 271 | 560 | 1150 |
| tmax (h) | 1.02 | 0.79 | 0.83 | 0.94 |
| kel (h−1) | 0.26* | 0.23 | 0.19 | 0.18 |
| t1/2 (h) | 2.62 | 2.96 | 3.73 | 3.84 |
| UK-103,320 | ||||
| AUC (ng ml−1 h) | 147* | 328 | 776 | 1822 |
| AUCt (ng ml−1 h) | 134 | 318 | 756 | 1772 |
| Cmax (ng ml−1) | 54 | 126 | 254 | 526 |
| tmax (h) | 0.97 | 0.78 | 0.77 | 0.88 |
| kel (h−1) | 0.24* | 0.20 | 0.15 | 0.14 |
| t1/2 (h) | 2.85* | 3.48 | 4.57 | 4.85 |
n = 30 per group.
Plasma concentrations of UK-103,320 (Cmax and AUC) were approximately 45% of those of the parent drug. Although no formal statistical analysis was conducted on the metabolite parameters, the relationship between sildenafil dose and UK-103,320 pharmacokinetics was similar to that for sildenafil itself. For all four doses, Cmax occurred within approximately 1 h of dosing, confirming the rapid production of UK-103,320 by first-pass metabolism [9].
Safety and tolerability
Sildenafil was well tolerated in all three studies. No subject discontinued treatment for any reason. The most commonly reported treatment-related adverse events were nausea, headache, abnormal vision, dizziness, limb pain, eye pain and vasodilatation; all were mild to moderate in severity and transient. All treatment-related adverse events resolved spontaneously. There were no reports of serious adverse events or treatment-related laboratory abnormalities. In the absolute bioavailability study, slight decreases from baseline in supine diastolic blood pressure were seen following both IV and PO administration of sildenafil; however, the lack of placebo control precludes any firm conclusions regarding the clinical relevance of these decreases. In the dose-proportionality trial, the incidence of adverse events increased with sildenafil dose. These were also mild in severity and transient, and resolved spontaneously. No consistent changes suggestive of a treatment effect in heart rate were observed, and no subject had a clinically significant change in ECG findings.
Discussion
The pharmacodynamic [4] and pharmacokinetic profiles of sildenafil are important contributing factors to its demonstrated effectiveness in the treatment of men with ED of various aetiologies [4, 6, 7]. The results of the present studies define the pharmacokinetic attributes that support the therapeutic efficacy of sildenafil, particularly when taken as needed.
In the present studies, sildenafil exhibited rapid oral absorption, as judged by the achievement of peak plasma concentrations at approximately 1 h postdose. This observation is consistent with the findings of Walker et al. who reported rapid and complete absorption of sildenafil from the gastrointestinal tract with a mean tmax of 1.2 h in man [9]. A modest, albeit statistically significant, reduction in the rate of absorption and no change in extent of absorption were observed when sildenafil was taken with food (high-fat meal), as compared to dosing in the fasting state. As a consequence of the effects of food on sildenafil pharmacokinetics, a similar picture was seen for its primary circulating metabolite, UK-103,320. These findings are in close agreement with those of a randomized, three-way crossover study in which 50-mg oral doses of sildenafil were administered in the fasted and nonfasted states to a group of nine healthy male volunteers [10]. The latter study also observed a statistically significant delay in sildenafil absorption (by 0.7 h, P <0.05) and a similar reduction in mean Cmax (−23%), with no significant alteration in the extent of absorption (AUC) [10]. The delay in sildenafil absorption is most likely due to delayed gastric emptying in the presence of food [11].
The mean absolute oral bioavailability of sildenafil was calculated to be 41%, in accordance with a value of 38% reported by other investigators [9]. The moderate oral bioavailability of sildenafil reflects gut wall and hepatic first-pass metabolism. Furthermore, the observed occurrence of maximum concentrations of the metabolite UK-103,320 within 1 h of dosing confirms the rapid first-pass biotransformation of sildenafil. Sildenafil was found to have a mean apparent steady state volume of distribution (approximately 105 l) that substantially exceeds the total volume of body water (approximately 42 l). This suggests drug distribution into tissues and possibly tissue binding. The mean plasma clearance rate of sildenafil observed in the absolute bioavailability study (41 l h−1) approximates the rate of hepatic blood flow in man, indicating that the liver is the predominant route of elimination of sildenafil as described by Hyland et al.[12]. The volume of distribution and clearance characteristics of sildenafil resulted in an apparent terminal half-life ranging from 3 to 4 h. The half-life of sildenafil provides for an appropriate duration of therapeutic effect and also prevents drug accumulation with chronic once-daily use.
Previous studies have shown that the pharmacokinetics of sildenafil and UK-103,320 were approximately dose proportional over the range of 1.25–200 mg, when administered orally [4, 10]. In the present dose-proportionality study, increases in Cmax and AUC were proportional to dose when consecutive dose increments were compared. Regression analysis predicted a 2.23-fold increase in AUC and a 2.08-fold increase in Cmax for every twofold increase in dose. Although this amounts to some statistical evidence of nonproportionality for both Cmax and AUC over the whole dose range (25–200 mg), the extent of the nonproportionality was considered pharmacokinetically and clinically nonsignificant.
Consistent with a ratio of 2.1 for Cmax sildenafil/UK-103,320 reported elsewhere [9], the present dose-proportionality study found plasma concentrations of UK-103,320 to be about 45% of those of the parent drug. At the 100-mg dose of sildenafil, the pharmacokinetic findings for UK-103,320 for the subjects in the dose-proportionality study and the fasting subjects in the food-effects study were in very close agreement.
The drug was well tolerated in all three studies. No subject was withdrawn from the trials for any reason related to treatment, and no serious adverse events were reported. Most adverse events were transient and mild in severity, and all adverse events resolved spontaneously. These observations are similar to the safety data from extensive double-blind and open-label studies in patients with ED [13, 14]. Headache and abnormal vision were the most common treatment-related adverse events. The abnormal vision reported after the 200-mg dose is consistent with findings from other clinical trials examining the effects of high doses of sildenafil on visual function in patients with ED [15]. These studies observed transient changes in the Farnsworth-Munsell 100-Hue test following a 200-mg dose of sildenafil that correlated with maximal plasma concentrations.
In summary, after oral single-dose administration, sildenafil was rapidly absorbed, reaching maximum plasma concentrations within approximately 1 h. Plasma sildenafil concentrations were generally proportional to the administered dose. Food slowed the rate but minimally affected the extent of drug absorption. The absolute bioavailability was 41% due to first-pass metabolism. Sildenafil was rapidly cleared from the body with a plasma elimination half-life of approximately 3–4 h over the dose range of 25 and 200 mg. These characteristics represent pharmacokinetic support for the effectiveness and appropriateness of sildenafil for the as-needed treatment of patients with ED. In addition, these studies showed that oral sildenafil is well tolerated over the approved dosage range (25–100 mg).
Acknowledgments
This study was funded by Pfizer Central Research.
References
- 1.National Institutes of Health. Consensus Development Panel on Impotence. JAMA. 1993;280:083–090. [Google Scholar]
- 2.Levine SB, Althof SE, Turner LA, et al. Side-effects of self-administration of intracavernous papaverine and phentolamine for the treatment of impotence. J Urol. 1989;141:54–57. doi: 10.1016/s0022-5347(17)40585-4. [DOI] [PubMed] [Google Scholar]
- 3.Eardley I, Brook J, Yates PK, Wulff MB, Boolell M. Sildenafil (Viagra®), a novel oral treatment with rapid onset of action for penile erectile dysfunction. Abstract. Br J Urol. 1997;79(Suppl 4):66. [Google Scholar]
- 4.Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 5.Jackson G, Benjamin N, Jackson N, Allen MJ. Effects of sildenafil citrate on human hemodynamics. Am J Cardiol. 1999;83:13C–20C. doi: 10.1016/s0002-9149(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 7.Dinsmore W, Hodges M, Hargreaves C, Osterloh I, Smith M, Rosen R. Sildenafil citrate (Viagra®) in erectile dysfunction: near normalization in men with broad-spectrum erectile dysfunction compared with age-matched healthy control subjects. Urology. 1999;53:800–805. doi: 10.1016/s0090-4295(98)00586-x. 10.1016/s0090-4295(98)00586-x. [DOI] [PubMed] [Google Scholar]
- 8.Cooper JDH, Muirhead DC, Taylor JE, Baker PR. Development of an assay for the simultaneous determination of sildenafil (Viagra®) and its metabolite (UK-103,320) using automated sequential trace enrichment of dialysates and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;701:87–95. doi: 10.1016/s0378-4347(97)00339-3. [DOI] [PubMed] [Google Scholar]
- 9.Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 10.Muirhead GJ, Allen MJ, James GC, et al. Pharmacokinetics of sildenafil (Viagra®), a selective cGMP PDE5 inhibitor, after single oral doses in fasted and fed healthy volunteers. Br J Clin Pharmacol. 1996;42:268P. [Google Scholar]
- 11.Sjöqvist Borgå O, Dahl M-L, Orme MLE. Fundamentals of clinical pharmacology. In: Speight TM, Holford NHG, editors. Avery's Drug Treatment. 4. Auckland, New Zealand: Adis International Limited; 1997. pp. 2–73. [Google Scholar]
- 12.Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-demethylation of sildenafil. Br J Clin Pharmacol. 2001;51:239–248. doi: 10.1046/j.1365-2125.2001.00318.x. 10.1046/j.1365-2125.2001.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales A, Gingell C, Collins M, Wicker PA, Osterloh IH. Clinical safety of oral sildenafil citrate (Viagra®) in the treatment of erectile dysfunction. Int J Impot Res. 1998;10:69–74. doi: 10.1038/sj.ijir.3900354. [DOI] [PubMed] [Google Scholar]
- 14.Sadovsky R, Miller T, Moskowitz M, Hackett G. Three-year update of sildenafil citrate (Viagra®) efficacy and safety. Int J Clin Pract. 2001;55:115–128. [PubMed] [Google Scholar]
- 15.Laties A, Ellis P, Koppiker N, Patat A, Stuckey B. Visual function testing in patients and healthy volunteers receiving Viagra®. Ophthalmic Res. 1998;30(Suppl 1):177. [Google Scholar]