Abstract
We previously demonstrated the excellent protective efficacy of DNA priming followed by Gag-expressing Sendai virus (SeV) boosting (DNA prime/SeV-Gag boost vaccine) against a pathogenic simian-human immunodeficiency virus (SHIV89.6PD) infection in macaques. Here we show that we established a practical, safer AIDS vaccine protocol, a single DNA priming followed by a single booster with a recently developed replication-defective F deletion SeV-expressing Gag, and show its protective efficacy against SHIV89.6PD infections.
Virus-specific cellular immune responses play an important role in the control of immunodeficiency virus infections (1, 4, 5, 7, 12, 19, 21, 23). DNA vaccines, recombinant-viral-vector-based vaccines, and their combinations are promising AIDS vaccine methods because of their potential for inducing cellular immune responses. Recently, some of these AIDS vaccines inducing virus-specific cellular immune responses have been reported to prevent AIDS progression in macaque models using pathogenic simian-human immunodeficiency viruses (SHIV) (2, 3, 17, 22, 25).
Members of our laboratory previously reported the potential of a recombinant Sendai virus (SeV) vector for inducing virus-specific cellular immune responses and the excellent protective efficacy of a vaccine system consisting of DNA priming and a Gag-expressing SeV (SeV-Gag) booster in a macaque AIDS model (9, 17). In a preclinical trial, DNA priming-SeV-Gag booster vaccination induced high levels of virus-specific T cells, the viremia in all the vaccinated macaques was controlled, and the animals were protected from AIDS progression after a pathogenic SHIV (SHIV89.6PD) challenge.
SeV is an enveloped virus with a negative-sense RNA genome. It causes fatal pneumonia in mice, its natural host, but is thought to be nonpathogenic in primates, including humans (6, 20). The recombinant SeV vector system has been shown to induce efficient gene transfer in vitro (10, 11). A recent analysis (8) confirmed that SeV infection is nonpathogenic in macaques and that its transmission between them is inefficient. Intranasal SeV inoculation of macaques induces antigen expression localized in the nasal mucosa and its primary lymph nodes (LN), the submandibular LN and the retropharyngeal LN. Furthermore, a safer replication-defective SeV vector lacking the F gene, F(−)SeV, has recently become available (14).
The DNA prime/SeV-Gag boost regimen reported previously consists of a series of four vaccinations with defective proviral DNA and a single intranasal booster with a replication-competent SeV-Gag (17). In this study, we established a practical, safer DNA prime/SeV boost system, a single DNA priming followed by a single booster with a replication-defective F(−)SeV-expressing simian immunodeficiency virus (SIV) Gag protein, F(−)SeV-Gag, for clinical use as an AIDS vaccine.
The DNA priming-boosting regimen, called DNA/F(−)SeV-Gag version 2, used in this study consists of a single DNA vaccination and an intranasal F(−)SeV-Gag booster at week 6 after the priming. The DNA used for the priming (referred to as CMV-SHIVdEN) was constructed from an env and nef deletion SHIV DNA (SIVGP1 DNA) (17, 24) by replacing the 5′ long terminal repeat region with a cytomegalovirus promoter with an immediate-early enhancer and the 3′ long terminal repeat region with simian virus 40 poly(A). At DNA vaccination, the animals received 5 mg of CMV-SHIVdEN DNA intramuscularly. At the booster vaccination, the animals received 6 × 109 cell-infectious units of F(−)SeV-Gag intranasally. All the animal experiments in this study were performed in accordance with the guidelines for laboratory animals of the National Institute of Infectious Diseases.
We measured virus-specific T-cell levels by flow-cytometric analysis of gamma interferon (IFN-γ) induction after specific stimulation as described previously (13, 17). In brief, lymphocytes were cocultured with autologous herpesvirus papio-immortalized B lymphoblastoid cell lines (26) infected with a recombinant vaccinia virus vector (16) expressing SIV Gag for Gag-specific stimulation. Alternatively, lymphocytes were cocultured with B lymphoblastoid cell lines infected with vesicular stomatitis virus G-pseudotyped SIVGP1 for SHIV-specific stimulation. Gag-specific T-cell levels and SHIV-specific T-cell levels were calculated by subtracting IFN-γ-positive T-cell frequencies after nonspecific stimulation from those after Gag-specific stimulation and SHIV-specific stimulation, respectively. Values exceeding 0.05% were considered positive.
In the first experiment, we examined the distribution of the F(−)SeV-Gag vector after the booster. Two cynomolgus macaques (Macaca fascicularis), C97-018 and C94-030, received the DNA prime/F(−)SeV-Gag boost vaccine. Neither of them showed apparent clinical symptoms. Both of them were euthanized 2 weeks after the booster. We extracted RNA from the cells prepared from each tissue taken at autopsy and examined F(−)SeV-Gag distribution by detection of SIV gag after nested reverse transcription-PCR amplification as previously described (8, 24). The vector was detected in the nasal mucosa, the tonsil, the submandibular LN, and the retropharyngeal LN but was undetectable in other lymphoid tissues, including peripheral LN (Table 1). Reverse transcription-PCR with SeV N-specific primers showed similar results (data not shown). These results indicate that F(−)SeV-Gag distribution was restricted to the nasal mucosa and its primary lymphoid tissues.
TABLE 1.
Distribution of SIV gag RNA 2 weeks after a F(−)SeV-Gag boostera
| Macaque | Detection of gag RNA in:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Nasal | Tonsils | SM LN | RP LN | Thymus | MC LN | Ax LN | Ing LN | |
| C97-018 | Positive | Positive | Positive | Positive | Negative | Negative | Negative | Negative |
| C94-030 | ND | ND | Positive | Positive | Negative | Negative | Negative | ND |
“Positive” indicates that the gag sequence was detected in 106 lymphocytes by nested RT-PCR. “Negative” indicates that the gag sequence was undetectable in 106 lymphocytes by nested RT-PCR. ND, not determined; nasal, nasal mucosa; SM LN, submandibular LN; RP LN, retropharyngeal LN; MC LN, meschenchymal LN; Ax LN, axillary LN; Ing LN, inguinal LN.
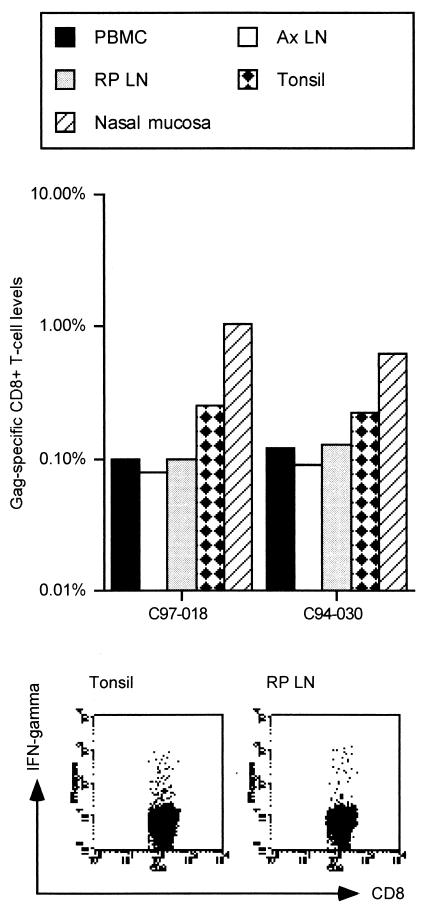
Flow-cytometric analysis of Gag-specific IFN-γ induction in peripheral blood mononuclear cells (PBMC) at the autopsy showed efficient Gag-specific T-cell induction in both of the vaccinated macaques. Then we examined the distribution of Gag-specific CD8+ T cells after the booster. While Gag-specific CD8+ T cells were detected in all the analyzed tissues (the nasal mucosa, the tonsil, the retropharyngeal LN, and the axillary LN), the levels in the nasal mucosa and the tonsil harboring the vector were higher than those in the axillary LN and PBMC (Fig. 1).
FIG. 1.
Gag-specific CD8+-T-cell levels in tissues after an intranasal F(−)SeV-Gag booster. Samples were obtained from two cynomolgus macaques, C97-018 and C94-030, which were euthanized 2 weeks after an intranasal F(−)SeV-Gag booster. Numbers of Gag-specific CD8+ T cells are shown as percentages of the total number of CD8+ T cells are shown as percentages of the total number of CD81 T cells. The lower panels show representative dot plots of IFN-g induction in CD81 T cells after Gag-specific stimulation. Dot plots gated on CD31 CD81 lymphocytes of the tonsil (left) and the retropharyngeal LN (right) in macaque C97-018 are shown. Ax LN, axillary LN; RP LN, retropharyngeal LN.
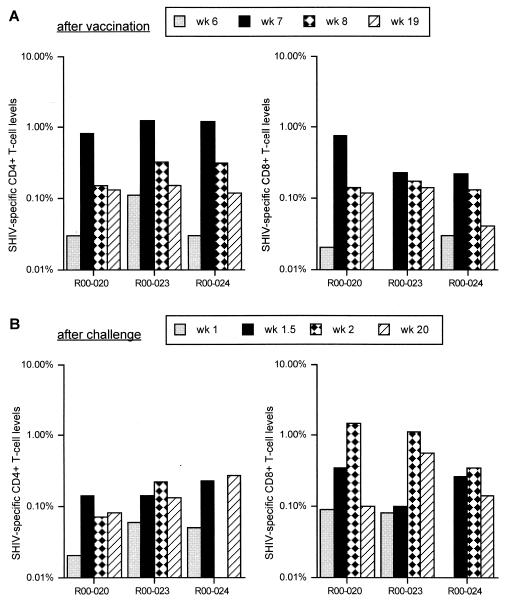
In the second experiment, we evaluated the protective efficacy of the DNA/F(−)SeV-Gag version 2 system in a SHIV model. Three rhesus macaques (Macaca mulatta), R00-020, R00-023, and R00-024, were vaccinated for the challenge experiment. None of them showed apparent clinical symptoms after the vaccination. At week 6, just before the F(−)SeV-Gag booster, SHIV-specific CD8+ T cells were undetectable in all three animals and significant levels of SHIV-specific CD4+ T cells were detected in one animal (R00-023) but not in the others. At week 7, 1 week after the booster, all the macaques showed high levels of SHIV-specific CD4+ and CD8+ T cells, indicating efficient expansion of the number of SHIV-specific T cells as a result of the F(−)SeV-Gag booster (Fig. 2A). The levels peaked at around 1 week after the booster. Macaques R00-020 and R00-023 maintained detectable levels of SHIV-specific CD8+ T cells as well as CD4+ T cells until challenge at week 19, about 3 months after the booster. Macaque R00-024 also maintained SHIV-specific CD4+ T cells until challenge, but its SHIV-specific CD8+-T-cell level declined to a marginal level before challenge.
FIG. 2.
SHIV-specific-T-cell levels in PBMC before (A) and after (B) challenge. For three vaccinated rhesus macaques, numbers of SHIV-specific CD4+ T cells (left panels) and numbers of SHIV-specific CD8+ T cells (right panels) are shown as percentages of the total numbers of CD4+ and CD8+ T cells, respectively. (A) The levels at week 6 [just before the F(−)SeV-Gag booster], week 7 (1 week after the booster), week 8 (2 weeks after the booster), and week 19 (just before SHIV challenge) after the initial DNA priming are shown. (B) The levels at weeks 1, 1.5, 2, and 20 after challenge are shown.
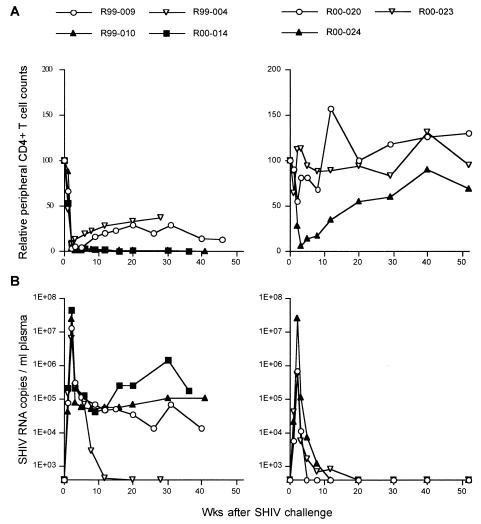
These three macaques were intravenously challenged with 10 50% tissue culture infective doses (TCID50) of SHIV89.6PD (15) 13 weeks after the booster. In the control experiment performed previously (17), all four naive control macaques showed acute depletion of peripheral CD4+ T lymphocytes 2 weeks after the challenge. After the acute phase, the viremia in three of the macaques was not controlled and they developed AIDS and were euthanized within a year. In contrast, all three macaques vaccinated with the DNA/F(−)SeV-Gag version 2 system were protected from acute AIDS progression (Fig. 3). Among them, two macaques, R00-020 and R00-023, were protected from acute CD4+-T-cell depletion. Their peak viral loads in plasma were greatly reduced (geometric mean, 5.9 × 105 copies/ml) compared to those in the controls (geometric mean, 1.7 × 107 copies/ml) and were undetectable at the set point. Acute viremia was not controlled in one macaque (R00-024), and peripheral CD4+ T cells were lost during the acute phase, but viremia was controlled and CD4+ T cells had recovered at the set point.
FIG. 3.
Changes in peripheral CD4+-T-cell counts and plasma viral loads after SHIV89.6PD challenge. The left panels show results for previously described naive control macaques (17), and the right panels show results for macaques vaccinated with the DNA prime/F(−)SeV-Gag boost vaccine. (A) Changes in relative peripheral CD4+-T-cell counts (per microliter). For each animal, the CD4 count at the challenge is considered to be 100, and the values for the CD4 counts relative to that at the challenge are shown. (B) Changes in plasma SHIV RNA copy numbers (numbers of copies per milliliter) quantified as described previously (18, 24).
We then examined SHIV-specific T-cell levels after challenge (Fig. 2B). In macaques R00-020 and R00-023, which showed higher levels of protection, SHIV-specific CD8+ T cells were detectable in PBMC even at week 1 after challenge and more vigorous secondary responses were observed after that. In contrast, SHIV-specific CD8+ T cells were undetectable at week 1 in macaque R00-024, which showed a lower level of protection, although vigorous secondary responses were observed after that. These results are compatible with those of a previous study, indicating that rapid secondary responses of virus-specific CD8+ T cells are important for controlling acute viremia (17).
Levels of SHIV-specific CD4+ T cells were augmented in all the vaccinated macaques at week 1.5 after challenge (Fig. 2B). Both of the macaques in which acute viremia was controlled maintained these levels after that. In macaque R00-024, SHIV-specific CD4+ T cells became undetectable at week 2, but the levels recovered after that. Thus, control of SHIV infections by the DNA/F(-)SeV-Gag vaccine led to maintenance of virus-specific CD4+ T cells at the set point.
Recently, several kinds of DNA prime/viral vector boost vaccines have been shown to induce protective efficacy against pathogenic SHIV infections (2, 17, 25). In those studies, DNA vaccinations were performed more than once. In the present study, we evaluated the efficacy of a DNA prime/SeV boost system with minimum numbers of vaccinations in an SHIV model.
Furthermore, this study is the first preclinical trial of a replication-defective F(−)SeV vector as a vaccine tool. In macaques, the vector distribution was localized in the nasal mucosa, its primary LN, and the tonsil after an intranasal F(−)SeV-Gag booster. The distribution was similar to that with replication-competent SeV-Gag. Gag-specific CD8+ T cells were detected predominantly in the nasal mucosa and the tonsil, suggesting that the vector-derived antigen presentation was predominant in these tissues. In addition to the local induction, the intranasal F(−)SeV-Gag injection induced Gag-specific CD8+ T cells to be produced systemically. These results indicate that the F(−)SeV vector is a promising vaccine tool for inducing virus-specific cellular immune responses.
In a previous study (17), members of our laboratory showed that the production of SHIV-specific T cells was efficiently induced in all four macaques vaccinated with the DNA/SeV-Gag version 1 system consisting of a series of four DNA vaccinations and a replication-competent SeV-Gag booster. All of them maintained SHIV-specific CD8+ T cells for more than 3 months until challenge, and acute viremia was controlled without acute CD4+-T-cell depletion after SHIV89.6PD challenge. Two of three macaques vaccinated with the DNA/F(−)SeV-Gag version 2 system in this study showed similar levels of protection; acute viremia was controlled without acute CD4+-T-cell depletion, and they were protected from AIDS progression. On the other hand, in the remaining macaque (R00-024) with a marginal level of SHIV-specific CD8+ T cells at challenge, acute viremia was uncontrolled and there was acute CD4+-T-cell depletion. However, even in macaque R00-024, SHIV89.6PD infections were controlled at the set point, CD4+ T cells recovered, and the animal was protected from AIDS progression. While two levels of protection were observed, this study indicates that even the DNA/F(−)SeV-Gag version 2 AIDS vaccine system with a minimum number of vaccinations can induce protective immune responses in a macaque AIDS model.
Acknowledgments
This work was supported by Health Sciences Research Grants from the Ministry of Health, Labour and Welfare, by grants from the Human Sciences Foundation, and by a grant from the Ministry of Education and Science in Japan.
We thank M. A. Martin for providing SHIVMD14YE DNA; Y. Lu for providing SHIV89.6PD; Y. Ami, F. Ono, K. Komatsuzaki, K. Oto, H. Ogawa, K. Mori, R. Mukai, A. Yamada, and K. Terao for assistance in the animal experiments; and A. Kato, A. Kojima, T. Sata, T. Takemori, N. Yamamoto, T. Kurata, and A. Nomoto for their help.
REFERENCES
- 1.Altfeld, M., and E. S. Rosenberg. 2000. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 12:375-380. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS in rhesus macaques by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M.-E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implication for vaccine development. Curr. Opin. Immunol. 11:451-459. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz, J. L., K. F. Soike, M. Y. Sangster, A. Portner, R. E. Sealy, D. H. Dawson, and C. Coleclough. 1997. Intranasal Sendai virus vaccine protects African green monkeys from infection with human parainfluenza virus-type one. Vaccine 15:533-540. [DOI] [PubMed] [Google Scholar]
- 7.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kano, M., T. Matano, A. Kato, H. Nakamura, A. Takeda, Y. Suzaki, Y. Ami, K. Terao, and Y. Nagai. 2002. Primary replication of a recombinant Sendai viral vector in macaques. J. Gen. Virol. 83:1377-1386. [DOI] [PubMed] [Google Scholar]
- 9.Kano, M., T. Matano, H. Nakamura, A. Takeda, A. Kato, K. Ariyoshi, K. Mori, T. Sata, and Y. Nagai. 2000. Elicitation of protective immunity against simian immunodeficiency virus infection by a recombinant Sendai virus expressing the Gag protein. AIDS 14:1281-1282. [DOI] [PubMed] [Google Scholar]
- 10.Kato, A., K. Kiyotani, Y. Sakai, T. Yoshida, and Y. Nagai. 1997. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 16:578-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato, A., Y. Sakai, T. Shioda, T. Kondo, M. Nakanishi, and Y. Nagai. 1996. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells 1:569-579. [DOI] [PubMed] [Google Scholar]
- 12.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavini, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, H.-O., Y. F. Zhu, M. Asakawa, H. Kuma, T. Hirata, Y. Ueda, Y.-S. Lee, M. Fukumura, A. Iida, A. Kato, A. Y. Nagai, and M. Hasegawa. 2000. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 74:6564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu, Y., C. D. Pauza, X. Lu, D. C. Montefiori, and C. J. Miller. 1998. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:6-18. [DOI] [PubMed] [Google Scholar]
- 16.Mackett, M., G. L. Smith, and B. Moss. 1982. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc. Natl. Acad. Sci. USA 79:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matano, T., M. Kano, H. Nakamura, A. Takeda, and Y. Nagai. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA-prime/Sendai virus vector boost regimen. J. Virol. 75:11891-11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matano, T., M. Kano, T. Odawara, H. Nakamura, A. Takeda, K. Mori, T. Sata, and Y. Nagai. 2000. Induction of protective immunity against pathogenic simian immunodeficiency virus by a foreign receptor-dependent replication of an engineered avirulent virus. Vaccine 18:3310-3318. [DOI] [PubMed] [Google Scholar]
- 19.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai, Y. 1999. Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev. Med. Virol. 9:83-99. [DOI] [PubMed] [Google Scholar]
- 21.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 22.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A., Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 24.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176: 362-373. [DOI] [PubMed] [Google Scholar]
- 25.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 26.Voss, G., S. Nick, C. Stahl-Hennig, K. Ritter, and G. Hunsmann. 1992. Generation of macaque B lymphoblastoid cell lines with simian Epstein-Barr-like viruses: transformation procedure, characterization of the cell lines and occurrence of simian foamy virus. J. Virol. Methods 39:185-195. [DOI] [PubMed] [Google Scholar]