Abstract
Aims
To characterize the pharmacokinetics and metabolism of oral midazolam in 15 preterm infants.
Methods
After an oral dose (0.1 mg kg−1), blood was drawn up to 24 h after administration. Midazolam and 1-OH-midazolam concentrations were determined with GC-MS. In 8 out of these 15 patients the pharmacokinetics of intravenous midazolam was also studied.
Results
Apparent oral clearance, apparent volume of distribution, plasma half-life and 1-OH-Midazolam/Midazolam AUC ratio were [median (range)]: 2.7 [0.67–15.5] ml kg−1 min−1, 1.4 [0.3–12.1] l kg−1, 7.6 [1.2–15.1], h and 0.03 [0.01–0.96], respectively. Absolute bioavailability was 0.49 [0.12–1.0].
Conclusions
Midazolam oral clearance is markedly decreased in preterm infants as compared with older children, probably because of immature CYP3A4 activity.
Keywords: CYP3A, midazolam, oral bioavailability, preterm infant
Introduction
Midazolam, a short-acting benzodiazepine, is used for sedation in newborn infants requiring prolonged mechanical ventilation and prior to invasive procedures [1]. Data describing midazolam disposition following oral administration in preterm infants are lacking. Midazolam undergoes extensive metabolism by the cytochrome P450 3A (CYP3A) subfamily to a major hydroxylated metabolite (1-OH-midazolam) and several minor metabolites [2]. In preterm infants, hepatic CYP3A activity is decreased, thereby resulting in prolonged plasma clearance of midazolam after intravenous administration [3]. Following oral administration, midazolam is subject to hepatic and intestinal metabolism by CYP3A [4]. As the ontogeny of CYP3A activity in the small intestine appears to mirror that observed for the liver, a decrease in the clearance of midazolam and the rate and extent of 1-OH-midazolam formation would be expected to occur in preterm infants [5].
In this investigation, we examined the pharmacokinetics and metabolism of oral midazolam in preterm infants who required the drug for preprocedural sedation.
Methods
The study was conducted in 15 preterm infants with gestational and postnatal ages ranging from 26 to 31 weeks and 3–13 days, respectively. Mean (±s.d.) study weights were 1076±240 g. Mean (±s.d.) Apgar scores at 1 and 5 min were 6.0±2.0 and 7.9±1.3, respectively. The infants were recruited from the Neonatal Intensive Care Unit of the Sophia Children's Hospital. Patients were excluded if they received morphine, dobutamine, dopamine or any drug known to affect CYP3A4 activity, or if they had significant underlying haemodynamic, renal, hepatic or neurologic dysfunction. This research protocol was approved by the Human Ethics Committee of the Sophia Children's Hospital and the Network Steering Committee of the Paediatric Pharmacology Research Unit Network. Written, informed consent was obtained from parents or legal guardians prior to enrolment of subjects in the study.
Drug administration and sample collection
A single oral dose (0.1 mg kg−1) of midazolam (Dormicum® injection, Roche Laboratories, the Netherlands) was given as a 0.5 ml glucose 5% solution via nasogastric tube, followed by 0.5 ml of glucose 5% to ensure complete drug delivery. In eight of these patients, midazolam was also administered as a single 0.1 mg kg−1 dose in a 5% glucose solution (0.03 mg ml−1) infused over 30 min. Serial arterial blood samples (0.2 ml each) were obtained from an indwelling arterial catheter up to 24 h after dosing.
Analytical methods
Plasma samples were analysed for midazolam and 1-OH-midazolam by gas chromatography with mass spectrometric detection (Hewlett Packard 6890, Agilent Technologies Inc., Palo Alto, CA). The column used was a J & W Scientific DB-17 EVDX [0.2 micron, 25 m (J & W Scientific, Folsom, CA)]. Diazepam (Elkins Sinn, Cherry Hill, NJ), 5 µl of 500 ng ml−1 solution, was added to each sample as an internal standard and solid phase extraction was performed using a Varian Bond Elut Column (Varian Inc., Palo Alto, CA). The interday and intraday coefficients of variation for the low standard (2 ng ml−1) were less than 10% for midazolam and 1-OH midazolam. The lower limit of quantification was 1 ng ml−1 for midazolam and 0.5 ng ml−1 for 1-OH-midazolam using a sample volume of 0.5 ml.
Pharmacokinetic analysis
Pharmacokinetic parameters were calculated using standard noncompartmental methods. The metabolite:parent AUC(0,t) ratio was used as a ‘surrogate’ marker of CYP3A activity, where t is the last sampling time-point. All pharmacokinetic analyses were performed using the Kinetica (version 2.0, Innaphase, Inc., Philadelphia, PA, USA) software package.
Statistical analysis
Results are expressed as mean±s.d. unless stated otherwise. The relationship between age and midazolam or 1-OH-midazolam pharmacokinetics were determined using Spearman's rank (rs) correlation test (SPSS software version 9.0.0, SPSS Inc., Chicago, Ill). The level of significance accepted for all statistical analysis was P = 0.05.
Results
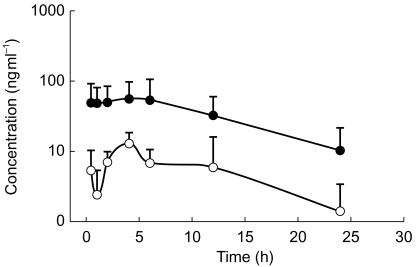
The mean plasma concentration-time curves for midazolam and 1-OH-midazolam are depicted in Figure 1. Midazolam and 1-OH-midazolam pharmacokinetics are summarized in Table 1. Apparent midazolam CL/F was [median (range)] 2.7 [0.7–15.5] ml kg−1 min−1, Vss/F was: 1.4 [0.3–12.1] l kg−1 and t½ was 7.6 [1.2–15.1] h. In 9 out of 15 patients, 1-OH-midazolam could be quantified over the entire post-dose sampling intervals.
Figure 1.
Concentration-time curve of midazolam (•) and 1-OH-midazolam (○) after 0.1 mg kg−1 oral midazolam: mean data (s.d.) from 15 and 4 patients for midazolam and 1-OH-midazolam, respectively.
Table 1.
Oral midazolam calculated pharmacokinetic parameters in preterm infants.
| Midazolam | 1-OH-midazolam | |
|---|---|---|
| AUC(0,t) (ng ml−1 h) | 613 (90–2286) | 68.9 (<0.01–272.6) |
| AUC(0,∞) (ng ml−1 h) | 642 (108–2465) | 71.8 (1.6–305.0)# |
| t½ (h) | 7.6 (1.2–15.1) | NA |
| Vss/F (l kg−1) | 1.4 (0.3–12.1) | NA |
| CL/F (l kg−1 h−1) | 0.16 (0.04–0.93) | NA |
| MRT (h) | 12.0 (3.7–22.7) | NA |
| Cmax (ng ml−1) | 64.4 (15.2–204.0) | 10.3 (<0.01–22.1) |
| tmax (h) | 2.0 (0.5–12.0) | 4.0 (0.5–24.0) |
| AUC ratio | 0.03 (<0.01–0.96) | |
| F (%) | 0.49 (0.12–1.0) |
Data are expressed as: median (range)
data of four patients.
Cmax = maximal concentration of drug in plasma, tmax = time to reach Cmax, AUC(0,t) = area under the concentration-time curve from time zero to the last sampling time point, AUC(0,∞) = area under the concentration-time curve from time zero to infinity, t½ = elimination half-life, CL/F = total apparent clearance and Vss/F = apparent volume of distribution at steady state, MRT = mean resident time, AUC ratio = 1-OH-midazolam AUC(0,t)/midazolam AUC(0,t), F = oral bioavailability, NA = not available.
No significant relationship was detected between age (postnatal, gestational or postconceptual age) and midazolam or 1-OH-midazolam pharmacokinetics.
Discussion
In preterm infants, midazolam CL/F following oral administration was nearly 10-fold lower than previously reported in older children and adults (14.0–40.0 ml kg−1 min−1). Accordingly, midazolam mean elimination half-life was longer in our patients as compared with values reported in older children and adults (1.9–3.2 h) [6, 7]. This ‘impaired’ midazolam elimination in preterm infants mirrors the recognized pattern for the ontogeny of CYP3A4 [5, 8, 9]. Moreover, in preterm infants, decreased midazolam clearance is also observed after intravenous administration consequent to low hepatic CYP3A activity shortly after birth [3]. However, while intravenous midazolam clearance mainly reflects hepatic CYP3A activity, oral midazolam clearance is dependent on both intestinal and hepatic CYP3A activity [4, 10]. Therefore, the decreased midazolam clearance after oral administration to preterm infants is in line with low hepatic ánd intestinal CYP3A activity directly after birth. This hypothesis is also supported by our observation that the median 1-OH-midazolam/midazolam AUC(0,t) ratio is substantially lower and bioavailability higher as compared with adults [0.43±0.03 (mean±s.d.) and 24–38%, respectively [10, 11]].
Whereas midazolam elimination shows a positive association with age over the first years of life [9], we did not find a relationship between age (postconceptional, gestational or postnatal) and either the CL/F or 1-OH-midazolam/midazolam AUC(0,t) ratio within our study cohort. This finding is in agreement with previous reports from preterm and term newborn infants with gestational ages ranging from 24 to 39 weeks [3, 12], which suggests that CYP3A4/5 activity increases only marginally during the first 2 weeks of postnatal life. The lack of a relationship between metabolism and age may also be influenced by a contribution from CYP3A7 to the metabolism of midazolam in this postnatal period [8].
Finally, owing to the technical limitations of a small sample volume, we were not able to measure plasma 1-OH-midazolam-glucuronide concentrations. Therefore, the AUC ratio we report is not ‘corrected’ for glucuronidation [13]. As the ontogenic pattern of 1-OH-midazolam glucuronidation is unknown, interpretation of data pertaining the use of this ratio as a surrogate ‘marker’ of CYP3A activity should be made with caution.
Acknowledgments
Roche Pharmaceuticals, Jerry Sepinwall, Ph.D., Director, Roche Records Office kindly provided 1-OH-midazolam. This work was supported in part by grant #216 (S.N.W.) from the Sophia Foundation for Medical Research, Rotterdam and the Erasmus Trust Fund, the Netherlands, a grant-in-aid from Roche Laboratories, Nutley, NJ, and grants #1U01HD31313-07 (G.L.K) and #5U01HD31316-08 (J.N.A), Paediatric Pharmacology Research Unit Network, National Institute of Child Health and Human Development, Bethesda, MD.
References
- 1.Jacqz-Aigrain E, Burtin P. Clinical pharmacokinetics of sedatives in neonates. Clin Pharmacokinet. 1996;31:390–443. doi: 10.2165/00003088-199631060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Gorski JC, Hall SD, Jones DR, VandenBranden M, Wrighton SA. Regioselective biotransformation of midazolam by members of the human cytochrome P450, 3A (CYP3A) subfamily. Biochem Pharmacol. 1994;47:1643–392. doi: 10.1016/0006-2952(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 3.Burtin P, Jacqz-Aigrain E, Girard P, et al. Population pharmacokinetics of midazolam in neonates. Clin Pharmacol Ther. 1994;56:615–625. doi: 10.1038/clpt.1994.186. [DOI] [PubMed] [Google Scholar]
- 4.Thummel KE, O'Shea D, Paine MF, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 5.Johnson TN, Tanner MS, Taylor CJ, Tucker GT. Enterocytic CYP3A4 in a paediatric population. Developmental changes and the effect of coelic disease and cystic fibrosis. Br J Clin Pharmacol. 2001;51:451–460. doi: 10.1046/j.1365-2125.2001.01370.x. 10.1046/j.1365-2125.2001.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbari B, Khoo K-C, Barsanti F, Pou S, Piscitelli D, Gillespie W. Pharmacokinetics of midazolam and 1-hydroxymidazolam in pediatric patients after a single oral dose of midazolam syrup. Clin Pharmacol Ther. 1998;63:PII 90A. [Google Scholar]
- 7.Smith MT, Eadie MJ, O'Rourke Brophy T. The pharmacokinetics of midazolam in man. Eur J Clin Pharmacol. 1981;19:271–178. doi: 10.1007/BF00562804. [DOI] [PubMed] [Google Scholar]
- 8.Lacroix D, Sonnier M, Monion A, Cheron G, Cresteil T. Expression of CYP3A in the liver. Evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem. 1997;247:625–634. doi: 10.1111/j.1432-1033.1997.00625.x. [DOI] [PubMed] [Google Scholar]
- 9.de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. Cytochrome P450, 3A. Ontogeny and drug disposition. Clin Pharmacokinet. 1999;37:485–505. doi: 10.2165/00003088-199937060-00004. [DOI] [PubMed] [Google Scholar]
- 10.Paine MF, Shen DD, Kunze KL, et al. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60:14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 11.Mandema JW, Tuk B, van Steveninck AL, Breimer DD, Cohen AF, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the central nervous system effects of midazolam and its main metabolite alpha-hydroxymidazolam in healthy volunteers. Clin Pharmacol Ther. 1992;51:715–728. doi: 10.1038/clpt.1992.84. [DOI] [PubMed] [Google Scholar]
- 12.Lee TC, Charles BG, Harte GJ, Gray PH, Steer PA, Flenady VJ. Population pharmacokinetic modeling in very premature infants receiving midazolam during mechanical ventilation: midazolam neonatal pharmacokinetics. Anesthesiology. 1999;90:451–457. doi: 10.1097/00000542-199902000-00020. [DOI] [PubMed] [Google Scholar]
- 13.van Rij KM, Compas D, Swart EL, de Goede PN, Touw DJ. Reversed-phase ion-pair HPLC method for the direct analysis of 1-OH midazolam glucuronide in human serum. Ther Drug Monit. 1999;21:416–420. doi: 10.1097/00007691-199908000-00006. 10.1097/00007691-199908000-00006. [DOI] [PubMed] [Google Scholar]