Abstract
Aims
Intravenous formulations of busulfan have recently become available. Although busulfan is used frequently in children as part of a myeloablative regimen prior to bone marrow transplantation, pharmacokinetic data on intravenous busulfan in children are scarce. The aim was to investigate intravenous busulfan pharmacokinetics in children and to suggest a limited sampling strategy in order to determine busulfan systemic exposure with the minimum of inconvenience and risk for the patient.
Methods
Plasma pharmacokinetics after the first administration was investigated in six children using nonlinear mixed effect modelling.
Results
Pharmacokinetics showed little variability and were described adequately with a one-compartment model (population estimates CL,av = 0.29 l h−1 kg−1; V,av = 0.84 l kg−1; t1/2 = 1.7–2.8 h). Combined with limited sampling and a Bayesian fitting procedure, the model can adequately estimate the systemic exposure to intravenous busulfan, which in children appears to be at the lower end of the adult range.
Conclusions
Busulfan systemic exposure in children during intravenous administration can be estimated adequately with limited sampling and a Bayesian fitting procedure from a one-compartment model. Intravenous busulfan pharmacokinetics in children should be the subject of more research.
Keywords: busulfan, paediatrics, pharmacokinetics
Introduction
Busulfan (Bu) is frequently included as part of a myeloablative regimen prior to bone marrow transplantation (BMT), especially in infants and young children to avoid total body irradiation. Therapeutic drug monitoring of Bu is recommended because of the variability in its pharmacokinetics after oral administration, and because of proven relationships between systemic exposure to the drug and both toxicity and success of treatment [1]. Several studies have suggested that Bu toxicity and a higher incidence of graft failure may be due to plasma drug concentration and/or to under or over dosing in children [2–6]. Recently several intravenous formulations of Bu have become available [7, 8]. The pharmacokinetics of intravenous Bu have been described mainly in adults [9–11]. In the present study, Bu pharmacokinetics were investigated in children receiving intravenous Bu as part of a myeloablative regimen (busulfan/cyclophosphamide) prior to BMT.
Methods
Patients
The parents of the children studied provided written informed consent for participation in the study and in the transplantation protocol, which was approved by the ethics committee of the Leiden University Medical Center. Six patients were studied between July 2000 and January 2001. Age ranged between 1.5 and 14 years, body weight between 12 and 40 kg. Five children were treated for myelodysplastic syndrome, one child for thalassaemia. The patients received busulfan (Busulfex, Orphan Medical Inc, USA) 0.8 mg kg−1 as an intravenous infusion (0.5 mg ml−1) during 2 h four times daily for 4 consecutive days. Busulfan was administered through a central double-lumen catheter. Blood was collected through the same catheter at 2.5, 3, 4 and 6 h after end of the first infusion at day 1 of treatment.
Drug analysis
Bu was analysed in plasma by a validated high-performance liquid chromatographic (h.p.l.c.) assay, involving precolumn derivatization, liquid/liquid-extraction and ultraviolet detection according to Chow et al.[12]. The assay was linear between 30 and 8000 µg l−1. The limit of quantification was 30 µg l−1. Precision at 200 and 1500 µg l−1 was 3.5 and 0.8%, respectively.
Pharmacokinetics
Bu pharmacokinetics were analysed with a one-compartment model as visual inspection indicated that no improvement could be obtained using a more complex model. Pharmacokinetic parameters were estimated with a population approach using NONMEM software (NONMEM Version V, NONMEM Project Group, UCSF, San Francisco, CA, USA), in the form of clearance per kg body weight (CL kg−1) and volume of distribution per kg body weight (V kg−1). A constant coefficient inter- and intraindividual variability model was used. First order conditional estimation with the ‘interaction’ option was used in parameter estimation, providing the most accurate numerical approximation to the model available within NONMEM. Empirical Bayes individual parameter estimates were generated that provide a weighted combination of previously derived population information (the NONMEM estimates) and the individual measurements.
In order to assess whether two concentration measurements would be sufficient to estimate busulfan AUCs, PK parameters were simulated in 1000 subjects. This was based on the population estimates with their between subject variability, for subjects with a body weight uniformly distributed between 10 and 40 kg. The simulated volume and clearance values were used to simulate plasma drug concentrations according to two different sampling schemes (2.5 and 4 h, and 2.5 and 6 h). The underlying simulated AUCs were calculated from the expression Dose/CL. Individual Bayesian estimates for the AUCs were subsequently obtained from the simulated data points using the NONMEM population estimates as prior information.
Simulated AUCs and corresponding Bayesian estimates were analysed using Pearsonapos;s correlation test.
Results
In general, intravenous Bu was well tolerated. Bu pharmacokinetics were described adequately with a one-compartment model. The population estimate for clearance corrected for body weight (CL kg−1) was 0.29 l h−1 kg−1 with an interindividual coefficient of variation (IICV) of 14%. The population estimate for volume of distribution corrected for body weight (V kg−1) was 0.84 l kg−1 with an IICV of 10%. Empirical Bayes estimates for (CL kg−1) ranged from 0.23 to 0.36 l h−1 kg−1 while V kg−1 estimates ranged from 0.73 to 0.95 l kg−1. Bu plasma half-life ranged from 1.7 to 2.8 h and estimated AUC from 2235 to 3419 µg l−1 h. In Table 1 individual pharmacokinetic data are listed. In response to the AUC values, the dose was increased in all six patients to an arbitrary maximum of 1.0 mg kg−1 four times a day.
Table 1.
Characteristics and individual pharmacokinetics parameters in children receiving intravenous busulfan.
| Subject | Age (years) | Weight (kg) | Disorder | CL (l h−1 kg−1) | V (l kg−1) | AUC (µg l−1 h) |
|---|---|---|---|---|---|---|
| 1 | 6 | 19 | MDS | 0.330 | 0.878 | 2424 |
| 2 | 6 | 20 | Thal. | 0.358 | 0.899 | 2235 |
| 3 | 14 | 40 | MDS | 0.273 | 0.734 | 2930 |
| 4 | 4 | 18 | MDS | 0.234 | 0.946 | 3419 |
| 5 | 9 | 25 | MDS | 0.276 | 0.809 | 2899 |
| 6 | 1.5 | 12 | MDS | 0.272 | 0.805 | 2941 |
MDS = Myelodysplastic syndrome; Thal. = Thalassaemia.
The maximal Bu plasma concentration according to the compartment model ranged between 600 and 800 µg l−1 (t = 2 h). The mean (±s.d.) concentration measured at t = 2.5 h was 545±46 µg l−1, while the concentrations measured at t = 4 and t = 6 h were 343±59 µg l−1 and 187±46 µg l−1, respectively. Inter-patient variability in the pharmacokinetic parameters appears to be low.
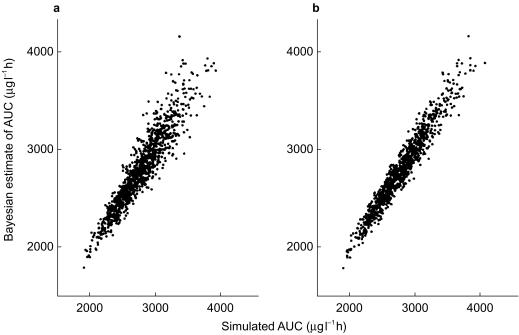
In Figure 1, the simulated and estimated (Bayesian) AUCs are shown for both combinations of sampling times. The performance of the model to estimate the AUC improves when the last sampling is at t = 6 h rather than 4 h, but the difference appears to be small (r = 0.934 for 2.5 and 4 h; r = 0.970 for 2.5 and 6 h).
Figure 1.
Bayesian estimates of AUCs following intravenous busulphan (y axis) vs simulated AUCs (x axis). Bayesian estimates were based on the concentrations at t = 2.5 and 4 h (a) and t = 2.5 and 6 h (b) after dosing.
Discussion
Interindividual variability in Bu exposure after oral administration is well known, and may be caused partially by differences in absorption. Bu pharmacokinetics during oral administration appears to be related to age, disease, and time after start of treatment [2–6]. In order to decrease variability in pharmacokinetics, various intravenous formulations have been developed in recent years. Data on the pharmacokinetics of Spartaject (a microsuspension formulation) in 12 patients older than 18 years prior to stem cell transplantation (STC) indicated little intrapatient variability, but interpatient variability in AUC at 1.0 mg kg−1 was still considerable [7].
The pharmacokinetics of Busulfex, a solution of Bu in dimethylacetamide, polyethylene glycol 400 and water, was studied in 59 (adult) patients participating in a prospective trial of a busulfan/cyclophosphamide (BuCy) preparatory regimen prior to SCT [9]. Patients received 0.8 mg kg−1 busulfan every 6 h for 4 days. The mean AUC at steady state was 4785 µg l−1 h (range 2279–6859 µg l−1 h), while clearance was 0.15 l kg−1 h−1 with a CV of 25%.
Systemic exposure of intravenous busulfan (Busulfex) in the children studied is at the lower end of the adult range, and variability in pharmacokinetics is very low. The latter could be due to a small homogenous subset of subjects, since only a small number of children was investigated. As blood withdrawal is difficult and forms a risk for infection because these children are rendered immuno-incompetent, it was decided to investigate only six patients with more than two blood sampling points. The relatively low systemic exposure of busulfan in these children could be caused by an increased clearance, which has been described earlier after oral Bu [2–6]. In contrast to these findings, Hassan et al. recently reported no apparent difference in pharmacokinetics of liposomal intravenous busulphan between adults and children [11].
Our finding of a relatively low AUC indicates that the intravenous dose in children should be higher than 0.8 mg kg−1, when the same target AUCs are used in children as in adults (4200–5650 µg l−1 h). Indeed, in all six children studied the dose was increased. However, dose adjustments were not followed by a second determination of systemic exposure. Because of this, and because of the small number of patients studied, the starting dose should remain 0.8 mg kg−1 until more patients are investigated, and dose adjustments are followed by a determination of plasma concentrations. In our hospital, a generally accepted target AUC of 4925 µg l−1 h is used during oral myeloablative therapy (four times daily). However, at the moment no specific target ranges of the AUC are established for intravenous administration of busulfan, especially in children. During oral administration, target AUCs can be reached by individualizing Bu dosing by monitoring of Bu concentrations [13]. During intravenous Bu administration, this should be accomplished with as limited blood sampling as possible. Therefore, two limited sampling schemes were investigated using a population model and a Bayesian fitting procedure. The estimation of the AUC seems adequate using plasma time concentrations at 2.5 and 4 h after start of infusion. Compared with the other scheme (sampling at 2.5 and 6 h), 4 h sampling has a considerable logistic advantage as the results of analyses can be obtained on the day of sample collection.
In conclusion, the pharmacokinetics of intravenous Bu (Busulfex) in children are described adequately by a one-compartment model. This combined with limited sampling at 2.5 and 4 h after the start of infusion and a Bayesian fitting procedure, can estimate systemic exposure of i.v. Bu. Compared with the pharmacokinetics of oral Bu, those of intravenous Bu show very little variability in children. Systemic exposure to intravenous Bu appears to be relatively low in children compared with adults. However, whether this should lead to an increased dose remains to be investigated in larger studies.
Acknowledgments
This study was funded from internal sources.
References
- 1.Slattery JT, Risler LJ. Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther Drug Monit. 1998;20:543–549. doi: 10.1097/00007691-199810000-00017. 10.1097/00007691-199810000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Grochow LB, Krivit W, Whitley CB, Blazar B. Busulfan disposition in children. Blood. 1990;75:1723–1727. [PubMed] [Google Scholar]
- 3.Hassan M, Öberg G, Bekassy AN. Pharmacokinetics of high-dose busulphan in relation to age and chronopharmacology. Cancer Chemother Pharmacol. 1991;28:130–134. doi: 10.1007/BF00689702. [DOI] [PubMed] [Google Scholar]
- 4.Vassal G, Fischer A, Challine D, et al. Busulfan disposition below the age of three: alteration in children with lysosomal storage disease. Blood. 1993;82:1030–1030. [PubMed] [Google Scholar]
- 5.Regazzi MB, Locatelli F, Buggia I, et al. Disposition of high-dose busulfan in pediatric patients undergoing bone marrow transplantation: a pharmacokinetic study of dose escalation. Blood. 1992;80:2425–2428. [Google Scholar]
- 6.Hassan M, Fasth A, Gerritsen B, et al. Busulphan pharmacokinetics and limited sampling model in children with leukemia and inherited disorders. Bone Marrow Transplantation. 1996;18:843–850. [PubMed] [Google Scholar]
- 7.Olavarria E, Hassan M, Eades A, et al. A phase I/II study of multiple-dose intravenous busulfan as myeloablation prior to stem cell transplantation. Leukemia. 2000;14:1954–1959. doi: 10.1038/sj.leu.2401921. [DOI] [PubMed] [Google Scholar]
- 8.Andersson BS, Gajewski J, Donato M, et al. Allogeneic stem cell transplantation (BMT) for AML and MDS following i.v. busulfan and cyclophosphamide (i.v. BuCy) Bone Marrow Transplantation. 2000;25(Suppl 2):S35–S38. doi: 10.1038/sj.bmt.1702351. [DOI] [PubMed] [Google Scholar]
- 9.Busulfex Product information. Minnetonka, Minnesota, USA: Orphan Medical Inc; 2000. [Google Scholar]
- 10.Andersson BS, Madden T, Tran HT, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6:548–554. doi: 10.1016/s1083-8791(00)70064-4. [DOI] [PubMed] [Google Scholar]
- 11.Hassan Z, Ljungman P, Ringden O, et al. Pharmacokinetics of liposomal busulphan in man. Bone Marrow Transplantation. 2001;27:479–485. doi: 10.1038/sj.bmt.1702823. [DOI] [PubMed] [Google Scholar]
- 12.Chow DSL, Bhagwatwar HP, Phadungpojna S, Andersson BS. Stability-indicating high-performance liquid chromatographic assay of busulfan in aqueous and plasma samples. J Chromatogr B. 1997;704:277–288. doi: 10.1016/s0378-4347(97)00419-2. [DOI] [PubMed] [Google Scholar]
- 13.Tran HT, Madden T, Petropoulos D, et al. Individualizing high-dose oral busulfan: prospective dose adjustment in a pediatric population undergoing allogeneic stem cell transplantation for advanced hematologic malignancies. Bone Marrow Transplantation. 2000;26:463–470. doi: 10.1038/sj.bmt.1702561. [DOI] [PubMed] [Google Scholar]