Abstract
Aims
Several studies have found that compliance with lipid-lowering drug (LLD) treatment is low. However, the results of these studies were based on crude measures of compliance. The aim of this study was to describe compliance with statin treatment by analysing prescription patterns on an individual level in a population-based prescription database over a 6 year period.
Methods
For incident statin users, all prescriptions for statins and drugs indicating cardiovascular disease or diabetes were retrieved from the OPED prescription database covering a population of about 470000 inhabitants. Treatment was considered discontinued if the interval between two prescriptions exceeded number of tablets prescribed, plus 30 days. Compliance was assessed in terms of persistence and continuity. Persistence was defined as the period from the first prescription date to the date of discontinuation. Continuity was defined as the number of days with treatment (= number of tablets) divided by the total number of days in the period of persistence.
Results
11% of the study cohort only received a single statin prescription. Survival analyses revealed a median persistence of 41 months. Less than 15% of the patients had more than 20% days without therapy within the period of persistence. Patients under 45 years without drug indicators of cardiovascular disease or diabetes presented the lowest compliance.
Conclusions
The study showed good compliance with statin treatment in terms of persistence and continuity. A high percentage of the youngest patients, however, seemed to discontinue treatment before obtaining the full benefit in terms of decreased risk of coronary heart morbidity and mortality.
Keywords: compliance, prescription database, statins
Introduction
It is generally accepted that lipid-lowering drug (LLD) treatment ought to be given on a long-term basis in order to obtain the benefits in terms of reduced cardiovascular morbidity and mortality. The 4S study [1] showed that a biochemical effect of simvastatin can already be observed after a few weeks, but the effect on ischaemic heart disease (IHD) related morbidity and mortality was observed after approximately 2 years at the earliest. Data from the WOSCOPS study [2] showed that mortality risk from coronary heart disease was significantly influenced by the degree of compliance. Several studies that have addressed the extent of noncompliance with LLD treatment [3–9] have found a considerable problem, but these studies have been based on rather crude measures of compliance.
The aim of this study was to describe compliance with statin treatment by analysing prescription patterns on an individual level in a population-based prescription database over a 6 year period using methodological assumptions based on clinical evidence.
Methods
Setting
For people receiving at least one prescription for LLD in the period 1993–1998, data were retrieved on all filled prescriptions for LLDs (ATC code C10, formerly B04) and cardiovascular (ATC codes B01 and C01 to C09) and antidiabetic medication (ATC code A10). Drug prescription data were retrieved from the Odense University Pharmaco-epidemiologic Database (OPED) [10]. The population covered in OPED (470000 inhabitants or approximately 10% of the Danish population) is considered representative of the total Danish population for a number of important factors including drug consumption [10]. The database provides the following information for each reimbursed prescription: identification of the dispensed product according to the ATC-classification [11], number of packages and number of Defined Daily Doses (DDD) dispensed, code of the prescriber, anonymous code of the patient and date of prescription. The patient code allows the reconstruction of each individual's drug history without identification of the individual.
The possible presence of cardiovascular disease or risk factors was assessed by identifying the LLD-treated individuals, who in the year preceding their first LLD prescription received at least one prescription for one or more drugs belonging to the following ATC groups: A10 (insulins and oral antidiabetics) as a marker for diabetes and B01 (antithrombotics), C01 (cardiac glycosides, antiarrhythmics, nitrates), C02 (antihypertensives), C03 (diuretics), C07 (beta-blockers), C08 (calcium antagonists) and C09 (ACE inhibitors) as markers for cardiovascular disease.
Data were analysed in such a way that the results were comparable with previous studies [3, 8].
Compliance with lipid-lowering drugs
We intended to describe two aspects of compliance with statin treatment by investigating persistence (treatment period as defined below) and continuity (compliance within the treatment period). Compliance was calculated for incident statin users only. An incident user was defined as a patient who filled his first statin prescription after a 1 year run-in period from 1 January 1993. Compliance was calculated for a patient's first period of treatment only. A treatment period was considered discontinued if the interval between two prescriptions exceeded a period covered by the number of tablets prescribed (one tablet per day) plus 30 days. Patients were allowed to save tablets for later use, whereas an excess of tablets received later in the period could not be included backwards.
Persistence of statin use was defined as the period from the date of the first prescription to the date of discontinuation. Observations were censored if they exceeded the end of the study period or at the time the patient died or moved out of the county, whichever event came first.
Continuity was defined as the number of days with treatment (= number of tablets) divided by the total number of days in the period of persistence. A limit between good and poor compliance in relation to continuity was set at 80% [3].
Statistical methods
The time course of discontinuation of statin treatment was illustrated by Kaplan-Meier survival curves. The log rank test was used to test equality of curves. The Cox proportional hazards model was used to estimate the effect of sex, age and comedication on the relative risk (RR) of discontinuation. A logistic regression model estimated the effect of the same factors on continuity. STATA version 6.0 was used for all analyses.
Ethics
Information consisted exclusively of anonymised data. Therefore, according to Danish law the project needed no approval by the regional scientific ethical committee. However, the committee was notified and the Danish Data Protection Agency approved the project.
Results
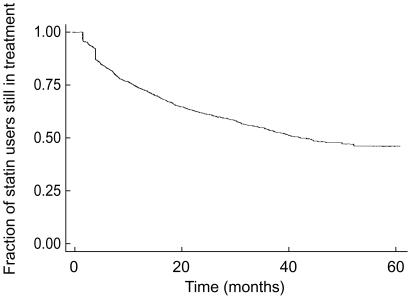
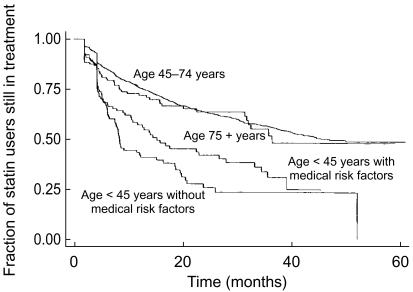
Among 3623 incident statin users observed, 406 (11.2%) filled only one prescription during the study period. Among 1207 patients receiving a prescription for a statin in 1993, 869 (72.0%) also received at least one such prescription in each of the following 5 years (85.6% of the patients receiving a statin prescription in 1993 also received at least one prescription in 1998). Figure 1 shows the overall survival curve for statin treatment (persistence) for patients filling more than one prescription in their first period of treatment. Median persistence was 41 months. A higher persistence was observed in patients starting treatment above the age of 65 (median: 50 vs 40 months). Table 1 shows the effect of six independent variables on persistence. The middle age group (i.e. 45–74 years) was used as reference. When adjusted for the other variables, there was no significant difference between males and females nor between the age group above 75 years and the reference group, whereas age <45 years predicted low persistence. In the low age group there was a significantly lower persistence in those who did not receive risk factor comedication. Patients who had cardiovascular drug risk factors tended to present higher persistence than those without such risk factors (median: 42 months). For patients treated with antidiabetics, this was not the case. Moreover, patients who received insulin had lower persistence than the rest, when adjusted for age and other cardiovascular comedication and insulin (median: 32 months). Figure 2 demonstrates the survival curves for persistence of statin treatment for the groups <45 years with and without overall risk factor comedication, and 75+ years compared with the age group 45–74 years. The age group <45 years with no risk drug indicators presented the lowest persistence (median: 8 months; n = 124), while the same age group with risk indicators presented a better, but still lower persistence than average (median: 15 months; n = 123). The age group above 75 years showed a slightly lower persistence than the reference group (median: 36 months) but still higher than the youngest age group. In this age group, only 14 persons (8.3%) did not receive drugs indicating cardiovascular disease or diabetes (22.1% in the total material).
Figure 1.
Overall persistence of statin treatment 1994–98 in Funen County, Denmark (Kaplan-Meier survival estimate).
Table 1.
Persistence and continuity of statin treatment analysed by Cox's proportional hazards model and a logistic regression model. Relative risk of discontinuation and odds ratio of continuity <80% by sex, age <45 years, age 75+ years and risk factor co-medication. The middle age group (i.e. 45–74 years) served as areference. (95% confidence intervals shown in brackets).
| Variable | n | Persistence Relative risk of discontinuation | P | Continuity OR for low continuity (<80%) | P |
|---|---|---|---|---|---|
| Sex | 3623 | 1.00 (0.89, 1.13) | 0.98 | 1.10 (0.79, 1.53) | 0.57 |
| Age 0–44 years | 247 | 2.24 (1.86, 2.71) | <0.001 | 0.46 (0.27, 0.78) | <0.01 |
| Age 75+ years | 168 | 1.15 (0.87, 1.53) | 0.32 | 1.17 (0.47, 2.92) | 0.74 |
| Cardiovascular co-medication | 2823 | 0.88 (0.77, 1.01) | 0.07 | 1.33 (0.91, 1.95) | 0.14 |
| Insulin | 98 | 1.43 (1.03, 1.98) | 0.03 | 0.62 (0.26, 1.45) | 0.27 |
| Oral antidiabetics | 193 | 1.05 (0.80, 1.38) | 0.73 | 0.67 (0.35, 1.27) | 0.22 |
Figure 2.
Persistence of statin treatment grouped by age <45 years without risk factor comedication, age <45 years with risk factor comedication and age 75+ years with and without risk factor comedication compared with age group 45–74 years (Kaplan-Meier survival estimate).
Patients starting statin treatment in 1994 showed significantly lower persistence than patients starting in any of the following years (median: 29 months; n = 235).
According to our definition of continuity, 95.1% of the incident statin users were 80% or more continuant within their first treatment period. When adjusted for the other variables, young age (<45 years) predicted a significantly lower continuity than the reference group (Table 1). Neither risk factor, comedication nor gender was predictive of continuity. Using a method that allowed 120 days of pause instead of 30 days before defining discontinuation resulted in a decrease in ≥80% continuity to 87.3%.
Discussion
In this study of a Danish population, we found that compliance with statin use was high. 50% of the incident users stayed on the drugs continuously for more than 3 years and over 90% of the users were more than 80% continuant measured by the number of tablets available (days' supply) during the first treatment period. Younger patients without drug indicators of cardiovascular or diabetic disease presented the lowest compliance, their median persistence being less than 1 year. Also, this age group was less continuant, although still 85% presented less than 20% days without statin supply.
Compliance with other lipid-lowering drugs than statins may differ from the results of this study. However, since statins have been shown to represent more than 90% of the lipid-lowering drug use in Denmark [12] we found it appropriate to estimate compliance for this group of drugs only. Our prescription database did not have information on prescribed daily doses, which would have been the ideal measure for continuity calculations. However, as tablets come in all clinically relevant strengths we found it unlikely that a patient took for instance half a tablet or two tablets a day and therefore calculated compliance with statin use on the assumption that one tablet a day was taken. In order to obtain a realistic setting, we also found it reasonable to define discontinuation of a treatment period from a clinical point of view. The effect of simvastatin on serum cholesterol has been reported to return to pretreatment level 5 weeks after withdrawal [13]. Thus, a 30 day period without statin supply was chosen to define discontinuation of treatment. This, naturally, underestimates persistence compared with a method using a longer period without supply. Very few patients of those aged 75 years or above were free of drugs indicating cardiovascular disease or diabetes, which made it impossible to investigate the effect of risk factor comedication on compliance within this group.
Our findings are consistent with the results of a previous comparative Danish study describing compliance with lipid-lowering drugs in Denmark and Italy [6] even though the results of that study were based on a simpler definition of the study cohort and on simpler compliance calculation methods. In their cross-national study Avorn et al. [1] found a 50% discontinuation rate for all lipid-lowering drugs over a 5 year period. However, these results were obtained for patients aged 65+ years who received treatment in the original study year (1990–1991) and also in the follow-up year (1995–1996). Pauses between these periods were not taken into account, which might have resulted in an even lower persistence. Using this method, we found a 5 year discontinuation rate of 14.4%, which, compared with our survival curves, shows that this is a very crude measure of persistence. Of all lipid-lowering drugs, Avorn et al. found the highest 1 year continuation rate for statins (64% days covered). In Australia, Simons et al. found a 60% discontinuation after 12 months [3], contrasting the discontinuation rate of 30% after 6–7 months recently found by the same main author [7]. In a controlled trial, Eriksson et al. [5] found nearly 90% continuation of pravastatin treatment in a 2 year closely followed-up study in Sweden. The study cohort was highly selected, and furthermore, it is well known that patients participating in controlled trials comply better with medication compared with patients observed in a natural setting.
In conclusion, the present study has shown good compliance with statin treatment in a Danish population measured by persistence as well as by continuity. However, a high percentage of the youngest group of patients without drug indicators of cardiovascular disease or diabetes discontinued treatment before obtaining the full benefit in terms of decreased risk of coronary heart morbidity and mortality. As opposed to previously used methods for the calculation of compliance, the one used in this study is considered appropriate due to a realistic setting.
Acknowledgments
The authors wish to thank secretary Lise Stark for proof reading the manuscript. The study was supported by the Danish Pharmacy Foundation, The Danish Medical Research Council (grants no. 9501767 and 9700814), and by the Danish Ministry of Research (grant no. 115–1996). The study complies with current laws in Denmark.
References
- 1.Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease. the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group [see comments] N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 3.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 4.Andrade SE, Walker AM, Gottlieb LK, et al. Discontinuation of antihyperlipidemic drugs – do rates reported in clinical trials reflect rates in primary care settings? N Engl J Med. 1995;332:1125–1131. doi: 10.1056/NEJM199504273321703. [DOI] [PubMed] [Google Scholar]
- 5.Simons LA, Levis G, Simons J. Apparent discontinuation rates in patients prescribed lipid-lowering drugs. Med J Aust. 1996;164:208–211. doi: 10.5694/j.1326-5377.1996.tb94138.x. [DOI] [PubMed] [Google Scholar]
- 6.Beck P, Downey W, Butler-Jones D, Lueck L. Abstract. Presented at the Third Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR); 1998. Pharmaceutical costs of patient non-adherence to lipid-lowering drug therapy. May 27–30. [Google Scholar]
- 7.Eriksson M, Hadell K, Holme I, Walldius G, Kjellstrom T. Compliance with and efficacy of treatment with pravastatin and cholestyramine: a randomized study on lipid-lowering in primary care. J Intern Med. 1998;243:373–380. doi: 10.1046/j.1365-2796.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- 8.Larsen J, Vaccheri A, Andersen M, Montanaro N, Bergman U. Lack of adherence to lipid-lowering drug treatment. A comparison of utilisation patterns in defined populations in Funen, Denmark and Bologna, Italy. Br J Clin Pharmacol. 2000;49:463–471. doi: 10.1046/j.1365-2125.2000.00192.x. 10.1046/j.1365-2125.2000.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simons LA, Simons J, McManus P, Dudley J. Discontinuation rates are high. Br Med J. 2000;321:1084. letter. [PMC free article] [PubMed] [Google Scholar]
- 10.Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997;44:445–448. [PubMed] [Google Scholar]
- 11.WHO Collaborating Centre for Drug Statistics Methodology. Oslo Norway: 1998. ATC index with DDDs. [Google Scholar]
- 12.Larsen J, Andersen M, Gram L, Kragstrup J. Changes in the utilization of lipid-lowering drugs over a six-year period in a Danish population. Eur J Clin Pharmacol. 1993–98 doi: 10.1007/s002280100307. in press. [DOI] [PubMed] [Google Scholar]
- 13.Sampietro T, Geletta F, Bionda A. Behavior of Lp (a) and apoproteins (A1, B, C2, C3, E) during and after therapy with simvastatin. Cardiovasc Drugs Ther. 1995;9:785–789. doi: 10.1007/BF00879872. [DOI] [PubMed] [Google Scholar]