Abstract
Aims
The purpose of this study was to investigate the tolerability, pharmacokinetics, and pharmacodynamics of tezosentan, an intravenous dual endothelin receptor antagonist, during chronic infusions in healthy male subjects.
Methods
Tezosentan was infused at a rate of 100 mg h−1 for 6 h (study A, six subjects) and at a rate of 5 mg h−1 for 72 h (study B, eight subjects). Both studies had a randomized, placebo-controlled, double-blind design. Tolerability and safety were monitored by the recording of vital signs, ECG, adverse events and clinical laboratory parameters. Blood samples were collected frequently for pharmacokinetic determinations and measurement of plasma endothelin-1 concentrations.
Results
In both studies tezosentan was well tolerated with headache the most frequently reported adverse event (incidence of 75–100% for tezosentan and 50% for placebo). Plasma concentrations of tezosentan rapidly approached steady state (3000 and 125 ng ml−1 in study A and B, respectively) and did not change upon prolonged infusion. A two-compartment model could describe its pharmacokinetic profile. The half-lives of the two disposition phases were approximately 0.10 and 3.2 h. Endothelin-1 concentrations increased rapidly 11- and 2-fold compared with pre-dose values in study A and B, respectively, during infusion of tezosentan and did not change during the 72 h infusion.
Conclusions
On the basis of these results, dose finding studies with tezosentan in acute heart failure can be initiated in the dose range 5–100 mg h−1.
Keywords: endothelin antagonist, endothelin-1, infusion, pharmacodynamics, pharmacokinetics, tezosentan
Introduction
Endothelin-1, which is synthesized predominantly by the vascular endothelium, is the most potent known constrictor of human resistance and capacitance vessels [1]. Since its discovery in 1988 [2], it has been the topic of numerous investigations. Endothelin-1 appears to play an important role in several pathological processes such as renal failure [3] and acute heart failure [4]. In atherosclerosis, myocardial infarction, pulmonary hypertension, heart failure, and renal failure, endothelin-1 concentrations are elevated in both tissue and plasma [5]. Patients with the most severe grade of heart failure appear to have the highest levels of endothelin-1 [6]. Endothelin receptor antagonists are currently being investigated for a wide spectrum of indications [7]. For the acute treatment of emergency indications, the water-soluble endothelin receptor antagonist tezosentan is being developed.
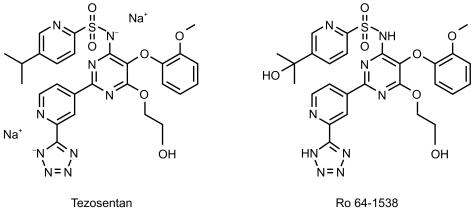
Tezosentan [Veletri™; Ro 61–0612; 5-isopropyl-pyridine-2-sulphonic acid 6-(2-hydroxy-ethoxy)-5-(2-methoxy-phenoxy)-2-(2-1H-tetrazol-5-yl-pyridin-4-yl)- pyrimidin-4-ylamide] is a new endothelin antagonist with a high affinity to both endothelin A and B receptors, and specifically designed for parenteral use [8]. The chemical structure of tezosentan is depicted in Figure 1. Intravenous (i.v.) injection of tezosentan is effective in animal models of hypertension, acute renal failure and heart failure in which it increases cardiac output and renal blood flow and decreases peripheral and pulmonary pressures, pulmonary oedema and induces coronary vasodilatation [8]. In the first clinical study tezosentan was administered in a dose range of 5–600 mg, as an i.v. infusion for 1 h to healthy male subjects [9]. The drug was shown to be well tolerated with a dose-dependent incidence of headache at doses of 100 mg and higher. Tezosentan followed two-compartmental pharmacokinetics with a pronounced first disposition phase (half-life 6 min) and a terminal elimination half-life of approximately 3 h. In vivo metabolism of tezosentan in rats is very limited. The predominant elimination mechanism is biliary excretion of unchanged compound. Preliminary data indicate that in humans tezosentan is also for the major part excreted unchanged into faeces whereas unchanged tezosentan in urine accounts for approximately 2% of the administered dose (Actelion Pharmaceuticals Ltd, data on file). Endothelin-1 levels increased in a dose- and concentration-dependent fashion. The findings in the entry-into-man study, in particular the good tolerability and the advantageous pharmacokinetic profile, warranted further clinical studies with tezosentan.
Figure 1.
Chemical structure of tezosentan and its hydroxylated metabolite Ro 64–1538.
The objectives of the two studies presented here were to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of tezosentan in healthy subjects when infused for a longer period of time, in line with anticipated clinical practice. The doses selected were the extremes of the dose regimens to be investigated in patient trials, i.e. 5 and 100 mg h−1. The infusion rate of 100 mg h−1 was maintained for 6 h because 100 mg infused for 1 h had been well tolerated and induced marked increases in endothelin-1 plasma concentrations [9]. The latter are thought to be caused by reduced endothelin-B-mediated clearance [10, 11]. Tezosentan has a higher affinity for endothelin A than for B receptors (pA2 values of 9.5 and 7.7, respectively) and therefore an infusion rate of 100 mg h−1 is probably at the higher end of the dose–effect relationship. The infusion rate of 5 mg h−1 for 1 h elicited marginal changes in endothelin-1 concentrations and in the present study this rate was maintained for 72 h.
Methods
Investigational site
This study was conducted at Hôpital Stell, Unité de Pharmacologie Clinique, 1 Rue Charles Drot, 92500 Rueil Malmaison, France with Dr Gérard Greig as medical investigator.
Subjects
In study A eight healthy male subjects (seven white, one black), in the age range 21–36 years and weighing 63–94 kg, and in study B 12 healthy male subjects (nine white, two oriental, and one of mixed race), in the age range 21–28 years and weighing 56–79 kg, were recruited. Both study protocols were approved by an Ethics Committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale de Versailles, France). All subjects gave written informed consent before any screening procedures were performed. Both studies were conducted in full conformity with the principles of the Declaration of Helsinki and its amendments. Subjects were assessed to be healthy on the basis of physical examination (including 12-lead ECG), medical history, and clinical laboratory tests (haematology, blood chemistry, and urinalysis) performed within 2 weeks before drug administration. Testing for illicit drugs in blood and urine was also performed. Subjects smoking more than 10 cigarettes a day were not included. No concomitant medications were allowed during the studies, with the exception of medications to treat adverse events. Restrictions were applied regarding the intake of methylxanthine-containing beverages and food as well as consumption of grapefruit juice. The subjects were hospitalized from approximately 12 h before until 24 h after drug infusion. An end-of-study examination was performed 6±3 days after discharge.
Design
Both studies had a double-blind, randomized, placebo-controlled design. In study A 100 mg h−1 tezosentan were infused for 6 h. In study B the infusion rate was 5 mg h−1 for a period of 72 h. A dose of 600 mg infused for 1 h had already been administered in the first clinical study with tezosentan and had shown an acceptable tolerability [9]. In study A, a group of eight subjects were randomized to receive either active drug (n = 6) or placebo (n = 2) whereas in study B eight subjects received active drug and four received placebo. After a light breakfast 2 h before start of the infusion, the drug was infused, using a calibrated infusion pump, and no food was allowed until 4 h after start of the drug infusion. A pharmacist, who was not further involved in the conduct of the study, prepared tezosentan infusions at a concentration of 0.25% (study A, 40 ml h−1) and 0.025% (study B, 20 ml h−1) in physiological saline, starting from 2.5% vials. A solution of 0.9% sodium chloride served as placebo.
Assessments
Tolerability
Adverse events were assessed by spontaneous reports, observations, and questioning at regular intervals. The intensity of the adverse events was rated on a three-point scale (mild, moderate, and severe) and the investigator assessed the potential relationship to drug, before breaking the code. Supine blood pressure, pulse rate, and body temperature were measured at frequent intervals. A two-lead ECG was recorded continuously for 4 h after start of the infusion in both studies as well as during the intervals 24–28 and 48–52 h in study B. Twelve-lead ECGs were recorded immediately before and at 3, 9, and 24 h after start of the infusion in study A and immediately before and at 3, 6, 12, 24, 27, 30, 36, 48, 51, 54, 60, and 72 h after start of the infusion in study B.
Pharmacokinetics
Blood samples of 4.5 ml were collected into EDTA-containing tubes, via a catheter inserted into a forearm vein, contralateral to the drug infusion arm. In study A, samples were collected at the following time points: immediately before and at 1.0, 3.0, 5.0, 6.0, 6.25, 6.5, 6.75, 7.0, 7.5, 8.0, 9.0, 10, 12, 15, and 18 h after start of the infusion. In study B blood samples were collected immediately before and at 1, 3, 6, 12, 24, 30, 36, 48, 54, 60, and 72 h after start of the infusion. Blood samples were not collected after termination of the infusion because the entry-into-man study had indicated that the sensitivity of the assay method was not sufficient to characterize both disposition phases following infusion rates≤20 mg h−1[9]. Plasma was separated and stored at −20° C pending analysis. Plasma concentrations of tezosentan and its hydroxy metabolite Ro 64–1538 (Figure 1) were simultaneously determined by means of a narrow bore liquid chromatography method coupled to tandem mass spectrometry operating in the positive ionization mode. The analytes and the internal standard Ro 61–0612/5 (an analogue of tezosentan deuterated in four positions) were extracted from acidified plasma with tert-butyl methyl ether. The compounds were chromatographed on a 2.1×50 mm reversed-phase column (mobile phase acetonitrile/water 1:1 with 0.02% trifluoroacetic acid) and introduced into the mass spectrometer with positive ionization detection at a flow rate of 0.4 ml min−1. The limit of quantification was 2.5 ng ml−1 for tezosentan and 5.0 ng ml−1 for Ro 64–1538 using 0.5 ml plasma aliquots. The performance of the chromatographic method was monitored by simultaneous analysis of independently prepared quality control samples of various concentrations. The assay was found to be linear in the concentration range 2.50–1000 and 5.00–1000 ng ml−1 for tezosentan and Ro 64–1538, respectively. The interassay coefficient of variation was always smaller than 12.7%, whereas the inaccuracy was between −6.5 and 6.3%.
Pharmacodynamics
Plasma endothelin-1 concentrations in study A were determined in samples collected immediately before and at 1, 3, 5, 6, 8, 10, 12, 15, and 18 h after start of the infusion. In study B samples were taken at the same time points as for the pharmacokinetic determinations. A validated enzyme linked immuno sorbent assay (ELISA) method with a limit of quantification of 0.25 pg ml−1 was used. The quantification of endothelin-1 was performed with a commercially available immunoassay kit (R & D Systems Europe, Oxon, UK) after extraction from plasma by reversed-phase column chromatography. Briefly, duplicate 500 µl aliquots of plasma were extracted on C18 solid phase columns after conditioning with methanol, followed by washing with water and elution with methanol–water (90:10, v/v). The eluates were dried and reconstituted in assay buffer, and sandwich ELISA performed. After washing, the bound endothelin-1 was detected enzymatically and quantified photometrically.
Evaluations
Tolerability
The adverse events and clinical laboratory data were evaluated descriptively. Vital signs were screened for values outside the normal range that was defined as 100–140 mmHg for systolic blood pressure, 45–90 mmHg for diastolic blood pressure, 45–90 beats min−1 for pulse rate, and 35.0–37.5° C for body temperature. Clinical laboratory values were compared with the normal range supplied by the analysing laboratory.
Pharmacokinetic data analysis
The pharmacokinetic evaluation for tezosentan and its metabolite in study A was performed with model-dependent and model-independent methods [12]. The peak plasma concentration (Cmax) was read directly from the concentration–time data. Area under the plasma concentration–time curve (AUC) was estimated with use of the linear trapezoidal rule and extrapolation to infinity with the help of the terminal elimination rate constant λz. The latter was determined by log-linear regression analysis of the terminal phase. The t½ was calculated by division of ln2 by λz. The systemic plasma clearance (CL) and volume of distribution at steady state (Vss) were calculated using standard techniques [12]. Plasma concentrations of tezosentan were fitted to an open two-compartment pharmacokinetic model, using the programme WinNonlin with a constant weight factor (w = 1). Pharmacokinetic parameters were analysed descriptively, calculating geometric mean and 95% confidence limits.
Pharmacodynamic data analysis
Plasma endothelin-1 concentrations were evaluated descriptively.
Results
Tolerability
All eight subjects in study A completed the study according to the protocol, whereas in study B one subject discontinued after 52 h of tezosentan infusion because of headache. Another subject who received placebo was withdrawn after 58 h because an incomplete right bundle branch block was evident on the ECG. The adverse events reported in the studies are presented in Table 1. In study A, adverse events were reported by one of two subjects treated with placebo and by all six subjects treated with active drug. In study B, adverse events were reported by two of four subjects treated with placebo and by six of eight subjects treated with tezosentan.
Table 1.
Overview of reported adverse events in the two studies.
| Study A | Study B | |||
|---|---|---|---|---|
| Tezosentan | Placebo | Tezosentan | Placebo | |
| Number of subjects | 6 | 2 | 8 | 4 |
| Number of subjects reporting adverse events | 6 | 1 | 6 | 2 |
| Total number of adverse events reported | 11 | 2 | 9 | 4 |
| Specific adverse events reported (number of cases) | ||||
| Headache | 6 | 1 | 6 | 2 |
| Flushing | 1 | – | 1 | – |
| Nausea | 1 | – | – | – |
| Abdominal pain | – | – | 1 | – |
| Dizziness | 1 | – | – | – |
| Palpitations | – | – | – | 1 |
| Haematoma | 1 | – | – | – |
| Eye pain | 1 | 1 | – | – |
| Back pain | – | – | 1 | 1 |
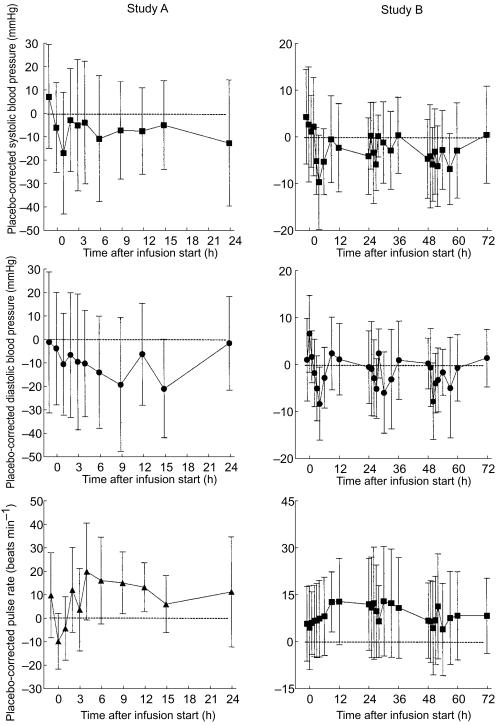
The most prominent adverse event was headache, which was reported by 12 out of 14 subjects treated with tezosentan and by three out of six subjects treated with placebo. Flushing was reported by two subjects treated with tezosentan in study A. No adverse event was rated as severe in intensity whereas in study B headache was rated as moderate by one subject treated with tezosentan and by one treated with placebo. All other events were rated as mild in intensity and all resolved quickly without sequelae. There was no pattern of abnormal laboratory values or vital signs observed during the study to suggest a treatment effect, and the abnormalities observed were judged to be clinically not relevant. Figure 2 displays the differences between tezosentan- and placebo-treated subjects (with their 95% confidence intervals) for blood pressure and pulse rate during and following the two infusions. Electrocardiogram findings did not show relevant differences to baseline with the exception of the placebo subject withdrawn from study B. Results of investigations at the end-of-study examination did not reveal relevant differences from baseline values.
Figure 2.
Time-course of differences between tezosentan- and placebo-treated subjects in systolic and diastolic blood pressure and pulse rate in study A (left panels) and B (right panels). Data are presented as mean±95% confidence intervals.
Pharmacokinetics
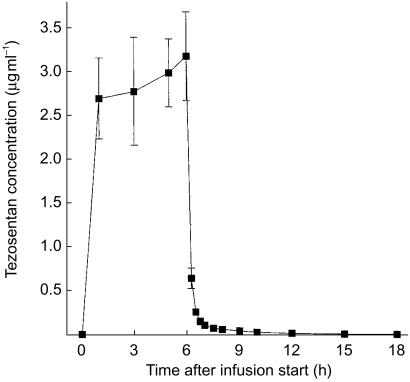
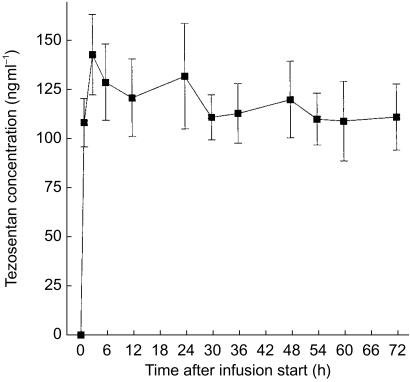
The metabolite Ro 64–1538 was detected only in some samples collected in study A. Its maximum concentration was lower than 1% of that of the parent compound and therefore Ro 64–1538 concentrations were not subjected to pharmacokinetic evaluation. Figures 3 and 4 present the mean plasma concentration–time profiles of tezosentan in studies A and B, respectively. Plasma concentrations reached steady-state conditions within a short period of time. After discontinuation of the infusion, plasma concentrations of tezosentan quickly decreased following a biphasic profile (Figure 3). Within 30 min after termination of the infusion, mean plasma concentrations were lower than 10% of those just before termination of the infusion. The pharmacokinetic parameters as determined by model-independent analysis are summarized in Table 2. Only the first half-life (t1/2,λ1) was estimated by compartmental evaluation.
Figure 3.
Plasma concentration–time profile of tezosentan during and following an i.v. infusion of 100 mg h−1 for 6 h. Data are presented as mean±s.e.mean (n = 6).
Figure 4.
Plasma concentration–time profile of tezosentan during an i.v. infusion of 5 mg h−1 for 72 h. Data are presented as mean±s.e.mean (n = 7).
Table 2.
Pharmacokinetic parameters of tezosentan following i.v. infusion.
| Study A 100 mg h−1 (6 h) | Study B 5 mg h−1 (72 h) | |
|---|---|---|
| Number of subjects | 6 | 8 |
| Cmax (µg ml−1) | 3.4 (2.7, 4.2) | 0.16 (0.12, 0.21) |
| t1/2,λ1 (h) | 0.10 (0.07, 0.15) | – |
| t1/2,λz (h) | 3.2 (2.1, 5.0) | – |
| AUC(0, ∞) (µg ml−1 h) | 16 (13, 20) | 7.9 (5.6, 11)a |
| CL (l h−1) | 38 (31, 47) | 44 (33, 60) |
| Vss (l) | 22 (14, 36) | – |
Data are presented as geometric mean (95% confidence interval).
AUC(0, 72 h) (n = 7).
Pharmacodynamics
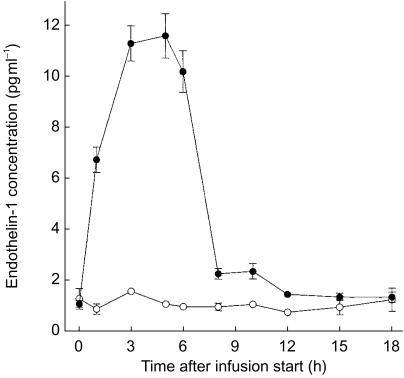
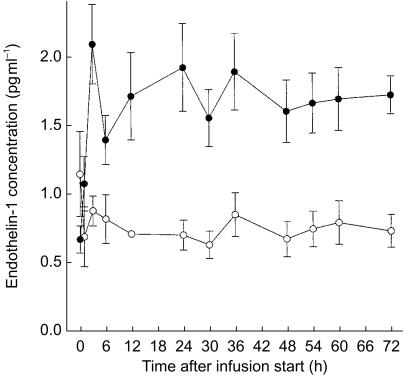
Endothelin-1 concentrations as a function of time are presented in Figures 5 and 6 for study A and B, respectively. Predose levels were approximately 0.9 pg ml−1 and quickly increased during the infusion of tezosentan approximately 11-fold in study A and 2–3 fold in study B. Within several hours endothelin-1 concentrations attained steady-state conditions. Levels decreased rapidly after cessation of the infusion.
Figure 5.
Plasma concentration–time profile of endothelin-1 during and following an i.v. infusion of 100 mg h−1 tezosentan for 6 h. Data are presented as mean±s.e.mean. •, tezosentan (n = 6); ○, placebo (n = 2).
Figure 6.
Plasma concentration–time profile of endothelin-1 during an i.v. infusion of 5 mg h−1 tezosentan for 72 h. Data are presented as mean±s.e.mean. •, tezosentan (n = 7); ○, placebo (n = 4).
Discussion
The experiments described above constitute the second set of clinical studies performed with tezosentan, a dual endothelin receptor antagonist. As drugs used in the treatment of acute heart and renal failure are often chronically infused, in these studies the infusions administered were of longer duration than in the entry-into-man study [9]. The infusion rates selected were 5 and 100 mg h−1, which, on the basis of the previous study, are expected to be the limits of the therapeutic dosing regimen.
The only adverse event, which clearly had a higher incidence with tezosentan than with placebo, was headache. This confirmed the results of the first clinical study in which doses of 100 mg and higher (infused in 1 h) also induced headache of mild/moderate intensity [9]. A nitrate-like headache has occurred in healthy subjects treated with other endothelin antagonists [13–15] but was less pronounced in patients [16]. Probably this phenomenon is caused by cerebral vasodilatation, which is in line with the absence of signs of systemic vasodilatation (Figure 2). Even upon infusion for 72 h, no local tolerability problems occurred which confirms that the physico–chemical properties of tezosentan are appropriate for chronic infusion.
Only slight decreases in systolic and diastolic blood pressure (without reflex tachycardia) were observed and these did not differ significantly from those after placebo. However, these findings should be viewed within the constraints of the study, i.e. a relatively small number of subjects. Dual endothelin receptor antagonists such as tezosentan and bosentan do not modify blood pressure in healthy subjects. This suggests that endogenous endothelin does not play a physiological role in the maintenance of normal blood pressure. However, selective antagonism of endothelin-A receptors reduces blood pressure in normal subjects, which can probably be attributed to excessive release of nitric oxide. Endothelin-A receptor antagonist-mediated vasodilatation can be attenuated by endothelin-B receptor blockade [17, 18]. An i.v. bolus injection of 10 mg kg−1 tezosentan decreased the mean arterial blood pressure in conscious normotensive rats by only 8 mmHg and did not change blood pressure in dogs [8]. Endothelin receptor blockade with the mixed endothelin-A and -B receptor antagonist SB 209670 also showed only a modest contribution of endothelin to basal renal vascular tone in healthy subjects [14].
Upon i.v. infusion of tezosentan, plasma concentrations quickly increased and approached constant levels in accordance with the data obtained previously [9]. Mean tezosentan concentrations in study A showed a trend for a small increase during the 6 h infusion, which might reflect the terminal elimination half-life, although interindividual variability was substantial. Following termination of the infusion, tezosentan plasma concentrations decreased in a biphasic fashion, similar to that observed following the 1 h infusion [9]. This indicates that the pharmacokinetics of tezosentan are not prone to changes upon chronic infusion. In study B, tezosentan plasma concentrations were only determined during the infusion and therefore a complete pharmacokinetic evaluation was not possible. However, the pharmacokinetic parameters are in agreement with those obtained in study A, which is in line with dose- and time-independent pharmacokinetics. The clearance was approximately 40 l h−1, which, at a blood/plasma ratio of approximately 0.6, is moderate in relation to hepatic blood flow. The volume of distribution was approximately 22 l, in accordance with the volume of extracellular water [19]. Ro 64–1538, a metabolite of tezosentan formed by hydroxylation of the isopropyl side chain, was present at only very low concentrations, which means that this metabolite does not accumulate upon chronic infusion. Because it is 10–20 fold less potent than the parent compound (M. Clozel, personal communication), its contribution to the overall pharmacological effect is negligible. The pharmacokinetic properties of tezosentan make it a good candidate for use in acute situations. Without the need for loading doses, efficacious drug levels can be quickly reached and termination of the infusion leads to a quick disappearance of drug from the systemic circulation, which is an asset in case of drug-related tolerability problems.
The increase in endothelin-1 plasma concentrations appeared to be dose related over the dose range covered in the present studies. Tezosentan prevents binding of endothelin-1 to its receptors, in particular endothelin-B receptors, which have been reported to be responsible for endothelin-1 clearance [10, 11]. This phenomenon has also been observed in patients treated with other nonselective endothelin antagonists [14, 20, 21]. Only upon more frequent measurement of endothelin-1 concentrations during the ascending and the descending phase, attempts can be undertaken to model the relationship between pharmacokinetics and pharmacodynamics of tezosentan (Dingemanse et al. in preparation). The constant levels of endothelin-1 during the infusion of tezosentan are, however, indicative of an unaltered concentration–effect relationship for up to 72 h. After termination of the infusion at a rate of 100 mg h−1 (study A), endothelin-1 concentrations decreased rapidly which reflects the rapid disposition of tezosentan. Endothelin-1 levels close to baseline were already attained approximately 6 h after discontinuation of the infusion. Endothelin-1 shows a relatively complicated pharmacokinetic profile with a terminal elimination half-life of several hours [22].
In conclusion, the two studies reported here with infusion durations of 6 and 72 h are in accordance with the tolerability, pharmacokinetics, and pharmacodynamics data reported in the entry-into-man study. Taken together, these studies provide a solid base for initiating clinical studies in the patient population. It is recommended that dose finding studies are performed in the range 5–100 mg h−1. There is an ongoing debate on whether selective or nonselective endothelin receptor antagonists are to be preferred in the (investigational) treatment of heart failure [23–25]. Because tezosentan is a potent dual endothelin receptor antagonist, is well tolerated, and does not cause a detectable effect on blood pressure or pulse rate in these small groups of healthy subjects, it may be an interesting probe for comparative studies with selective endothelin receptor antagonists.
References
- 1.Pernow J, Ahlborg G, Lundberg JM, Kaijser L. Long-lasting coronary vasoconstrictor effects and myocardial uptake of endothelin-1 in humans. Acta Physiol Scand. 1997;159:147–153. doi: 10.1046/j.1365-201X.1997.578350000.x. [DOI] [PubMed] [Google Scholar]
- 2.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 3.Rabelink TJ, Kaasjager KA, Stroes ES, Koomans HA. Endothelin in renal pathophysiology: from experimental to therapeutic application. Kidney Int. 1996;50:1827–1833. doi: 10.1038/ki.1996.502. [DOI] [PubMed] [Google Scholar]
- 4.Kaddoura S, Poole-Wilson PA. Endothelin-1 in heart failure. A new therapeutic target? Lancet. 1996;50:1827–1833. doi: 10.1016/S0140-6736(05)64531-X. [DOI] [PubMed] [Google Scholar]
- 5.Lüscher TF, Barton M. Endothelins and endothelin receptor antagonists. Therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 6.Wei C, Lerman A, Rodeheffer R, et al. Endothelin in human congestive heart failure. Circulation. 1994;89:1580–1586. doi: 10.1161/01.cir.89.4.1580. [DOI] [PubMed] [Google Scholar]
- 7.Ray A, Hegde LG, Chugh A, Gupta JB. Endothelin-receptor antagonists. current and future perspectives. Drug Discovery Today. 2000;5:455–464. doi: 10.1016/s1359-6446(00)01557-9. 10.1016/s1359-6446(00)01557-9. [DOI] [PubMed] [Google Scholar]
- 8.Clozel M, Ramuz H, Clozel JP, et al. Pharmacology of tezosentan, new endothelin receptor antagonist for parenteral use. J Pharmacol Exp Ther. 1999;290:840–846. [PubMed] [Google Scholar]
- 9.Dingemanse J, Clozel M, van Giersbergen PLM. Entry-into-man study with tezosentan, an intravenous dual endothelin receptor antagonist. J Cardiovasc Pharmacol. doi: 10.1097/00005344-200206000-00004. in press. [DOI] [PubMed] [Google Scholar]
- 10.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 11.Plumpton C, Ferro CJ, Haynes WG, Webb DJ, Davenport AP. The increase in human plasma immunoreactive endothelin but not big endothelin-1 or its C-terminal fragment induced by systemic administration of the endothelin antagonist TAK-044. Br J Pharmacol. 1996;119:311–314. doi: 10.1111/j.1476-5381.1996.tb15987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker; 1982. [Google Scholar]
- 13.Weber C, Schmitt R, Birnboeck H, et al. Pharmacokinetics and pharmacodynamics of the endothelin-receptor antagonist bosentan in healthy human subjects. Clin Pharmacol Ther. 1996;60:124–137. doi: 10.1016/S0009-9236(96)90127-7. [DOI] [PubMed] [Google Scholar]
- 14.Freed MI, Wilson DE, Thompson KA, Harris RZ, Ilson BE, Jorkasky DK. Pharmacokinetics and pharmacodynamics of SB 209670, an endothelin receptor antagonist: effects on the regulation of renal vascular tone. Clin Pharmacol Ther. 1999;65:473–482. doi: 10.1016/S0009-9236(99)70066-4. [DOI] [PubMed] [Google Scholar]
- 15.Verhaar MC, Grahn AY, van Weerdt AW, et al. Pharmacokinetics and pharmacodynamic effects of ABT-627, an oral ETA selective endothelin antagonist, in humans. Br J Clin Pharmacol. 2000;49:562–573. doi: 10.1046/j.1365-2125.2000.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. N Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 17.Verhaar MC, Strachan FE, Newby DE, et al. Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation. 1998;97:752–756. doi: 10.1161/01.cir.97.8.752. [DOI] [PubMed] [Google Scholar]
- 18.Reinhart GA, Preusser LC, Opgenorth TJ, Wegner CD, Cox BF. Endothelin and ET (A) receptors in long-term arterial pressure homeostasis in conscious nonhuman primates. Am J Physiol Regul Integr Comp Physiol. 2000;279:701–706. doi: 10.1152/ajpregu.2000.279.5.R1701. [DOI] [PubMed] [Google Scholar]
- 19.Rowland M, Tozer TN. Clinical PharmacokineticsConcepts and Applications. 3. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 20.Kiowski W, Sütsch G, Hunziker P, et al. Evidence for endothelin-1 mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–736. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- 21.Sütsch G, Kiowski W, Yan XW, et al. Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation. 1998;98:2262–2268. doi: 10.1161/01.cir.98.21.2262. [DOI] [PubMed] [Google Scholar]
- 22.Parker JD, Thiessen JJ, Reilly R, Tong JH, Stewart DJ, Pandey AS. Human endothelin-1 clearance kinetics revealed by a radiotracer technique. J Pharmacol Exp Ther. 1999;289:261–265. [PubMed] [Google Scholar]
- 23.Love MP, McMurray JJ. Endothelin in heart failure: a promising therapeutic target? Heart. 1997;77:93–94. doi: 10.1136/hrt.77.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu C. Recent discovery and development of endothelin receptor antagonists. Exp Opin Ther Patents. 2000;10:1653–1668. [Google Scholar]
- 25.Suresh DP, Lamba S, Abraham WT. New developments in heart failure: role of endothelin and the use of endothelin receptor antagonists. J Card Fail. 2000;6:359–368. doi: 10.1054/jcaf.2000.20560. [DOI] [PubMed] [Google Scholar]