Abstract
Aims
The pharmacokinetics of omeprazole and its metabolites in healthy subjects were evaluated to determine if a single dose of moclobemide inhibited CYP2C19 activity.
Methods
Sixteen volunteers, of whom eight were extensive metabolizers (EM) and eight were poor metabolizers for CYP2C19, participated in two studies. Venous blood samples were collected for 24 h after oral ingestion of 40 mg omeprazole with or without 300 mg moclobemide coadministration. The pharmacokinetic change of omeprazole, omeprazole sulphone and 5-hydroxyomeprazole concentrations were assessed to test for an interaction between omeprazole and moclobemide.
Results
The coadministration of moclobemide in EMs approximately doubled the mean AUC (from 1834 to 3760 ng ml−1 h) and Cmax (from 987 to 1649 ng ml−1) of omeprazole, and increased the AUC of omeprazole sulphone without changing AUC ratio of omeprazole to omeprazole sulphone. Moclobemide coadministration more than doubled the AUC ratio of omeprazole to 5-hydroxyomeprazole (from 2.5 to 5.3) in EMs, too. There was a significant decrease in Cmax and AUC of 5-hydroxyomeprazole in PMs but no significant changes were seen in the results for omeprazole and omeprazole sulphone AUCs.
Conclusions
A single dose of moclobemide resulted in significant suppression of CYP2C19 activity in EMs. We conclude that physicians prescribing moclobemide should pay attention to its pharmacokinetic interactions even on the first day of coadministration with CYP2C19 substrates.
Keywords: CYP2C19, interaction, moclobemide
Introduction
Significant pharmacokinetic interactions between moclobemide and CYP450 pathways have been documented [1–6]. That moclobemide inhibits CYP2D6 activity was suggested in a report on depressed patients undergoing moclobemide therapy [7] and by an in vitro microsomal study [8]. In a study using several probe drugs as a cocktail [9], 1 week of moclobemide therapy appeared to inhibit CYP2C19 and CYP1A2. Such a wide inhibitory spectrum on CYP450 isozymes may be understood in relation to its various metabolic pathways involving C-oxidation, deamination, N-oxidation, aromatic hydroxylation, et cetera [6]. Recently, we conducted a drug interaction study using healthy volunteers possessing homozygotic extensive metabolizer (EM) and poor metabolizer (PM) genotypes to test the influence of omeprazole on the pharmacokinetics of moclobemide [10].
5-hydroxyomeprazole and omeprazole sulphone, the two major metabolites of omeprazole, are produced by CYP2C19 and CYP3A4, respectively, and the affinity of omeprazole for CYP2C19 is known to be approximately 10 times greater than its affinity for CYP3A4 [11]. In the study above mentioned, we also measured concentrations of omeprazole and 5-hydroxyomeprazole to confirm their CYP2C19 phenotype and observed that the magnitude of omeprazole 5-hydroxylation in the EMs was much lower than expected. To investigate this phenomenon further, we performed another set of pharmacokinetic interaction studies of moclobemide and omeprazole in the same subjects.
Methods
Subjects
Sixteen healthy, nonsmoking or moderately smoking (less than 10 cigarettes/day) volunteers (20–36 years old Koreans within ±15% range of their ideal body weight; 13 men/3 women) were recruited after CYP2C19 genotyping. Eight were homozygotic EM (wt/wt) and the other eight were homozygotic PM (three were m1/m1 and five were m1/m2) genotypes. The genotyping was performed using a PCR based RFLP method [12, 13] to detect m1 (exon 5) and m2 (exon 4) mutations with the restriction enzymes SmaI and BamHI, respectively.
Drug administration and pharmacokinetic study
A randomized crossover design was implemented. Pharmacokinetic studies of omeprazole with and without the coadministration of 300 mg moclobemide were performed over a 2 week interval. The subjects ingested two 20 mg omeprazole capsules (Losec®, Yuhan Co., Seoul, Korea) at 08.00 h after fasting overnight. For those who were taking both moclobemide and omeprazole, moclobemide was administered simultaneously with the omeprazole. Venous blood (10 ml) was drawn into heparinized tubes at 0, 0.33, 0.66, 1, 1.5, 2, 3, 4, 5, 6, 8, 12 and 24 h after administration and the separated plasma was stored frozen (−80° C) until analysis. Two of the eight EMs and one of the eight PMs did not participate in the ‘omeprazole-only’ study due to personal reasons. Pharmacokinetic results of the ‘omeprazole-only’ and the omeprazole and moclobemide coadministration were compared. The study protocol was approved by the ethics committee of Ghil hospital. All of the subjects gave written consent after full explanation of the protocol.
Assay of omeprazole and its metabolites
Concentrations of omeprazole, omeprazole sulphone, and 5-hydroxyomeprazole in plasma were determined at u.v. 302 nm on the basis of column-switching h.p.l.c. with semimicro columns [14]. The detection limits for omeprazole and metabolites were 10 ng ml−1. Interbatch variations (coefficient of variation) of QC samples spanned less than 10% and intrabatch variation less than 2%. Pure compounds of omeprazole, omeprazole sulphone and 5-hydroxyomeprazole were kindly provided by Astra-Zeneca (Sweden).
Pharmacokinetic and statistical analysis
Pharmacokinetic analysis was performed with noncompartmental methods using WinNonlin® Ver. 3.0. A Wilcoxon signed rank test performed with SAS® statistical software Ver. 6.0 was used to estimate the influence of moclobemide coadministration.
Results
Extensive metabolizers
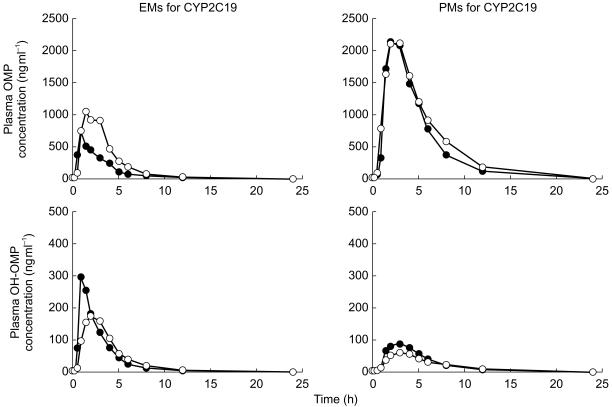
Moclobemide significantly increased (about two fold) the AUC and the Cmax of omeprazole. The AUC of omeprazole sulphone also increased secondarily to the increase of omeprazole AUC, but the AUC ratio of omeprazole to metabolite did not. In addition, the coadministration of moclobemide increased the AUC ratio of omeprazole to 5-hydroxymeprazole to more than double that of ‘omeprazole only’ (Figure 1, Table 1).
Figure 1.
Plasma concentration profiles of omeprazole and 5-hydroxyomeprazole with (○) or without (•) moclobemide. OMP: omeprazole; OH-OMP: 5-hydroxyomeprazole.
Table 1.
Pharmacokinetic parameters of omeprazole and its metabolites with and without moclobemide (mean and 95% confidence interval).
| EM | PM | |||
|---|---|---|---|---|
| Omeprazole only (6 subjects) | +Moclobemide (8 subjects) | Omeprazole only (7 subjects) | +Moclobemide (8 subjects) | |
| Omeprazole | ||||
| tmax (h) | 1.83 (0.43, 3.23) | 2.18 (1.25, 3.11) | 2.14 (1.53, 2.75) | 2.45 (1.59, 3.31) |
| Cmax (ng ml−1) | 986.56 | 1649.31 | 2651.93 | 2609.33 |
| (413.65, 1559.47) | (1053.90, 2244.72)† | (1983.98, 3319.88) | (2136.78, 3081.88) | |
| t½ (h) | 1.58 (0.88, 2.28) | 1.66 (0.61, 2.71) | 2.06 (1.31, 2.81) | 2.70 (2.25, 3.15) |
| AUC(0,24 h) (ng ml−1 h) | 1834.34 | 3759.82 | 10424.84 | 12137.77 |
| (444.21, 3224.47) | (2268.22, 5251.42)† | (8103.49, 12746.19) | (9329.81, 14945.73) | |
| AUC(0,∞) (ng ml−1 h) | 1821.37 | 3774.26 | 10348.26 | 12112.38 |
| (448.94, 3193.8) | (2257.95, 5290.57)† | (7933.80, 12762.72) | (9267.89, 14956.87) | |
| Vd/F (l) | 104.11 (0, 235.24)* | 26.46 (13.52, 39.40)† | 11.63 (7.79, 15.47) | 13.48 (9.88, 17.08) |
| CL/F (l h−1) | 36.63 (6.16, 67.1) | 13.01 (6.90, 19.12)† | 4.02 (3.29, 4.75) | 3.55 (2.63, 4.47) |
| MRT (h) | 2.74 (1.40, 4.08) | 3.32 (2.05, 4.59) | 4.58 (3.56, 5.60) | 5.29 (4.52, 6.06) |
| Omeprazole sulphone | ||||
| Cmax (ng ml−1) | 123.89 (80.04, 167.74) | 247.53 (98.39, 396.67)† | 479.11 (319.68, 638.54) | 549.17 (351.13, 747.21) |
| AUC(0,24 h) (ng ml−1 h) | 798.98 | 2141.60 | 5648.47 | 7501.15 |
| (123.86, 1474.10) | (877.28, 3405.92)† | (4296.86, 7000.08) | (5278.57, 9723.73) | |
| AUC(0,∞) (ng ml−1 h) | 797.03 | 2215.67 | 6449.61 | 9349.84 |
| (102.82, 1491.24) | (1021.32, 3410.02)† | (5172.79, 7726.43) | (6896.11, 11803.57) | |
| AUC ratio (OMP/OMP-S) | 2.45 (1.97, 2.93) | 2.15 (1.50, 2.80) | 1.94 (1.24, 2.64) | 1.77 (1.13, 2.41) |
| 5-Hydroxyomeprazole | ||||
| Cmax (ng ml−1) | 350.34 (187.51, 513.17) | 206.03 (169.18, 242.88)† | 95.73 (56.34, 135.12) | 49.43 (30.88, 67.98)† |
| AUC(0,24 h) (ng ml−1 h) | 711.29 (550.14, 872.44) | 720.79 (576.59, 864.99) | 451.18 (297.02, 605.34) | 365.17 (227.30, 503.04)† |
| AUC(0,∞) (ng ml−1 h) | 711.71 (558.58, 864.84) | 710.23 (570.81, 849.65) | 436.88 (287.83, 585.93) | 356.09 (214.71, 497.47)† |
| AUC ratio (OMP/OH) | 2.50 (0.84, 4.56) | 5.31 (0, 14.76)†* | 25.67 (15.56, 35.78) | 38.02 (21.48, 54.56) |
P value<0.05 by Wilcoxon signed rank test when compared with omeprazole only data in the same genotype group.
lower margin of confidence interval smaller than 0.
OMP: Omeprazole.
OH: 5-Hydroxyomeprazole.
OMP-S: Omeprazole sulphone.
Poor metabolizers
The Cmax and the AUC of 5-hydroxyomeprazole were the only parameters changed (decreased) significantly by moclobemide. The mean AUC ratio of omeprazole to 5-hydroxyomeprazole also increased after moclobemide coadministration, but it was not statistically significant.
Discussion
It is known that moclobemide does not cause irreversible inhibition of CYP450 isozymes [15], unlike some early MAO inhibitors [16–19]. The inhibition of its elimination into the metabolite Ro 12–8095 by 1 week of omeprazole therapy in our previous report confirmed CYP2C19 as its main elimination pathway [10].
Though moclobemide is regarded as a relatively safe agent, its pharmacokinetic interaction with various CYP450 substrate drugs needs to be delineated for efficient and safe pharmacotherapy.
The implications of this study on our understanding of the CYP2C19 genotype
Moclobemide-induced increase of omeprazole sulphone concentrations in EM subjects seems in accord with the assumption of metabolic shunt of omeprazole to CYP3A4 due to the inhibition of CYP2C19. That CYP3A4 activity (represented by the AUC ratio of omeprazole to omeprazole sulphone in the present report) remains fairly constant whether moclobemide was present or not may be seen as supporting evidence that moclobemide does not influence CYP3A4.
In PM subjects, we observed significantly decreased Cmax and AUC of 5-hydroxyomeprazole. Omeprazole is a racemate of R- and S-isomers and the intrinsic clearance by CYP2C19 is known to be about 10 fold higher in R-omeprazole [20]. Tybring and colleagues have shown that 5-hydroxylation is significantly greater for R-omeprazole than for S-omeprazole in PMs [21], thus CYP2C19 activity is also minimally detectable in phenotypic PMs. Taking these reports into consideration, we may interpret the decrease in 5-hydroxyomeprazole AUC in PMs in the current report as indicating that moclobemide has inhibited the hydroxylation of R-omeprazole, the substrate of CYP2C19.
The half-life of omeprazole in EMs did not change in spite of the AUC increase. This may be interpreted to show that the influence of moclobemide is relatively greater on the absorption phase (first pass effect) of omeprazole, rather than on its elimination phase. A comparative trial of i.v. and oral omeprazole coadministered with moclobemide seems necessary to delineate the interaction mechanism.
In conclusion, we confirmed that moclobemide is an inhibitor of CYP2C19 in EM subjects even when administered in a single dose. Though further research is necessary to evaluate the interaction of moclobemide and other CYP2C19 substrates, the current results alert physicians to possible pharmacokinetic interaction even on the first day of moclobemide coadministration.
References
- 1.Fulton B, Benfield P. Moclobemide, an update of its pharmacological properties and therapeutic use. Drugs. 1996;52:450–474. doi: 10.2165/00003495-199652030-00013. [DOI] [PubMed] [Google Scholar]
- 2.Mayersohn M, Guentert TW. Clinical pharmacokinetics of the monoamine oxidase-A inhibitor moclobemide. Clin Pharmacokinet. 1995;29:292–332. doi: 10.2165/00003088-199529050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Gram LF, Guentert TW, Grange S, et al. Moclobemide, a substrate of CYP2C19 and an inhibitor of CYP2C19, CYP2D6, and CYP1A2: a panel study. Clin Pharmacol Ther. 1995;57:670–677. doi: 10.1016/0009-9236(95)90230-9. [DOI] [PubMed] [Google Scholar]
- 4.Tiller JW. Clinical overview on moclobemide. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:703–712. doi: 10.1016/0278-5846(93)90054-v. [DOI] [PubMed] [Google Scholar]
- 5.Haefely W, Burkard WP, Cesura A, et al. Pharmacology of moclobemide. Clin Neuropharmacol. 1993;16(Suppl 2):S8–S18. [PubMed] [Google Scholar]
- 6.Jauch R, Griesser E, Oesterhelt G, et al. Biotransformation of moclobemide in humans. Acta Psychiatr Scand Suppl. 1990;360:87–90. doi: 10.1111/j.1600-0447.1990.tb05344.x. [DOI] [PubMed] [Google Scholar]
- 7.Gram LF, Brosen K. Moclobemide treatment causes a substantial rise in the sparteine metabolic ratio. Br J Clin Pharmacol. 1993;35:649–652. doi: 10.1111/j.1365-2125.1993.tb04196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhadyalla AS, Lennard MS, Tucker GT, et al. Data on File. Basel: F. Hoffmann-La Roche Ltd; 1991. In vitro inhibition of sparteine oxidation by moclobemide. [Google Scholar]
- 9.Gram LF, Guentert TW, Grange S, et al. Moclobemide, a substrate of CYP2C19 and an inhibitor of CYP2C19, CYP2D6, and CYP1A2: a panel study. Clin Pharmacol Ther. 1995;57:670–677. doi: 10.1016/0009-9236(95)90230-9. [DOI] [PubMed] [Google Scholar]
- 10.Yu KS, Yim DS, Cho JY, et al. Effect of omeprazole on the pharmacokinetics of moclobemide according to the genetic polymorphism of CYP2C19. Clin Pharmacol Ther. 2001;69:266–273. doi: 10.1067/mcp.2001.114231. [DOI] [PubMed] [Google Scholar]
- 11.Meyer UA. Metabolic interactions of the proton-pump inhibitors lansoprazole, omeprazole and pantoprazole with other drugs. Eur J Gastroenterol Hematol. 1996;8(Suppl 1):S21–S25. doi: 10.1097/00042737-199610001-00005. [DOI] [PubMed] [Google Scholar]
- 12.de Morais SM, Wilkinson GR, Blaisdell J, et al. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594–598. [PubMed] [Google Scholar]
- 13.de Morais SM, Wilkinson GR, Blaisdell J, et al. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 14.Yim DS, Jeong JE, Park JY. Assay of omeprazole and omeprazole sulfone by semi-microcolumn liquid chromatography with mixed-function precolumn. J Chromatogr B. 2001;754:487–493. doi: 10.1016/s0378-4347(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 15.Callingham BA, Valoti M, Sgaragli P. Biological Psychiatry, Proceedings of the 5th World Congress of Biological Psychiatry; 1991 June 9–14. Vol. 2. Florence Amsterdam: Elsevier Science; 1991. Drug and enzyme interactions with moclobemide; pp. 846–849. [Google Scholar]
- 16.Bélanger PM, Atitse-Gbeassor A. Inhibitory effect of tranylcypromine on hepatic drug metabolism in the rat. Biochem Pharmacol. 1982;31:2679–2683. doi: 10.1016/0006-2952(82)90719-5. [DOI] [PubMed] [Google Scholar]
- 17.Dupont H, Davies DS, Strolin-Benedetti M. Inhibition of cytochrome P-450-dependent oxidation reactions by MAO inhibitors in rat liver microsomes. Biochem Pharmacol. 1987;36:1651–1657. doi: 10.1016/0006-2952(87)90050-5. [DOI] [PubMed] [Google Scholar]
- 18.Jonen HG, Werringloer J, Prough RA, et al. The reaction of phenylhydrazine with microsomal cytochrome P-450. Catalysis of heme modification. J Biol Chem. 1982;257:4404–4411. [PubMed] [Google Scholar]
- 19.Moloney SJ, Snider BJ, Prough RA. The interactions of hydrazine derivatives with rat-hepatic cytochrome P-450. Xenobiotica. 1984;14:803–814. doi: 10.3109/00498258409151479. [DOI] [PubMed] [Google Scholar]
- 20.Abelo A, Andersson TB, Antonsson M, et al. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 2000;28:966–972. [PubMed] [Google Scholar]
- 21.Tybring G, Bottiger Y, Widen J, et al. Enantioselective hydroxylation of omeprazole catalyzed by CYP2C19 in Swedish subjects. Clin Pharmacol Ther. 1997;62:129–137. doi: 10.1016/S0009-9236(97)90060-6. [DOI] [PubMed] [Google Scholar]