Abstract
Aims
To determine the pharmacokinetic profile of norelgestromin (NGMN) and ethinyloestradiol (EE) following application of the contraceptive patch, Evra™/ Ortho Evra™, at each of four anatomic sites (abdomen, buttock, arm, and torso).
Methods
Thirty-seven healthy, nonpregnant women aged 20–45 years participated in this open-label, four-period crossover study. Subjects were randomized to one of four treatment (site of application) sequences. Each patch was worn for 7 days, with a 1 month washout between treatments. Blood samples were collected before and at various times up to 240 h after application of each patch. Serum samples were assayed for NGMN and EE by validated methods.
Results
The serum concentration reference ranges for NGMN and EE are 0.6–1.2 ng ml−1 and 25–75 pg ml−1, respectively, based on studies of the mean Cave of oral norgestimate 250 µg and EE 35 µg. For all application sites, mean concentrations of NGMN and EE remained within these ranges during the 7 day wear period. Absorption of NGMN and EE during patch application on the buttock, arm, and torso was equivalent. Absorption of NGMN and EE during patch application on the abdomen was approximately 20% less than observed for the other three sites, although mean serum concentrations were still within reference ranges. A previous study demonstrated therapeutic equivalence of patches worn on the abdomen vs other sites.
Conclusions
Serum concentrations of NGMN and EE from the contraceptive patch remain within the reference ranges throughout the 7 day wear period, regardless of the site of application (abdomen, buttock, arm, or torso).
Keywords: contraception, ethinyloestradiol, Evra™, norelgestromin, Ortho Evra™, pharmacokinetics
Introduction
Failure rates during typical use of reversible contraceptive methods are high [1,2]. Combination oral contraceptives, the most common method of birth control, have a typical use failure rate of 5% [3] to as high as 8.5% [1]. Half of all pregnancies in the United States in 1994 were unintended, and of these, nearly 50% occurred in women who were using a contraceptive method during the month they became pregnant [4]. New and innovative methods of birth control that provide advantages in terms of simplicity of use, user compliance, efficacy, and/or safety are clearly needed.
A contraceptive patch containing the progestin norelgestromin (NGMN) and the oestrogen ethinyloestradiol (EE) has recently been developed. NGMN (previously known as 17-deacetylnorgestimate) is the primary active metabolite of norgestimate [5], the progestin used in several combination oral contraceptives [6]. The patch is applied once weekly for 3 consecutive weeks (21 days) followed by 1 patch-free week per cycle. Thus, with this simple dosing schedule users always remove or apply patches on the same day of the week. The patch is not affected by gastrointestinal disturbances, provides steady drug concentrations, is user controlled, and can be easily reversed. The primary objective of the study reported here was to determine the pharmacokinetic profile of NGMN and EE following successive application of the patch at each of four anatomic sites (abdomen, buttock, arm, and torso).
An abstract was presented at 2000 Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, March 14–18, Los Angeles, California [7].
Methods
Study population
A total of 37 healthy women were enrolled in the study. Subjects had a mean age of 31.0 ± 7.2 years (range: 20–45 years), a mean weight of 65.0 ± 7.8 kg, and a mean height of 166.6 ± 5.3 cm. Most subjects (89%) were white. Subjects had to be nonsmoking; nonpregnant; nonlactating; have regular menstrual cycles; have no evidence of cervical dysplasia; have a haematocrit ≥36%; have body weight ≥50 kg and within 35% of the ideal weight based on frame size and height; have seated systolic/diastolic blood pressure ≤140/90 mmHg; agree not to use any other topical or oral medication (prescription or nonprescription) or alcohol within 3 days of the first patch application through the 240 h blood draw of each treatment period; agree not to apply oils, creams, or cosmetics on or around the area of patch placement; and provide written informed consent. Exclusion criteria included any disorders that were contraindications to steroid hormonal therapy; history or presence of dermal hypersensitivity in response to topical application; receipt of steroid hormonal therapy or hepatic enzyme-inducing drugs within 30 days of admission; use of Norplant® or an experimental drug or device within 60 days of admission; use of a steroid hormone-containing intrauterine device within 3 months of admission; receipt of Depo-Provera® within 6 months of admission; and alcohol or substance abuse within 12 months of admission. All subjects were to have either had a tubal ligation or agreed to use condoms and foam, a diaphragm, or a cervical cap throughout the study.
Study design
This was an open-label, four-period, randomized, crossover study conducted at one centre (Corning Besselaar Clinical Research Units, Inc., Madison, Wisconsin, US). The study protocol was approved by the Corning Besselaar Institutional Review Board. After subjects gave informed consent, they were randomized to one of four treatment (site of application) sequences: lower abdomen, buttock, upper arm, then upper torso (excluding breast tissue); buttock, upper torso, lower abdomen, then upper arm; upper arm, lower abdomen, upper torso, then buttock; or upper torso, upper arm, buttock, then lower abdomen. Each patch (Evra™/Ortho Evra™; The R.W. Johnson Pharmaceutical Research Institute, Raritan, New Jersey, US) was applied according to the treatment sequence by study site personnel and worn for 7 days. Normal daily activities, such as bathing, swimming, and exercising, were not restricted. Patches were removed by study site personnel following the 168 h blood draw. Treatment periods were separated by a 1 month washout. Subjects were admitted to the study unit the evening prior to dosing on day 1 through the morning of day 2, and from the morning of day 8 through the morning of day 11 of each treatment period. Subjects visited the study unit on days 3 and 4 of each treatment period.
Description of the patch
The patch is 20 cm2 and delivers NGMN 150 µg and EE 20 µg daily to the systemic circulation [8]. The patch is thin and consists of three layers: an outer protective layer; a medicated, adhesive middle layer; and a clear release liner that is removed prior to patch application.
Pharmacokinetics
Serial blood samples were collected immediately before and at 3, 6, 12, 24, 48, 72, 168, 171, 174, 180, 192, 204, 216, and 240 h after application of each patch.
Serum samples were analysed for NGMN at Primedica (Worcester, Massachusetts) using a validated high-performance liquid chromatographic/tandem mass spectrometric (LC/MS/MS) method that was similar to a previously described method [9]. The assay procedure differed from that of the published method in that the chromatographic elution of extracted serum was accomplished via isocratic rather than gradient mode. The mobile phase consisted of water and acetonitrile (38 : 62, v/v) at a flow rate of 1.0 ml min−1. A Perkin Elmer Sciex API-III Plus (Toronto, Ontario) triple quadrupole mass spectrometer was operated in the positive atmospheric pressure chemical ionization (APCI) mode with the use of a heated nebulizer interface. The mass spectrometer was operated in the multiple reaction monitoring (MRM) mode. Argon was utilized as the collision gas to detect the diagnostic precursor to product ion transition: m/z 328 (M−H)+ → 124 (NGMN; the protonated pseudo-molecular ion of m/z 328 derived from the loss of the 17β-acetyl group from parent compound). Serum concentrations were determined using the slope and intercept of the standard curve obtained from a linear least squares regression (weighted as 1/concentration2) for the analyte/internal standard peak area ratio vs calibration standard concentration. The method described in this communication demonstrated a lower limit of quantification of 0.1 ng ml−1 and was linear over a concentration range from 0.1 to 5.0 ng ml−1 for NGMN. During validation, the extraction recovery of NGMN from serum was at least 75%.
Serum concentrations of EE were determined at PPD Development (Richmond, Virginia) using a validated gas chromatographic (GC)/MS method [10]. Briefly, following a sample preparation procedure entailing liquid–liquid extraction with an organic solvent mixture, chemical derivatization, and subsequent solid-phase cleanup steps, the samples (1.0 ml) were analysed using GC separation coupled with single quadrupole MS detection. The mass spectrometer was operated in the negative ion mode. The assay was validated over a concentration range from 2.0 to 1000 pg ml−1. The overall recovery of EE from serum was ∼50%.
For both NGMN and EE, precision and accuracy were determined from the analysis of quality control samples prepared in serum at four concentrations spanning the calibration range. Inter-assay precision values (%CV) ranged from 3% to 11%, while intra-assay precision values ranged from 2% to 12%. Inter-and intra-assay accuracy values were consistently within ±10% of target.
Peak concentrations (Cmax) for each treatment period and the time to reach Cmax (tmax) were determined by inspection. Steady-state serum concentrations (Css) were calculated as the average of the concentrations at 48, 72, and 168 h after patch application. The area under the serum concentration vs time (Csvs t) curves from 0 to 168 h (AUC(0, 168 h)) and from 0 to 240 h (AUC(0, 240 h)) were calculated by linear trapezoidal summation method. Terminal half-life (t½) was calculated by linear regression of the terminal elimination phase of the Csvs t curve. Median values were calculated for tmax, and mean values were calculated for other pharmacokinetic parameters.
Safety evaluation
Adverse events, both those reported by subjects and those observed by the investigators, were collected throughout the study. Clinical laboratory tests (haematology, serum chemistry, and urinalysis) and physical and gynecological examinations were performed prestudy and on day 11 of treatment period 4 (or at early withdrawal). Vital signs (blood pressure and pulse rate) were determined prestudy and at various times throughout the study.
Statistical methods
Serum concentration data for NGMN and EE were summarized with the mean, s.d., and coefficient of variation at each time point. Analysis of variance models were fit to the log-transformed (natural logarithm) AUC(0, 240 h) and Css. Ninety percent confidence intervals were constructed for the ratio of mean bioavailability parameters from each pair of application sites.
Reference ranges
The reference concentration ranges for NGMN and EE were calculated from the average serum concentrations (Cave) in 90% of the individual subjects (i.e. excluding the upper and lower 5%) taking oral norgestimate 250 µg plus EE 35 µg in a previously performed pharmacokinetic study (data on file, RWJPRI). These were computed from AUC(0, τ)/τ values for these compounds at steady-state over a 24 h interval following administration of the norgestimate/EE oral contraceptives. The reference ranges are 0.6–1.2 ng ml−1 and 25–75 pg ml−1, respectively. These are not necessarily the upper and lower limits of the therapeutic concentration ranges for the patch, but were established as a developmental tool to identify efficacious concentrations of NGMN and EE. Css following transdermal administration and Cave following oral administration are pharmacokinetically distinct parameters which would not be directly compared. However, such a comparison is valid as a developmental tool.
Results
Disposition
Thirty-one of 37 subjects completed the study (all procedures); 5 subjects discontinued due to personal choice and 1 subject discontinued due to adverse events (see Safety section). One of the subjects who discontinued due to personal choice did so on day 1 of treatment period 1 and was replaced by a new subject. The other 5 subjects who discontinued were not replaced. No patch completely fell off during the study.
Pharmacokinetics
Mean serum concentrations of NGMN and EE remained within their reference range during the entire 7 day wear period, regardless of the site of application (Figures 1 and 2, respectively). Mean pharmacokinetic parameters are shown in Tables 1 and 2 for NGMN and EE, respectively.
Figure 1.
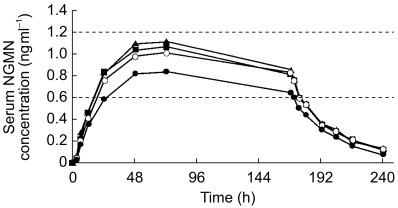
Mean serum concentration vs time profile of norelgestromin (NGMN) following successive applications of the contraceptive patch for 7 days at each of the four anatomical sites (• abdomen; ▴ arm; ▪ buttock; ○ torso). Dashed horizontal lines indicate reference range.
Figure 2.
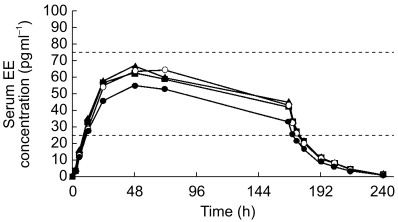
Mean serum concentration vs time profile of ethinyl oestradiol (EE) following successive applications of the contraceptive patch for 7 days at each of the four anatomical sites (• abdomen; ▴ arm; ▪ buttock; ○ torso). Dashed horizontal lines indicate reference range.
Table 1.
Pharmacokinetic parameters for norelgestromin.
| Parameter | Abdomen | Arm | Buttock | Torso |
|---|---|---|---|---|
| Cmax, (ng ml−1)* | 0.88 (0.28) | 1.18 (0.35) | 1.17 (0.50) | 1.07 (0.38) |
| Median tmax (interquartile range) (h)* | 72 (48–72) | 72 (48–72) | 72 (48–72) | 72 (48–72) |
| Css(48, 168 h) (ng ml−1)* | 0.78 (0.25) | 1.02 (0.27) | 0.99 (0.38) | 0.95 (0.32) |
| AUC(0, 168 h) (ng ml−1 h)* | 117 (37.6) | 155 (44.3) | 150 (57.9) | 143 (50.4) |
| AUC(0, 240 h) ng ml−1 h)* | 136 (43.6) | 177 (46.4) | 174 (64.6) | 166 (56.7) |
| t½, (h)† | 27.6 (10.4) | 26.1 (7.0) | 28.0 (6.9) | 30.1 (21.4) |
Values presented are means (s.d.) unless otherwise stated.
n = 33, 32, 36, and 35 for abdomen, arm, buttock, and torso, respectively.
n=32, 31, 35, and 33 for abdomen, arm, buttock, and torso, respectively.
Table 2.
Pharmacokinetic parameters for ethinyloestradiol.
| Parameter | Abdomen | Arm | Buttock | Torso |
|---|---|---|---|---|
| Cmax (pg ml−1)* | 58.7 (19.9) | 69.5 (20.6) | 66.3 (23.9) | 71.2 (32.2) |
| Median tmax (interquartile range) (h)* | 48 (48–72) | 48 (48–72) | 48 (36–48) | 48 (48–72) |
| Css(48, 168 h) (pg ml−1)* | 46.6 (14.0) | 57.0 (14.9) | 54.0 (16.5) | 57.1 (20.3) |
| AUC(0, 168 h) (pg ml−1 h)* | 7163 (2211) | 8751 (2272) | 8391 (2622) | 8599 (3161) |
| AUC(0, 240 h) (pg ml−1 h)* | 7766 (2332) | 9540 (2437) | 9189 (2755) | 9523 (3354) |
| t½ (h)† | 16.1 (3.0) | 16.4 (3.5) | 18.1 (6.4) | 17.1 (3.8) |
Values presented are means (s.d.) unless otherwise stated.
n = 33, 32, 36, and 35 for abdomen, arm, buttock, and torso, respectively.
n = 31, 32, 35, and 33 for abdomen, arm, buttock, and torso, respectively.
The treatment sequence group effect was not significant for AUC(0, 240 h) or Css for either analyte, whereas the period and site of application effects were found to be significant for AUC(0, 240 h) and Css for both analytes. The absorption of NGMN and EE during patch application on the buttock, arm, and torso was equivalent, as demonstrated by 90% confidence intervals for the ratio of the mean AUC(0, 240 h) and Css values falling within the 80% to 125% range (Table 3). The absorption of NGMN and EE during patch application on the abdomen was approximately 20% less than that observed for the other three sites and was not bioequivalent to the other three sites based on serum concentrations and the standard statistical criteria, although mean serum concentrations were still within reference ranges.
Table 3.
90% Confidence intervals for the ratio of the mean AUC and Css values.
| Norelgestromin | Ethinyloestradiol | ||||
|---|---|---|---|---|---|
| Test/Reference | Parameter | 90% CI† | Ratio (%)* | 90% CI† | Ratio (%)* |
| Arm/Abdomen | Css | 130.5 | 120.0, 142.0 | 124.6 | 114.0, 136.2 |
| AUC(0, 240 h) | 130.2 | 119.6, 141.7 | 125.4 | 115.0, 136.7 | |
| Buttock/Abdomen | Css | 125.0 | 114.9, 135.9 | 117.3 | 107.3, 128.2 |
| AUC(0, 240 h) | 125.8 | 115.5, 136.9 | 119.9 | 110.0, 130.7 | |
| Torso/Abdomen | Css | 125.4 | 115.3, 136.3 | 124.4 | 113.8, 136.0 |
| AUC(0, 240 h) | 124.6 | 114.5, 135.7 | 124.4 | 114.1, 135.7 | |
| Arm/Buttock | Css | 104.5 | 96.1, 113.6 | 106.3 | 97.2, 116.2 |
| AUC(0, 240 h) | 103.5 | 95.1, 112.7 | 104.6 | 95.9, 114.0 | |
| Torso/Buttock | Css | 100.3 | 92.2, 109.1 | 106.1 | 97.1, 116.0 |
| AUC(0, 240 h) | 99.1 | 91.0, 107.9 | 103.8 | 95.2, 113.2 | |
| Torso/Arm | Css | 96.0 | 88.3, 104.4 | 99.9 | 91.3, 109.2 |
| AUC(0, 240 h) | 95.7 | 88.0, 104.2 | 99.2 | 91.0, 108.2 | |
CI=confidence interval.
Ratio of geometric mean for test/reference.
90% CI for ratio of geometric mean for test/reference.
Safety evaluation
Treatment was generally well tolerated. The most common adverse events were nausea, mild-to-moderate application site reactions, and headache. One subject discontinued the study on day 94 due to migraine headaches, which were considered to be possibly related to the study drug. One subject experienced a serious adverse event that was considered to be probably related to the study drug. This subject noted heaviness of the left upper and lower extremities on day 115, which had resolved the next day. The investigator reported the adverse event as a right hemispheric transient ischaemic attack. Approximately 2 weeks later, the subject was hospitalized for recurrence of left arm and leg heaviness and pain. A computed axial tomography (CAT) scan was normal, and symptoms were attributed by her physician to be the result of an enlarged blood vessel and not to a cerebrovascular accident. There were no clinically relevant changes from baseline in laboratory test evaluations, gynaecological examination findings, or vital sign measurements.
Discussion
The objective of the present study was to assess the pharmacokinetics of NGMN and EE following successive application of the contraceptive patch at each of four different anatomical sites. The results demonstrated that mean serum concentrations of NGMN and EE remained within the reference ranges (0.6–1.2 ng ml−1 and 25–75 pg ml−1, respectively) during the 7 day wear period, regardless of whether the patch was worn on the abdomen, buttock, arm, or torso. These findings confirm and extend those of previous studies in which serum concentrations of NGMN and EE remained in the reference ranges during patch application to the abdomen [11–13] or buttock [12]. Absorption of NGMN and EE was approximately 20% less and not strictly bioequivalent when the patch was worn on the abdomen compared with the other three sites. However, because mean serum concentrations during patch application to the abdomen were still within the reference ranges, application of the patch to the abdomen is therapeutically equivalent to application at the other three sites. Moreover, in a separate parallel-group multiple application pharmacokinetic study, Css and AUC for the buttock and abdomen were not statistically different [14] and, in a previous four cycle study, patch application to the abdomen suppressed ovulation (defined as serum progesterone concentrations ≥3 ng ml−1) as effectively as oral norgestimate 250 µg plus EE 35 µg [15]. In pivotal clinical studies, application of the patch on any of the four anatomic sites evaluated in the present trial resulted in excellent contraceptive efficacy [16–18], which was comparable with that of combined oral contraceptives [17,18].
Since the patch is applied transdermally, first-pass metabolism (via the gastrointestinal tract and/or liver) of NGMN and EE that would be expected with oral administration is avoided. Hepatic metabolism of NGMN, the primary active metabolite of norgestimate, occurs and metabolites include norgestrel, which is highly bound to sex-hormone binding globulin (SHBG) thereby limiting its biologic availability, and various hydroxylated and conjugated metabolites [5, 19, 20]. EE is also metabolized to various hydroxylated products and their glucuronide and sulphate conjugates [21].
In conclusion, serum concentrations of NGMN and EE from the contraceptive patch remain within the reference ranges throughout the 7 day wear period, regardless of the site of application (abdomen, buttock, arm, or torso). Therapeutic concentrations will result from application at any of these four sites. All patches remained attached until removed, and were generally well tolerated by study subjects.
This study was supported financially by the R.W. Johnson Pharmaceutical Research Institute, Raritan, New Jersey, US.
References
- 1.Fu H, Darroch JE, Haas T, Ranjit N. Contraceptive failure rates; new estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:56–63. [PubMed] [Google Scholar]
- 2.Trussell J, Vaughan B. Contraceptive failure, method-related discontinuation and resumption of use: results from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:64–72. 93. [PubMed] [Google Scholar]
- 3.Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart F, editors. Contraceptive Technology. Seventeenth edition. New York: Ardent Media, Inc; 1998. pp. 779–844. [Google Scholar]
- 4.Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(24–29):46. [PubMed] [Google Scholar]
- 5.McGuire JL, Phillips A, Hahn D, Tolman EL, Flor S, Kafrissen ME. Pharmacologic and pharmacokinetic characteristics of norgestimate and its metabolites. Am J Obstet Gynecol. 1990;163:2127–2131. doi: 10.1016/0002-9378(90)90552-i. [DOI] [PubMed] [Google Scholar]
- 6.Corson SL. Efficacy and safety of a monophasic and a triphasic oral contraceptive containing norgestimate. Am J Obstet Gynecol. 1994;170:1556–1561. doi: 10.1016/s0002-9378(94)05019-2. [DOI] [PubMed] [Google Scholar]
- 7.Skee D, Abrams LS, Natarajan J, Anderson GD, Wong F. Pharmacokinetics of a contraceptive patch at 4 application sites. Clin Pharmacol Ther. 2000;67:159. doi: 10.1046/j.0306-5251.2001.01532.x. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams L, Skee D, Talluri K, Wong F. Bioavailability of 17-deacetylnorgestimate (17D-NGM) and ethinyl estradiol (EE) from a contraceptive patch. FASEB J. 2000;14:A1479. (Abstract) [Google Scholar]
- 9.Wong FA, Edom RW, Duda M, et al. Determination of norgestimate and its metabolites in human serum using high-performance liquid chromatography with tandem mass spectrometric detection. J Chromatogr B Biomed Sci Appl. 1999;734:247–255. doi: 10.1016/s0378-4347(99)00353-9. [DOI] [PubMed] [Google Scholar]
- 10.PPD Development. PPD Pharmaco. Richmond, Virginia; 1995. Validation report. GC/MS analysis of 17 α-ethinyl estradiol and norethindrone in human serum and plasma. [Google Scholar]
- 11.Abrams LS, Skee D, Natarajan J, Lasseter KC, Wong F. Dose proportionality study of a contraceptive patch. Clin Pharmacol Ther. 2000;67:105. (Abstract) [Google Scholar]
- 12.Abrams LS, Skee D, Bridson WE, Wong F. Pharmacokinetics of a contraceptive patch. Clin Pharmacol Ther. 2000;67:106. (Abstract) [Google Scholar]
- 13.Abrams LS, Skee DM, Wong FA, Anderson NJ, Leese PT. 2001;41:1232–1237. doi: 10.1177/00912700122012788. Pharmacokinetics of norelgestromin and ethinyl estradiol from two consecutive contraceptive patchesJ Clin Pharmacol. [DOI] [PubMed] [Google Scholar]
- 14.Abrams L, Skee D, Natarajan J, Wong F, Lasseter KC. Pharmacokinetics of multiple cycles of a contraceptive patch. J Clin Pharmacol. 2000;40:1047. (Abstract) [Google Scholar]
- 15.Dittrich R, Parker L, Rosen JB, Shangold G, Creasy GW, Fisher AC. Transdermal contraception. Evaluation of three norelgestromin/ethinyl estradiol doses in a randomized, multicenter, dose–response study. Am J Obstet Gynecol. 2001 doi: 10.1067/mob.2002.118844. [DOI] [PubMed] [Google Scholar]
- 16.Smallwood GH, Meador ML, Lenihan JP, Jr, Shangold GA, Fisher AC, Creasy GW. Efficacy and safety of a transdermal contraceptive system. Obstet Gynecol. 2001;98:799–805. doi: 10.1016/s0029-7844(01)01534-4. [DOI] [PubMed] [Google Scholar]
- 17.Hedon B, Helmerhorst FM, Cronje HS, Shangold G, Fisher A, Creasy G. Comparison of efficacy, cycle control, compliance, and safety in users of a contraceptive patch vs an oral contraceptive. Int J Gyn Obstet. 2000;70(Suppl 1):78. (Abstract) [Google Scholar]
- 18.Audet M-C, Moreau M, Koltun WD, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. JAMA. 2001;285:2347–2354. doi: 10.1001/jama.285.18.2347. [DOI] [PubMed] [Google Scholar]
- 19.Alton KB, Hetyei NS, Shaw C, Patrick JE. Biotransformation of norgestimate in women. Contraception. 1984;29:19–29. doi: 10.1016/0010-7824(84)90055-6. [DOI] [PubMed] [Google Scholar]
- 20.Madden S, Back DJ. Metabolism of norgestimate by human gastrointestinal mucosa and liver microsomes in vitro. J Steroid Biochem Mol Biol. 1991;38:497–503. doi: 10.1016/0960-0760(91)90338-6. [DOI] [PubMed] [Google Scholar]
- 21.Fotherby K. Pharmacokinetics of ethynyloestradiol in humans. Meth Find Exp Clin Pharmacol. 1982;4:133–141. [PubMed] [Google Scholar]


