Abstract
Aims
Sildenafil, an effective oral treatment for erectile dysfunction, is predominantly metabolized by the cytochrome P450 isozyme 3A4, which is inhibited by a number of the macrolide antibiotics. Therefore, two placebo-controlled, parallel-group studies were conducted to evaluate the effects of multiple doses of erythromycin and azithromycin on the pharmacokinetics, safety and tolerability of a single oral 100-mg dose of sildenafil.
Methods
In the erythromycin interaction study, 26 male volunteers (18–45 years of age) received open-label sildenafil 100 mg on day 1. Half received blinded erythromycin (500 mg) twice daily on days 2–6, and the other half received placebo. On day 6, all subjects received a second 100-mg dose of sildenafil. In the azithromycin interaction study, 24 male volunteers (19–33 years of age) received open-label 100 mg sildenafil on day 1. Half then received blinded azithromycin (500 mg) once daily on days 2–4, and the other half received placebo. On day 4, all subjects received another 100-mg dose of sildenafil. In both studies, blood samples were collected on the first and last study day for the analysis of plasma concentrations of sildenafil and its primary metabolite, UK-103,320.
Results
Repeated dosing with erythromycin caused statistically significant increases in the AUC and Cmax of sildenafil (2.8-fold and 2.6-fold, respectively) but had no effect on Tmax, kel or t1/2. A statistically significant 1.4-fold increase in the AUC of UK-103,320 was also observed, as well as a significant decrease in kel, resulting in an increase of about 1 h in t1/2. In contrast, repeated dosing with azithromycin caused no significant change in any pharmacokinetic parameter of either sildenafil or UK-103,320. Erythromycin, azithromycin and sildenafil were well tolerated; adverse events were mild and transient. No subject withdrew from either trial for any reason related to study drug.
Conclusions
These results indicate that erythromycin modifies the pharmacokinetics of sildenafil by inhibiting its CYP3A4-mediated first-pass metabolism. Given these data, a lower starting dose of sildenafil (25 mg) may be considered for patients receiving erythromycin or other potent CYP3A4 inhibitors. Azithromycin did not affect the pharmacokinetics of sildenafil; therefore, no adjustment in dosage is necessary for patients receiving these drugs concomitantly.
Keywords: sildenafil; UK-103,320; pharmacokinetics; antibiotics; erythromycin; azithromycin; safety
Introduction
Sildenafil citrate (Viagra®, Pfizer Inc) is a selective inhibitor of cyclic guanosine monophosphate–specific phosphodiesterase (PDE) type 5 and is an effective oral treatment for erectile dysfunction of various aetiologies [1–3]. Sildenafil is absorbed rapidly after oral administration and has a clearance of 41 l h−1, a mean steady-state volume of distribution of 105 l and a terminal half-life of approximately 4 h. The mean absolute bioavailability after oral dosing of a 50-mg capsule is 41% [1]. In vitro studies have shown that sildenafil is metabolized via the cytochrome P450 (CYP450) system [4, 5]. A major route of metabolism is N-demethylation to UK-103,320, which is mediated predominantly by the low-affinity, high-capacity isozyme CYP3A4, particularly during the absorption phase, when hepatic portal vein drug concentrations are high [4].
Erythromycin is a commonly prescribed macrolide antibiotic that is bacteriostatic against aerobic gram-positive bacteria [6]. It is a potent inhibitor of CYP3A4 and has been reported to produce clinically significant interactions with a number of other therapeutic agents that are substrates for this isozyme [7, 8]. Azithromycin, a newer synthetic azalide derivative of the macrolide antibiotics, has a somewhat broader range of activity than erythromycin, together with an improved pharmacokinetic profile [9]. In addition, it has been shown not to inactivate cytochrome P450 isozymes [10] and not to have any significant effect on the hepatic metabolism of a range of co-administered drugs [11]. Therefore, azithromycin was predicted to be less likely than erythromycin to interact with sildenafil.
Erythromycin and azithromycin are widely used antibiotics, making it likely that a large number of patients will take them concurrently with sildenafil. Because sildenafil and these antibiotics share a common metabolic pathway, the potential exists for drug–drug interactions. Two placebo-controlled, parallel-group studies were conducted to evaluate the effects of multiple doses of erythromycin or azithromycin on the pharmacokinetics of a single, open-label, oral 100-mg dose of sildenafil. The safety and tolerability of sildenafil in combination with these agents was also evaluated.
Methods
Subjects
Healthy male volunteers between the ages of 18 and 45 years were eligible for inclusion in these placebo-controlled, parallel-group studies, provided that they weighed between 60 and 100 kg and had a body mass index between 18 and 28 kg m−2. Subjects were excluded from both trials if they had evidence of any clinically significant disease or laboratory test abnormality, if the results of hepatitis B surface antigen tests were positive, if they had taken any prescription or over-the-counter medication (except paracetamol) in the 3 weeks before the study or any experimental drug in the 4 months before the study, if they had any contraindications to the use of either macrolide antibiotic, if they consumed more than 28 units of alcohol per week or if they smoked more than 10 cigarettes per day. Men with positive test results for hepatitis C, hepatitis B core antibody, or human immunodeficiency virus were also excluded. Twenty-six subjects were recruited into the erythromycin interaction study, and 24 were recruited into the azithromycin interaction study.
Approval for the erythromycin study was obtained from the Tayside Committee on Medical Research Ethics and approval for the azithromycin study from the Royal Masonic Hospital Ethics Committee. All subjects gave their written informed consent.
Protocol
In the 3 weeks before each study, all subjects underwent a screening process, including a full physical examination, a 12-lead electrocardiogram, vital sign measurement, a range of laboratory safety tests and a urine screen for drugs of abuse. Subjects were instructed to refrain from beverages containing caffeine or methylxanthines, alcohol and unaccustomed exercise in the 2 days before the study.
On admission to the study centre on day 0, subjects from both studies underwent a urine drug screen and an alcohol breath test. They fasted from all food and drink except water from 22:00 h until the next day (day 1). On day 1, they received a single 100-mg dose of sildenafil 2 h after a light breakfast. Subjects received a standard lunch and dinner after the 4- and 10-h blood samples (see below), respectively. Noncaffeinated beverages were allowed ad libitum after the 4-h sample.
In the first study, subjects were randomly assigned to receive either erythromycin (500 mg) or placebo twice daily approximately 12 h apart (after a light breakfast and small evening snack) on days 2–6. On day 6, all subjects received a second 100-mg dose of sildenafil about 1 h after receiving erythromycin or placebo. In the second study, the subjects were randomly assigned to receive either azithromycin (500 mg) or placebo once daily, after a light meal, on days 2–4. On day 4, all subjects received a second 100-mg dose of sildenafil about 1 h after the azithromycin or placebo dose. In both studies, subjects and investigators were blind to the erythromycin/azithromycin or placebo assignments; all subjects knew they were receiving sildenafil.
Pharmacokinetic assessments
On the first and last day of both studies, blood samples were collected at baseline (time 0) and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 18 and 24 h after study drug administration. Plasma concentrations of the parent drug and metabolite (UK-103,320) were determined by a fully validated Automated Sequential Trace Enrichment of Dialysis method, followed by high-performance liquid chromatography with ultraviolet detection [12]. The detection range for both assays was 1–250 ng ml−1. The overall imprecision (CV) was 5.1%, 3.2% and 3.0% for sildenafil and 3.4%, 3.1% and 2.9% for UK-103,320 concentrations of 3, 125 and 200 ng ml−1, respectively. The inaccuracy (bias) of the assay at all concentrations ranged from −2.3% to 3.5% for sildenafil and from −7.0% to 4.8% for UK-103,320.
The following pharmacokinetic parameters were derived by noncompartmental analysis from the individual concentration-time curves for both sildenafil and UK-103,320 using WinNonlin™ version 1.1 (Pharsight Corporation): the maximum observed plasma concentration (Cmax), the time to achieve Cmax (Tmax), the apparent terminal elimination phase rate constant (kel), the mean terminal half-life (t1/2) and the area under the plasma concentration-time curve from zero time extrapolated to infinity (AUC).
Safety and tolerability
In each study, before dosing on the morning of day 1, a physical examination was performed, vital signs were checked and blood and urine samples were collected for laboratory safety tests, including a standard blood panel (i.e. haemoglobin, RBC, WBC and platelet counts, haematocrit, corpuscular volume), clinical chemistry (i.e. total bilirubin, protein, albumin, AST/SGOT, ALT/SGPT, alkaline phosphatase, urea, creatinine, sodium, potassium, calcium) and standard urinalysis (i.e. stick test for pH, protein, glucose, ketones, blood). After the 24-h blood sample was collected, the physical examination, vital sign measurements and laboratory safety tests were repeated. Subjects in the second study also underwent this battery of tests after their first dose of azithromycin or placebo (day 2) and on the evening of day 3. In both studies, observed or volunteered adverse events (AEs) were recorded at each visit.
Statistical evaluation
In both studies, a sample size of 24 subjects (12 per treatment group) was determined to be sufficient to detect a difference of 50% in the AUC and Cmax of sildenafil with a probability of 0.80 when testing at the 5% level (two-tailed).
For each study, the AUC, Cmax, kel and Tmax data for sildenafil and UK-103,320 were subjected to analysis of variance (anova). The anova model took into account the variability due to subject and the treatment-by–day interaction (i.e. the four combinations of treatment and day). Using this anova, the mean differences between last day and first day were estimated for each treatment separately. These differences between the two treatments were then compared, which gave the comparison of primary interest, with 95% confidence intervals (CIs) calculated. Because AUC and Cmax data were log transformed before analysis, mean differences and 95% CI values were back-transformed and presented in the form of ratios. All statistical analyses were performed using SAS version 6.0 (SAS Institute Inc.).
Results
Subjects
Twenty-six male volunteers (18–45 years) entered the first study; 24 subjects completed the study and two subjects discontinued treatment for reasons unrelated to study drug. Twenty-four male volunteers (19–33 years) entered and completed the second study. In each study, the two treatment groups (i.e. erythromycin/azithromycin and placebo) were well matched with respect to demographic variables (Table 1).
Table 1.
Subject demographics in erythromycin and azithromycin studies.
| Sildenafil+ Erythromycin (n = 13) | Sildenafil+ Placebo (n = 13) | Sildenafil+ Azithromycin (n = 12) | Sildenafil+ Placebo (n = 12) | |
|---|---|---|---|---|
| Age range (y) | 18–43 | 19–45 | 19–33 | 19–25 |
| Mean | 29 | 28 | 24 | 22 |
| Weight range (kg) | 58–94 | 62–87 | 60–82 | 57–87 |
| Mean | 72 | 73 | 71 | 71 |
| Height range (cm) | 165–195 | 169–194 | 164–186 | 165–186 |
| Mean | 179 | 180 | 179 | 174 |
Effects of erythromycin
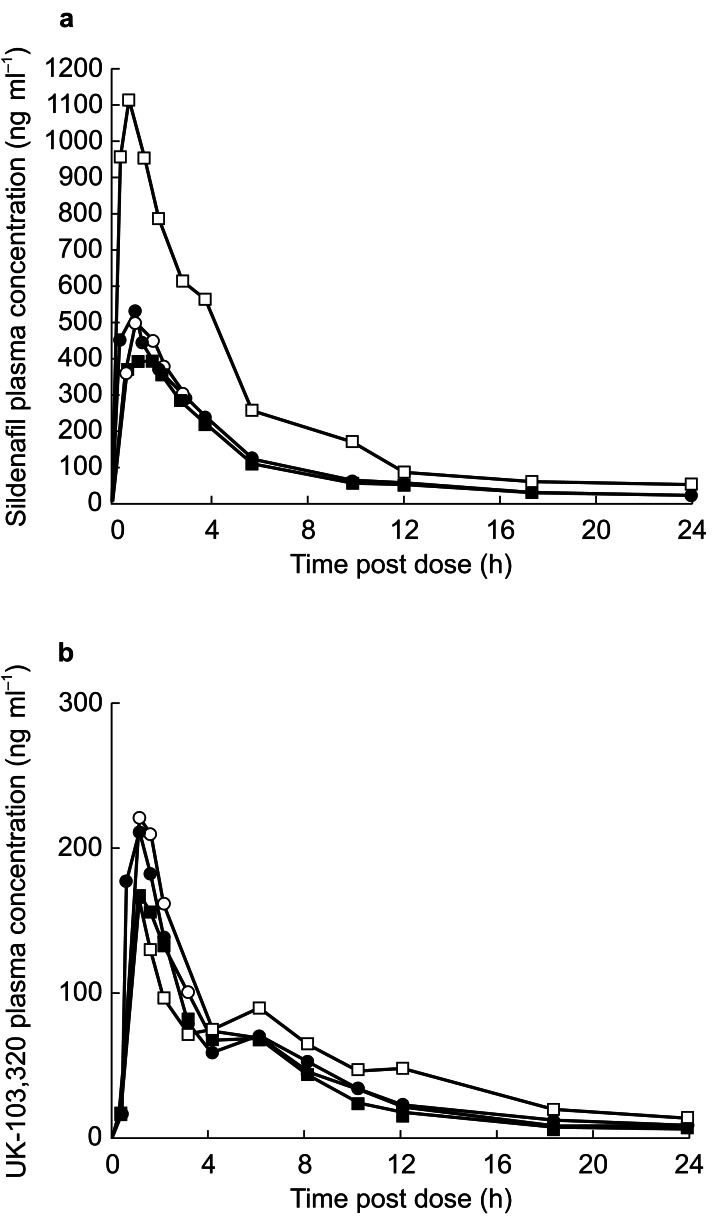
Mean pharmacokinetic parameters for sildenafil and UK-103,320 before and during co-administration of erythromycin or placebo are summarized in Table 2. The corresponding plasma concentration profiles for sildenafil and UK-103,320 are shown in Figure 1.
Table 2.
Mean pharmacokinetic parameters for sildenafil and UK-103,320 on days 1 and 6 in the erythromycin study. Geometric means are presented for AUC and Cmax, arithmetic means are presented for Tmax and kel and harmonic means are presented for t1/2.
| Group 1 | Group 2 | |||
|---|---|---|---|---|
| Parameter | Day 1 Sildenafil | Day 6 Sildenafil+ Erythromycin | Day 1 Sildenafil | Day 6 Sildenafil+ Placebo |
| Sildenafil | ||||
| AUC, (ng ml−1 h) (range) | 1904 (1336–3073) | 4911 (3184–9376) | 1893 (1358–3396) | 1732 (931–2815) |
| Cmax (ng ml−1) (range) | 596 (373–1292) | 1245 (831–1833) | 639 (462–1016) | 512 (327–727) |
| Tmax (h) (range) | 1.06 (0.25–3.00) | 0.79 (0.50–1.50) | 1.00 (0.50–3.00) | 1.17 (0.50–3.00) |
| kel (h−1) (range) | 0.20 (0.18–0.26) | 0.17 (0.11–0.20) | 0.21 (0.15–0.31) | 0.20 (0.15–0.29) |
| t1/2 (h) (range) | 3.47 (2.69–3.91) | 4.06 (3.51–6.29) | 3.26 (2.25–4.54) | 3.48 (2.36–4.53) |
| UK-103,320 | ||||
| AUC (ng ml−1 h) (range) | 905 (549–2349) | 1099 (508–2310) | 867 (463–1946) | 769 (435–1551) |
| Cmax (ng ml−1) (range) | 270 (151–489) | 169 (76–290) | 277 (163–379) | 227 (144–296) |
| Tmax (h) (range) | 1.13 (0.50–3.00) | 0.88 (0.50–1.00) | 0.92 (0.50–2.00) | 1.17 (0.50–3.00) |
| kel (h−1) (range) | 0.16 (0.09–0.28) | 0.13 (0.07–0.18) | 0.18 (0.14–0.24) | 0.19 (0.12–0.36) |
| t1/2 (h) (range) | 4.22 (2.52–7.55) | 5.30 (3.81–10.14) | 3.91 (2.84–5.11) | 3.61 (1.92–5.86) |
Figure 1.
(a) Plasma concentration profiles of sildenafil following administration of sildenafil 100 mg alone (baseline placebo group •; baseline erythromycin group ○) (day 1) or in combination with erythromycin 500 mg (□) or placebo (▪) (day 6). (b) Plasma concentration profiles of UK-103,320 following administration of sildenafil 100 mg alone (baseline placebo group •; baseline erythromycin group ○) (day 1) or in combination with erythromycin 500 mg (□) or placebo (▪) (day 6). Co-administration of sildenafil plus erythromycin produced significantly higher plasma concentrations of sildenafil on day 6 compared with co-administration of sildenafil plus placebo; n = 12 per group.
Absorption of sildenafil was rapid when taken alone or in the presence of either erythromycin or placebo (mean Tmax range 0.8–1.2 h). Compared with placebo, neither the Tmax (P = 0.132) nor the kel (P = 0.196) value for sildenafil was significantly affected by concomitant erythromycin treatment. As a result, the mean terminal half-lives were similar on day 1 and day 6 for both groups (mean t1/2 range, 3.3–4.1 h). For the metabolite, multiple dosing with erythromycin resulted in a statistically significant decrease in kel (P < 0.02) relative to placebo, resulting in an approximate mean increase of 1 h in t1/2. Plasma concentrations of sildenafil and UK-103,320 were reduced to subtherapeutic concentrations (<2 ng ml−1) 24 h after dosing in both groups.
Relative to placebo, administration of erythromycin significantly (P < 0.0001) increased the AUC of sildenafil by a factor of 2.82 (95% CI: 2.19–3.63) and significantly (P = 0.0004) increased the AUC of UK-103,320 by a factor of 1.37 (95% CI: 1.17–1.60). In addition, erythromycin increased the Cmax of sildenafil by a factor of 2.60 (95% CI: 1.93–3.51) compared with placebo (P < 0.0001) and decreased UK-103,320 Cmax, with a ratio of 0.76 (95% CI: 0.55–1.05), although this was not statistically significant.
Effects of azithromycin
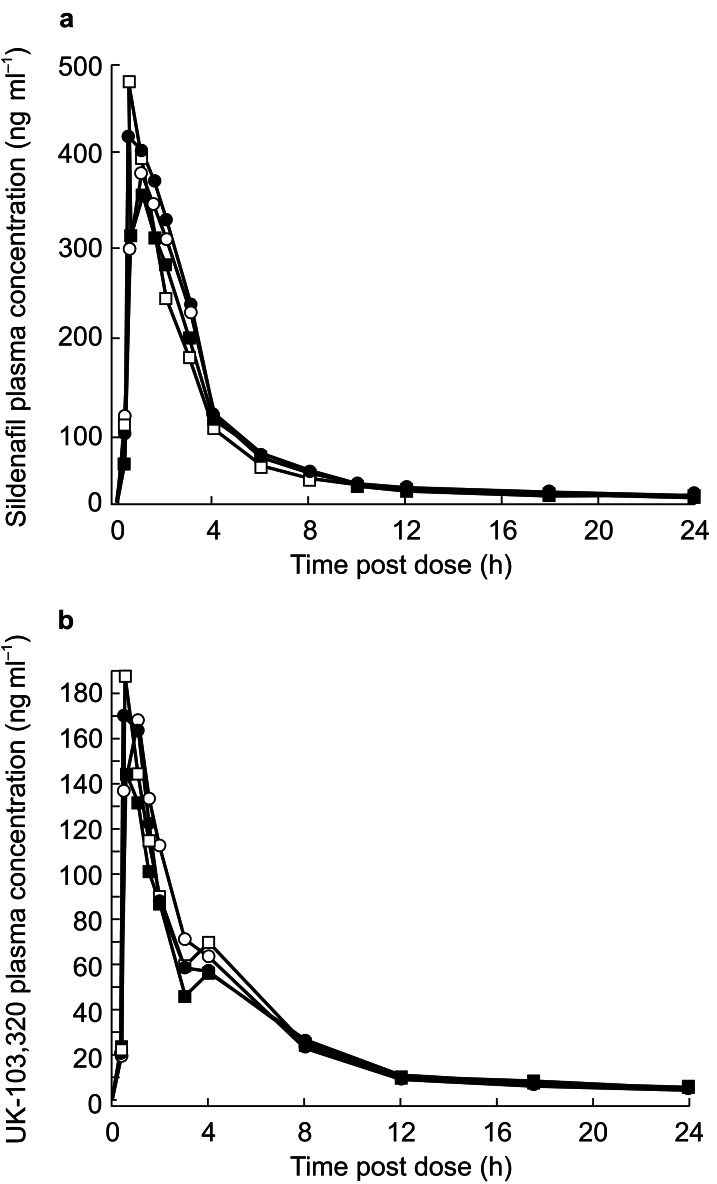
The mean pharmacokinetic parameters for sildenafil and UK-103,320 before and during co-administration of azithromycin or placebo are summarized in Table 3, and the corresponding plasma concentration profiles are shown in Figure 2. Absorption of sildenafil was rapid when taken alone or in combination with azithromycin or placebo (mean Tmax range, 0.8–1.2 h). However, unlike erythromycin, statistical analyses revealed no significant effect of azithromycin on any pharmacokinetic parameter of either sildenafil or UK-103,320. Compared with placebo, the kel values for sildenafil and UK-103,320 were not significantly affected by concomitant azithromycin treatment. Likewise, the Tmax values for sildenafil or UK-103,320 were not significantly affected by azithromycin treatment. Relative to placebo, administration of azithromycin did not significantly change the AUC of sildenafil or UK-103,320, and it did not significantly affect the Cmax of sildenafil or UK-103,320.
Table 3.
Mean pharmacokinetic parameters for sildenafil and UK-103,320 on days 1 and 4 in the azithromycin study. Geometric means are presented for AUC and Cmax, arithmetic means are presented for Tmax and kel and harmonic means are presented for t1/2.
| Group 1 | Group 2 | |||
|---|---|---|---|---|
| Parameter | Day 1 Sildenafil | Day 4 Sildenafil+ Azithromycin | Day 1 Sildenafil | Day 4 Sildenafil+ Placebo |
| Sildenafil | ||||
| AUC (ng ml−1 h) (range) | 1327 (929–2012) | 1216 (536–1676) | 1423 (1011–2311) | 1242 (804–2121) |
| Cmax (ng ml−1) (range) | 453 (257–843) | 526 (155–804) | 459 (264–869) | 403 (250–633) |
| Tmax (h) (range) | 1.15 (0.25–3.00) | 0.79 (0.50–2.00) | 0.96 (0.50–2.00) | 0.96 (0.50–2.00) |
| kel (h−1) (range) | 0.15 (0.11–0.18) | 0.15 (0.11–0.20) | 0.15 (0.11–0.21) | 0.16 (0.11–0.24) |
| t1/2 (h) (range) | 4.53 (3.79–6.14) | 4.50 (3.07–6.58) | 4.53 (3.23–6.57) | 4.28 (3.14–6.20) |
| UK-103,320 | ||||
| AUC (ng ml−1 h) (range) | 665 (368–1148) | 639 (403–1065) | 586 (353–1195) | 537 (395–920) |
| Cmax (ng ml−1) (range) | 194 (85–324) | 234 (186–284) | 181 (104–457) | 172 (112–382) |
| Tmax (h) (range) | 1.04 (0.50–2.00) | 0.79 (0.50–2.00) | 0.88 (0.50–1.50) | 0.88 (0.50–2.00) |
| kel (h−1) (range) | 0.11 (0.08–0.20) | 0.15 (0.11–0.27) | 0.11 (0.08–0.14) | 0.15 (0.10–0.26) |
| t1/2 (h) (range) | 6.37 (3.46–10.18) | 4.59 (2.58–6.28) | 6.43 (4.78–9.16) | 4.57 (2.69–6.94) |
Figure 2.
(a) Plasma concentration profiles of sildenafil following administration of sildenafil 100 mg alone (baseline placebo group •; baseline azithromycin group ○) (day 1) or in combination with azithromycin 500 mg (□) or placebo (▪) (day 4). (b) Plasma concentration profiles of UK-103,320 following administration of sildenafil 100 mg alone (baseline placebo group •; baseline azithromycin group ○) (day 1) or in combination with azithromycin 500 mg (□) or placebo (▪) (day 4); n = 12 per group.
Safety and tolerability
The increase in systemic exposure (AUC) to sildenafil and UK-103,320, which contributes approximately 20% of PDE inhibitory activity, resulting from the co-administration of erythromycin was not associated with an increase in the incidence of AEs. No subject discontinued treatment for any reason related to study drug, and no serious AEs were reported. The most common AEs following administration of sildenafil alone were headache and flushing; all but one of these events were mild or moderate in nature. One subject reported severe headache and vomiting. All AEs resolved by the end of the study.
Azithromycin also had no effect on the incidence or severity of AEs or laboratory abnormalities. Headache, dizziness and flushing were the most common side-effects, and overall AEs were reported at similar frequencies for both treatment groups. All AEs were mild or moderate and resolved by the end of the study. No serious AEs occurred, and no subject withdrew from the trial for any reason. Four subjects had abnormal laboratory test results during the study that included a positive urine haemoglobin value, elevated eosinophils (two subjects), and an elevated total bilirubin level. Two of these subjects, one with elevated eosinophils and one with an elevated bilirubin level, exhibited these laboratory test abnormalities throughout the study regardless of treatment. No causal relationship could be determined between the laboratory test abnormalities and study medication.
Discussion
Sildenafil metabolism is dependent primarily on two cytochrome P450 isozymes [4, 5]. During the absorption phase, when drug concentrations are high in the hepatic portal vein, the metabolism is mediated by CYP3A4, a high-capacity, low-affinity enzyme. During the elimination phase, when drug concentrations are lower, metabolism is mediated primarily via the low-capacity, high-affinity isozyme CYP2C9. An interaction of sildenafil with erythromycin was therefore predicted based on the specific and potent inhibition of hepatic microsomal CYP3A4 by erythromycin [10, 11, 13]. The significant increases in the Cmax and AUC of sildenafil, with no significant change in t1/2, suggest that erythromycin inhibits the first-pass metabolism of sildenafil but has little effect on its systemic elimination. This inhibition of presystemic metabolism probably occurs in the liver and the small intestinal wall because CYP3A4 has been shown to be present in both tissues [14, 15]. This conclusion is further supported by the reduction in UK-103,320 Cmax associated with co-administration of erythromycin: although this reduction in Cmax did not reach statistical significance, it suggests reduced formation of the metabolite during first-pass metabolism. The increase in AUC and t1/2 of UK-103,320 produced by erythromycin suggests that its metabolism and systemic clearance also are mediated via the CYP3A4 isozyme. The lack of effect of azithromycin on sildenafil pharmacokinetic parameters is in agreement with other clinical studies showing that azithromycin has no effects on the CYP450-mediated metabolism of co-administered drugs [16, 17] and suggests that azithromycin does not form stable complexes with CYP3A4 as does erythromycin.
Although the number of subjects in this study was relatively small and any safety conclusions are thus tentative, the increase in systemic exposure to sildenafil and its metabolite when sildenafil is administered concomitantly with erythromycin did not appear to affect the safety and tolerability of sildenafil. These findings are in agreement with those obtained from the analysis of population pharmacokinetic data and safety data obtained from sildenafil clinical trials, which showed a reduction in sildenafil clearance when the drug was taken concomitantly with CYP3A4 inhibitors, but no increase in the incidence of AEs [18–20].
The 2- to 3-fold increase in systemic exposure to sildenafil with concomitant erythromycin use is similar to that reported with concomitant saquinavir use (increases in sildenafil AUC and Cmax of 3.1-fold and 2.4-fold, respectively) but less than that reported with concomitant ritonavir use (increases in sildenafil AUC and Cmax of 11-fold and 3.9-fold, respectively) [21]. Like erythromycin, both of these protease inhibitors are known to inhibit CYP3A4, but the additional potent CYP2C9 inhibitory activity of ritonavir may account for its more pronounced effect on sildenafil pharmacokinetics. In contrast, a lesser effect was reported for co-administration of sildenafil with cimetidine, a nonspecific inhibitor of the CYP450 system [22]. Co-administration with cimetidine produced an increase in sildenafil plasma levels; however, this increase was not considered clinically significant.
The recommended therapeutic dose range of sildenafil is 25–100 mg, with a starting dose of 50 mg. Because sildenafil has a wide therapeutic window, the extent of the interaction with CYP3A4 inhibitors is not likely to represent a clinical safety concern, as its dose can be adjusted if required to avoid overexposing subjects to the drug. Prescribing physicians should consider a lower starting dose of sildenafil for patients receiving potent CYP3A4 inhibitors, such as erythromycin, concomitantly.
In conclusion, these studies indicate that multiple dosing of erythromycin (500 mg twice daily) significantly increases plasma concentrations of sildenafil and its major metabolite in healthy male volunteers. However, these increases do not appear to affect the incidence or nature of reported AEs. Although the extent of the interaction is not believed to constitute a safety concern, clinicians prescribing sildenafil may consider a lower starting dose of sildenafil (25 mg) for patients receiving potent CYP3A4 inhibitors. In contrast, multiple dosing of azithromycin (500 mg every day) has no effect on sildenafil pharmacokinetics; therefore, no adjustment in dosage is required for patients receiving this macrolide antibiotic.
References
- 1.Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 2.Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 3.Moreland RB, Goldstein I, Traish A. Sildenafil, a novel inhibitor of phosphodiesterase type 5 in human corpus cavernosum smooth muscle cells. Life Sci. 1998;62:309–318. doi: 10.1016/s0024-3205(98)00158-1. [DOI] [PubMed] [Google Scholar]
- 4.Hyland R, Roe EGH, Jones BC. Smith DA. Identification of the cytochrome P450 enzymes involved in the N-demethylation of sildenafil. Br J Clin Pharmacol. 2001;51:239–248. doi: 10.1046/j.1365-2125.2001.00318.x. 10.1046/j.1365-2125.2001.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warrington JS, Shader RI, Von Moltke LL, Greenblaat DJ. In-vitro biotransformation of sildenafil (Viagra®): identification of human cytochromes and potential drug interactions. Drug Metab Dispos. 2000;28:392–397. [PubMed] [Google Scholar]
- 6.Steigbigel NH. Macrolides and clindamycin. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 4. New York: Churchill Livingstone; 1995. pp. 334–346. [Google Scholar]
- 7.Olkkola KT, Aranko K, Luurila H, et al. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther. 1993;53:298–305. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]
- 8.Honig PK, Woosley RL, Zamani K, Conner DP, Cantilena LR. Changes in the pharmacokinetics and electrocardiographic pharmacodynamics of terfenadine with concomitant administration of erythromycin. Clin Pharmacol Ther. 1992;52:231–238. doi: 10.1038/clpt.1992.135. [DOI] [PubMed] [Google Scholar]
- 9.Lode H, Borner K, Koeppe P, Schaberg T. Azithromycin – review of key chemical, pharmacokinetic and microbiological features. J Antimicrob Chemother. 1996;37(Suppl C):1–8. doi: 10.1093/jac/37.suppl_c.1. [DOI] [PubMed] [Google Scholar]
- 10.Periti P, Mazzei T, Mini E, Novelli A. Pharmacokinetic drug interaction of macrolides. Clin Pharmacokinet. 1992;23:106–131. doi: 10.2165/00003088-199223020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Nahata M. Drug interactions with azithromycin and the macrolides: an overview. J Antimicrob Chemother. 1996;37(Suppl C):133–142. doi: 10.1093/jac/37.suppl_c.133. [DOI] [PubMed] [Google Scholar]
- 12.Cooper JDH, Muirhead DC, Taylor JE, Baker PR. Development of an assay for the simultaneous determination of sildenafil (Viagra®) and its metabolite (UK-103,320) using automated sequential trace enrichment of dialysates and high-performance liquid chromatography. J Chromatog. 1997;B 701:87–95. doi: 10.1016/s0378-4347(97)00339-3. [DOI] [PubMed] [Google Scholar]
- 13.Honig PK, Wortham DC, Zamani K, Cantilena LR. Comparison of the effect of the macrolide antibiotics erythromycin, clarithromycin and azithromycin on terfenadine steady-state pharmacokinetics and electrocardiographic parameters. Drug Invest. 1994;7:148–156. [Google Scholar]
- 14.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–423. [PubMed] [Google Scholar]
- 15.Kolars JC, Schmiedlin-Ren P, Schuetz JD, Fang C, Watkins PB. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992;90:1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rapp RP. Pharmacokinetics and pharmacodynamics of intravenous and oral azithromycin: enhanced tissue activity and minimal drug interactions. Ann Pharmacother. 1998;32:785–793. doi: 10.1345/aph.17299. [DOI] [PubMed] [Google Scholar]
- 17.Mattila MJ, Vanakoski J, Idanapaan-Heikkila JJ. Azithromycin does not alter the effects of oral midazolam on human performance. Eur J Clin Pharmacol. 1994;47:49–52. doi: 10.1007/BF00193477. [DOI] [PubMed] [Google Scholar]
- 18.Milligan PA, Marshall SF, Karlsson MO. A population pharmacokinetic analysis of sildenafil citrate in patients with erectile dysfunction. Br J Clin Pharmacol. 2002;53(Suppl 1):53–60. doi: 10.1046/j.0306-5251.2001.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales A, Gingell C, Collins M, Wicker PA, Osterloh IH. Clinical safety of oral sildenafil citrate (Viagra™) in the treatment of erectile dysfunction. Int J Impot Res. 1998;10:69–74. doi: 10.1038/sj.ijir.3900354. [DOI] [PubMed] [Google Scholar]
- 20.Zusman RM, Morales A, Glasser DB, Osterloh IH. Overall cardiovascular profile of sildenafil citrate. Am J Cardiol. 1999;88(Suppl 5a):35C–44C. doi: 10.1016/s0002-9149(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 21.Muirhead GJ, Wulff MB, Fielding A, Kleinermans D, Buss N. Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. Br J Clin Pharmacol. 2000;50:99–107. doi: 10.1046/j.1365-2125.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilner K, Labey L, LeBel M. The effects of cimetidine and antacid on the pharmacokinetic profile of sildenafil citrate in healthy male volunteers. Br J Clin Pharmacol. 2002;53(Suppl 1):31–35. doi: 10.1046/j.0306-5251.2001.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]