Abstract
Aims
To investigate the role of basal nitric oxide (NO) production in regulating large artery stiffness in vivo.
Methods
Incremental doses of the NO synthase inhibitor L-NG-monomethyl arginine (LNMMA: 0.1, 0.3, 1.0 and 3.0 mg kg−1 min−1) or placebo were infused in eight healthy men. Arterial stiffness was assessed noninvasively by pulse wave analysis.
Results
Compared with placebo, infusion of LNMMA led to a dose-dependent increase in mean arterial pressure, peripheral vascular resistance, and aortic and systemic arterial stiffness. There was an accompanying reduction in heart rate and cardiac index. The highest dose of LNMMA resulted in an increase of 25% in AIx (95% confidence limits; 12, 38) and of 16 mmHg in mean arterial pressure (9, 23) compared with infusion of saline.
Conclusions
These data indicate functional regulation of large artery stiffness in vivo by NO, and may provide new therapeutic strategies for cardiovascular risk reduction.
Keywords: arterial stiffness, LNMMA, nitric oxide, pulse wave analysis
Introduction
Large artery stiffness is an important determinant of cardiovascular risk [1]. Structural components within the arterial wall, mainly collagen and elastin, together with transmural pressure, are key determinants of large vessel stiffness [2,3]. However, stiffness also depends on smooth muscle tone [4], suggesting a degree of functional regulation of arterial stiffness by local and circulating vasoactive substances. Although endothelium-derived nitric oxide (NO) contributes to resting tone in resistance vessels, whether it also regulates large artery stiffness is unclear. Indeed, data concerning the effect of intra-arterial infusion of the NO synthase inhibitor L-NG-monomethyl arginine (LNMMA) on local arterial stiffness are conflicting [5,6].
We hypothesized that inhibition of basal NO synthesis would increase arterial stiffness in vivo. Indeed, McVeigh et al.[7] have recently reported that systemic infusion of L-NG nitro-arginine methyl ester (LNAME) reduces small artery compliance in healthy male volunteers. However, they were unable to detect any change in large artery compliance, despite an increase in mean arterial pressure. The aim of the present study was to assess the effect of inhibition of local endogenous NO synthesis on aortic and systemic arterial stiffness noninvasively using the technique of pulse wave analysis.
Methods
Eight healthy male subjects, mean age 30 years (range 21–42), were studied on two occasions separated by at least 1 week in a double-blind, randomized protocol. The study was approved by the Local Research Ethics Committee, and written informed consent was obtained from each participant. After 30 min supine rest, baseline haemodynamic recordings were made and LNMMA (Clinalfa; Laufelfingen; Switzerland) at 0.1, 0.3, 1.0 and 3.0 mg kg−1 min−1, or matching placebo (0.9% saline) were given intravenously. Each dose was infused for 15 min and haemodynamic measurements were made over the last 5 min of this period. Blood pressure was determined with an oscillometric sphygmomanometer (HEM-705CP, Omron Corporation, Japan) and cardiac index by transthoracic bioimpedance (BoMed; NCCOM3-R7; Irvine, USA). Radial artery waveforms were recorded from the wrist by applanation tonometry and corresponding central artery waveforms were generated and analysed using the technique of pulse wave analysis (SCOR; Atlor Medical, Sydney, Australia), as described in detail previously [8]. In brief, high fidelity peripheral pressure waveforms were recorded by applanation tonometry and a validated transfer function [9–12] was then used to generate corresponding ascending aortic waveforms from which augmentation index (AIx) and the timing of the reflected waveform (TR) were calculated. Although the transfer function has not been assessed in the context of NO synthesis inhibition, it has been validated for dynamic changes in blood pressure [10–12]. Augmentation index is a measure of the contribution of wave reflection to the aortic waveform and depends on the pulse wave velocity, and magnitude and site of the reflected pressure wave, and thus provides a composite measure of large artery (systemic) stiffness [13]. In contrast, the timing of the reflected waveform provides an estimate of aortic pulse wave velocity [14,15], and thus aortic stiffness.
All measurements were made in duplicate, and data were analysed as changes from values at baseline using analysis of variance (ANOVA). Significance was accepted at P< 0.05.
Results
Baseline haemodynamics did not differ significantly between the two visits and there was no significant change in any parameter during infusion of placebo. Compared with saline, infusion of LNMMA resulted in a dose-dependent increase in AIx (Figure 1), mean arterial pressure and peripheral vascular resistance, and a corresponding reduction in heart rate, cardiac index and TR (Table 1). Whereas central pulse pressure increased following LNMMA, there was no change in peripheral pulse pressure.
Figure 1.
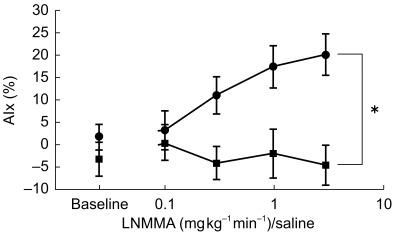
Effect of LNMMA on augmentation index. Effect of incremental infusion of LNMMA (•) and saline (▪) on augmentation index (AIx). Values represent means±s.e. mean; *P < 0.001 (ANOVA); n=8.
Table 1.
Haemodynamic response to infusion of saline and incremental doses of LNMMA.
| Saline | LNMMA (mg kg−1 min−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | – | – | – | – | Baseline | 0.1 | 0.3 | 1.0 | 3.0 | Significance | |
| Mean arterial pressure (mmHg) | 86 ± 2 | 86 ± 2 | 87 ± 1 | 86 ± 2 | 88 ± 2 | 86 ± 2 | 91 ± 2 | 93 ± 2 | 97 ± 2 | 105 ± 3 | < 0.001 |
| Peripheral pulse pressure (mmHg) | 47 ± 2 | 45 ± 2 | 46 ± 2 | 47 ± 1 | 47 ± 2 | 48 ± 2 | 48 ± 2 | 44 ± 2 | 45 ± 2 | 45 ± 2 | 0.3 |
| Central pulse pressure (mmHg) | 29 ± 2 | 28 ± 1 | 28 ± 1 | 29 ± 1 | 29 ± 1 | 30 ± 1 | 30 ± 2 | 31 ± 1 | 34 ± 2 | 36 ± 3 | 0.04 |
| Heart rate (beats min−1) | 58 ± 2 | 60 ± 2 | 59 ± 2 | 59 ± 2 | 62 ± 3 | 60 ± 2 | 55 ± 1 | 52 ± 2 | 49 ± 2 | 45 ± 2 | < 0.001 |
| Peripheral vascular resistance (AU) | 31 ± 2 | 30 ± 2 | 31 ± 2 | 30 ± 2 | 32 ± 2 | 29 ± 2 | 35 ± 2 | 39 ± 2 | 46 ± 3 | 57 ± 4 | < 0.001 |
| Cardiac index (l min−1 m−2) | 2.9 ± 0.2 | 3.0 ± 0.2 | 2.8 ± 0.2 | 3.0 ± 0.2 | 2.8 ± 0.2 | 3.0 ± 0.1 | 2.7 ± 0.1 | 2.4 ± 0.1 | 2.2 ± 0.1 | 1.9 ± 0.1 | < 0.001 |
| TR (ms) | 160 ± 5 | 160 ± 5 | 160 ± 5 | 162 ± 8 | 160 ± 5 | 168 ± 6 | 159 ± 5 | 162 ± 3 | 157 ± 5 | 154 ± 5 | < 0.001 |
Values represent means±s.e. mean; AU=arbitrary units, and significance was determined using ANOVA compared with the placebo phase, n=8. There were no significant differences in baseline values or changes during infusion of saline.
Discussion
The shape of the aortic pressure waveform depends on the pattern of left ventricular ejection, aortic stiffness and also on wave reflection within the arterial tree. AIx is a quantitative measure of the contribution of the reflected pressure wave to the central pressure waveform and depends on the amplitude and velocity of the reflected wave, both of which are influenced by arterial stiffness. Therefore, AIx provides a noninvasive measure of systemic arterial stiffness. Similarly, TR provides an estimate of the aortic pulse wave velocity and thus aortic stiffness.
As hypothesized, intravenous infusion of LNMMA significantly increased AIx and TR, indicating aortic and systemic arterial stiffening. LNMMA is a specific substrate-analogue inhibitor of NO synthase and intra-arterial infusion into the forearm vascular bed reduces forearm blood flow by ∼40%, indicating that there is basal NO production in resistance vessels [16]. Moreover, systemic administration of LNMMA increases mean arterial pressure and reduces heart rate [17]. However, previous data concerning the effect of inhibition of NO synthesis in vivo on local arterial stiffness are conflicting. Indeed, both unchanged [6] and reduced [5] radial artery stiffness has been described following intrabrachial administration of LNMMA. Moreover, although McVeigh et al.[7] demonstrated increased small artery stiffness following systemic infusion of LNAME, they were unable to detect any alteration in large artery stiffness, which is perhaps surprising since there was an accompanying increase in mean arterial pressure, a key determinant of large artery stiffness.
In the present study, the increase in AIx was accompanied by a fall in heart rate and rise in mean arterial pressure. Although changes in heart rate per se do not influence arterial stiffness we have previously shown that AIx, but not TR, is inversely related to heart rate, due to an alteration in the relative arrival-time of the reflected waveform in the ascending aorta [8]. However, our previous data [8] indicate that the observed change in heart rate only accounts for < 25% of the increase in AIx in the present study. In contrast, distending pressure is a key determinant of arterial stiffness and therefore the accompanying increase in mean arterial pressure may, in part, account for the rise in systemic and aortic stiffness. Indeed, we have previously shown that systemic infusion of angiotensin II or noradrenaline results in an increase in mean arterial pressure, AIx and TR[18]. Moreover, for a given increase in mean pressure, LNMMA produced a similar increment in AIx and TR to that observed with angiotensin II and noradrenaline. However, this does not necessarily mean that all the effect of LNMMA is indirect, i.e. passive stretching of vessels in response to a rise in distending pressure, since angiotensin II and noradrenaline may also alter wave reflection and large artery stiffness independently of mean arterial pressure through direct smooth muscle constriction.
Pulse pressure is often regarded as a surrogate measure of large artery stiffness. However, we have previously shown that changes in peripheral pulse pressure do not always predict accompanying changes in central pulse pressure or arterial stiffness [18]. Indeed, in the present study, despite an increase in central pulse pressure and stiffness in response to LNMMA, there was no change in peripheral pulse pressure, which is likely to be due to the observed decline in cardiac index. Therefore, peripheral pulse pressure should not always be considered a reliable indicator of systemic arterial stiffness, especially when investigating the effects of vasoactive drugs.
In conclusion, the present results demonstrate that basal NO synthesis modulates large artery stiffness in vivo Therefore, it may be possible to reduce the arterial stiffening associated with ageing and other cardiovascular risk factors, such as diabetes mellitus, either by improving endothelial function or by developing drugs that act selectively on the smooth muscle of large arteries.
References
- 1.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 2.Avolio AP, Chen S-G, Wang R-P, Zahang C-L, Li M-F, O'Rourke MF. Effects of ageing on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 3.Avolio A, Jones D, Tafazzoli-Shadpour M. Quantification of alterations in structure and function of elastin in the arterial media. Hypertension. 1998;32:170–175. doi: 10.1161/01.hyp.32.1.170. [DOI] [PubMed] [Google Scholar]
- 4.Gow BS. The influence of vascular smooth muscle on the viscoelastic properties of blood vessels. In: Bergel DH, editor. Cardiovascular Fluid Dynamics. London: Academic Press; 1972. pp. 66–97. [Google Scholar]
- 5.Joannides R, Richard V, Haefeli WE, et al. Role of nitric oxide in the regulation of the mechanical properties of peripheral conduit arteries in humans. Hypertension. 1997;30:1465–1470. doi: 10.1161/01.hyp.30.6.1465. [DOI] [PubMed] [Google Scholar]
- 6.Leeson CMP, Whincup PH, Cook DG, et al. Cholesterol and arterial distensibility in the first decade of life: a population-based study. Circulation. 2000;101:1533–1538. doi: 10.1161/01.cir.101.13.1533. [DOI] [PubMed] [Google Scholar]
- 7.McVeigh GE, Allen PB, Morgan DR, Hanratty CG, Silke B. Nitric oxide modulation of blood vessel tone identified by arterial waveform analysis. Clin Sci. 2001;100:387–393. [PubMed] [Google Scholar]
- 8.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 10.Takazawa K, O'Rourke M, Fujita M. Estimation of ascending aortic pressure from radial arterial pressure using a generalized transfer function. Z Kardiol. 1996;85:137–139. [PubMed] [Google Scholar]
- 11.Soderstrom S, Nyberg G, Ponten J, Sellgren J, O'Rourke M. Substantial equivalence between ascending aortic pressure waveforms and waveforms derived from the radial pulse using a generalized transfer function? FASEB J. 1998;A712:4131. [Google Scholar]
- 12.Segers P, Qasem A, DeBacker T, Carlier S, Verdonck P, Avolio A. Peripheral ‘oscillatory’ compliance is associated with aortic augmentation index. Hypertension. 2001;37:1434–1439. doi: 10.1161/01.hyp.37.6.1434. [DOI] [PubMed] [Google Scholar]
- 13.Safar ME, London GM. Therapeutic studies and arterial stiffness in hypertension: recommendations of the European Society of Hypertension. The Clinical Committee of Arterial Structure and Function. Working Group on Vascular Structure and Function of the European Society of Hypertension. J Hypertens. 2000;18:1527–1535. doi: 10.1097/00004872-200018110-00001. [DOI] [PubMed] [Google Scholar]
- 14.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure waveforms. Circulation. 1980;62:105–116. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 15.Marchais SJ, Guerin AP, Pannier BM, Levy BI, Safar M, London GM. Wave reflections and cardiac hypertrophy in chronic uremia. Hypertension. 1993;22:876–883. doi: 10.1161/01.hyp.22.6.876. [DOI] [PubMed] [Google Scholar]
- 16.Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;ii:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- 17.Haynes WG, Noon JP, Walker BR, Webb DJ. L-NMMA increases blood pressure in man. Lancet. 1993;342:931–932. doi: 10.1016/0140-6736(93)91981-q. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson IB, MacCallum H, Hupperetz PC, Van Thoor CJ, Cockcroft JR, Webb DJ. Changes in the derived central pressure waveform and pulse pressure in response to angiotensin II and noradrenaline in man. J Physiol. 2001;530:541–550. doi: 10.1111/j.1469-7793.2001.0541k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


