Abstract
Aims
The objectives of the present investigation were: (a) to determine the correlation between lignocaine and midazolam pharmacokinetics following intravenous administration in healthy volunteers, (b) to determine the effects of treatment with an inhibitor of CYP3A4 (erythromycin) on this correlation and (c) to assess the precision of the MEGX-test as a sole predictor of lignocaine and midazolam pharmacokinetics.
Methods
The study was conducted in four male and four female healthy volunteers, aged between 21 and 26years, who received 1mgkg−1 lignocaine HCl i.v. on days 1, 3, 5, 9 and 10 of the investigation. On days 5 and 10 they also received midazolam, 0.075mgkg−1 i.v. and from days 6–10 they took erythromycin 500mg orally, four times daily. Following administration of lignocaine and midazolam, frequent venous blood samples were obtained for determination of the concentrations of lignocaine, MEGX and midazolam.
Results
In the absence of erythromycin a statistically significant linear correlation was observed between the clearance of lignocaine and midazolam (CLmidazolam= 0.41×CLlignocaine+1.2; r2=0.857; P<0.001). Erythromycin cotreatment resulted in a loss of the correlation between the two clearances (r2=0.39; P=0.1). Erythromycin caused a statistically significant reduction in midazolam clearance from the original value of 3.8 to 2.5 (95% CI for the difference −2.27, −0.35) mlkg−1min−1. Interestingly there was no significant change in the clearance of lignocaine (6.4 vs 5.8 (95% CI for the difference −2.74, −1.51) mlkg−1min−1). Furthermore no correlation at all was observed between the MEGX-test and lignocaine or midazolam clearances. Considering the data on day 1, 3 and 5 the intra-individual coefficient of variation in the MEGX-test was 45.3% at 15min and 23.5% at 30min, respectively.
Conclusions
It is concluded that there is a significant correlation between lignocaine and midazolam clearances but this correlation is lost after CYP3A4 inhibition by erythromycin. The MEGX-test is of no value in assessing intra-and inter-individual variability in midazolam clearance.
Keywords: CYP3A4, erythromycin, lignocaine, MEGX, midazolam, pharmacokinetics
Introduction
Midazolam is frequently used in intensive care medicine as an intravenous sedative [1–5]. The management of sedation levels in these patients with midazolam is complicated by the wide variability in dose requirements, which makes frequent dose adjustments necessary [3–6]. There are indications that the observed variability in dose requirement is, at least in part, related to variability in liver function [2, 7–9]. It would be helpful to have a liver function test available, which is predictive for midazolam clearance and which might be used to guide a priori dose requirements. Ideally such a liver function test should be readily available, rapid, sensitive and specific for cytochrome P-450 mediated oxidation.
In a study by Thummel et al.[10], it was shown that in liver transplant patients total midazolam clearance in vivo is highly correlated with the hepatic cytochrome P-450 3A4 (CYP3A4) content. In addition it was demonstrated that midazolam clearance could be accurately predicted on the basis of the 1-hydroxymidazolam/midazolam concentration ratio in plasma at 30 min following an intravenous bolus dose. However a limitation of this test is that it can only be applied in subjects unexposed to midazolam, which means that it cannot be used as a basis for dose adjustments during chronic therapy. Furthermore, the assay of midazolam and its metabolite 1-hydroxymidazolam is complex and relatively time-consuming [11, 12].
In recent years, the so-called ‘MEGX-test’ has been proposed as a dynamic liver function test in liver transplantation [13–16]. This test is based on estimation of the metabolic conversion rate of lignocaine into its metabolite monoethylglycinexylidide (MEGX). Typically the serum MEGX concentration at 15 or 30 min following intravenous administration of a single bolus dose of 1 mg kg−1 of lignocaine is measured [13–16]. An important practical factor in this respect is that the assay of serum MEGX concentrations is relatively simple and straightforward [13].
Interestingly both the 1-hydroxylation of midazolam and the oxidative N-deethylation of lignocaine are mediated by CYP3A4 [9, 17]. This means that the MEGX-test might potentially be useful in predicting variability in midazolam elimination.
The objectives of the present study were therefore: (a) to determine the correlation between lignocaine and midazolam pharmacokinetics following intravenous administration in healthy volunteers, (b) to determine the effects of treatment with an inhibitor of CYP3A4 (erythromycin) on this correlation and (c) to assess the precision of the MEGX-test as a sole predictor of lignocaine and midazolam pharmacokinetics. To this end the pharmacokinetics of both lignocaine and midazolam were determined in a longitudinal study in eight healthy volunteers. The longitudinal design was chosen in order to assess the intraindividual variability in MEGX concentrations first and then the influence of midazolam on these values. If coadministration of midazolam would significantly alter the outcome of the MEGX-test, the latter could not be used to predict the pharmacokinetics of the former in ICU patients.
Methods
We studied four male and four female volunteers with normal laboratory tests for hepatic function, aged between 21 and 26 years, bodyweight 58–90 kg, who were deemed healthy on the basis of routine clinical examination and normal ECGs. The volunteers were not taking drugs in the month prior the study except for oral contraceptives. Subjects taking gestodene containing contraceptives were excluded. One male subject was taking pravastatin (20 mg once daily) for familial hypercholesterolaemia, but pravastatin has little potential for drug interactions with substrates, inhibitors or inducers of the CYP3A4 system [18]. Chronic use of alcohol more than 3 units a day was excluded and no alcohol or grapefruit juice was allowed during the study period.
Approval was obtained from the hospital's ethical review board and the study was carried out according to the Declaration of Helsinki. Written informed consent was obtained from every subject.
On days 1, 3, 5, 9 and 10 the volunteers received intravenous lignocaine (Lidocaine HCl 10 mg ml−1 Miniplasco, Braun, Uden, the Netherlands) 1 mg kg−1, given over 1 min. On days 5 and 10 they also received midazolam (Dormicum 3 mg ml−1, Roche, Mijdrecht, the Netherlands) 0.075 mg kg−1 intravenously 1 min before lignocaine administration. On days 6–10 the volunteers took erythromycin 500 mg orally, four times daily (Erythrocine 500 mg tablets, Centrafarm, Etten-Leur, the Netherlands).
Venous blood samples were taken at 5, 10, 15, 20, 25 and 30 min and 1, 2, 4 and 8 h after the injection of lignocaine. During the first hour after lignocaine injection the volunteers were in a recumbent position and their ECG's and blood pressure were monitored.
Blood samples were centrifuged immediately, transferred into plastic tubes and the plasma was stored at −30 ° C until analysis. Lignocaine and MEGX were measured with a highly specific fluorescence polarization immunoassay (Abbott Laboratories, Amstelveen, the Netherlands). Midazolam and 1-hydroxymidazolam were measured by h.p.l.c. (modified method as published by Vletter et al.[12]). The lower limit of quantification for lignocaine was 100 µg l−1, for MEGX 10 µg l−1 and for midazolam and 1-hydroxymidazolam 10 µg l−1. The intra-and inter-assay coefficients of variation were below 5.2% for all assays in the used concentration range.
The following pharmacokinetic parameters were calculated using the extended least squares program WinNonlin (Professional Network Edition version 1.5, Pharsight, Mountain View, California, USA): rate constant of elimination, AUC, total body clearance and volume of distribution for both lignocaine and midazolam and the first two for MEGX. A two-compartment model best described the data from all subjects.
A power analysis, based on the coefficients of variation previously obtained for the 30-60 min MEGX concentrations [15] and midazolam concentrations [12], indicated that eight subjects should be sufficient to detect differences of 30% with a significance level (α) of 0.05 and a power (1-α) of 90%. Midazolam clearance is expected to be reduced by at least 30% due to the interaction with erythromycin [19–21]. Statistical evaluation was carried out by anova. When the analysis indicated that the results were different, a paired t-test was used to compare them. Linear regression was used to assess correlations. The inter-and intra-subject variability were defined as s.d./mean×100% (coefficient of variation).
Results
The laboratory results for albumin, bilirubin, ASAT, ALAT, LDH and PT were within the normal range for all volunteers. Following lignocaine administration all subjects experienced transient side-effects during and immediately after the injections. Typical symptoms were increased aural acuity, light-headedness and dizziness. In addition, heart rate slowed down occasionally. No dysrhythmias were observed. In all cases adverse effects were transient, usually persisting for about 2–5 min, with a maximum duration of 10 min.
Figure 1 shows the plasma concentration vs time profiles of lignocaine and MEGX on day 5 (without erythromycin) and day 10 (during erythromycin cotreatment) of the study. Figure 2 shows the plasma concentration vs time profiles of midazolam. The pharmacokinetic parameter estimates of lignocaine and midazolam are given in Tables 1 and 2, respectively.
Figure 1.
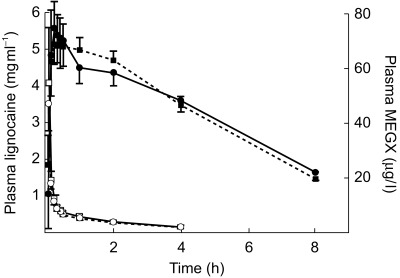
MEGX (solid symbols) and lignocaine (open symbols) plasma concentration vs time profiles on study day 5 (lignocaine and midazolam, solid line) and study day 10 (lignocaine and midazolam co-administered with erythromycin, dotted line) (mean value and 95% CI).
Figure 2.
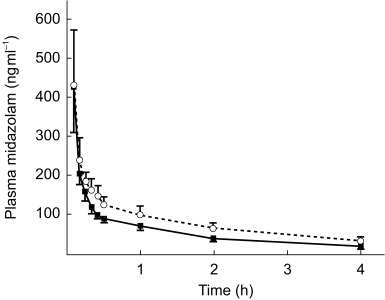
Midazolam concentration vs time profile on study day 5 (midazolam and lignocaine, solid line) and on study day 10 (midazolam and lignocaine co-administered with erythromycin, dotted line) (mean value and 95% CI).
Table 1.
Pharmacokinetic measurements of lignocaine on study day 5 in the absence of erythromycin and study day 10 during erythromycin co-treatment.
| Day 5 | Day 10 | |||||
|---|---|---|---|---|---|---|
| Subject | CL (ml kg−1 min−1) | t1/2(h) | V(l kg−1) | CL (ml kg−1 min−1) | t1/2(h) | V(l kg−1) |
| 1 | 4.8 | 2.1 | 1.3 | 5.1 | 2.1 | 1.3 |
| 2 | 10.7 | 1.2 | 1.1 | 6.0 | 2.9 | 1.5 |
| 3 | 6.4 | 2.1 | 1.1 | 3.9 | 4.1 | 1.3 |
| 4 | 6.5 | 1.8 | 1.0 | 4.2 | 2.3 | 0.9 |
| 5 | 5.8 | 2.3 | 0.9 | 6.9 | 2.4 | 0.8 |
| 6 | 5.8 | 1.7 | 0.9 | 7.8 | 2.4 | 1.6 |
| 7 | 5.3 | 3.7 | 0.9 | 8.0 | 2.7 | 1.8 |
| 8 | 5.7 | 1.9 | 1.0 | 4.2 | 2.1 | 0.7 |
| Average | 6.4 | 2.1 | 1.0 | 5.8 | 2.6 | 1.3 |
| s.d. | 1.8 | 0.7 | 0.1 | 1.7 | 0.7 | 0.4 |
Table 2.
Pharmacokinetic measurements of midazolam on study day 5 in the absence of erythromycin and study day 10 during erythromycin co-treatment.
| Day 5 | Day 10 | |||||
|---|---|---|---|---|---|---|
| Subject | CL (ml kg−1 min−1) | t1/2(h) | V(l kg−1) | CL (ml kg−1 min−1) | Day 10 t1/2(h) | V(l kg−1) |
| 1 | 3.0 | 2.8 | 0.7 | 2.2 | 2.5 | 0.5 |
| 2 | 5.5 | 1.4 | 0.7 | 3.5 | 2.4 | 0.7 |
| 3 | 3.5 | 2.4 | 0.9 | 1.8 | 2.6 | 0.8 |
| 4 | 4.5 | 1.7 | 0.6 | 1.5 | 2.1 | 0.4 |
| 5 | 3.0 | 1.6 | 0.9 | 2.9 | 3.1 | 0.8 |
| 6 | 3.8 | 1.9 | 0.6 | 2.2 | 4.6 | 0.9 |
| 7 | 3.4 | 2.9 | 0.8 | 3.9 | 4.1 | 0.8 |
| 8 | 3.3 | 2.1 | 0.6 | 2.6 | 2.7 | 0.6 |
| Average | 3.8 | 2.1 | 0.7 | 2.5* | 3.0* | 0.7 |
| s.d. | 0.8 | 0.6 | 0.1 | 0.9 | 0.9 | 0.2 |
Difference between day 5 and 10 95% CI −2.27, −0.35 ml kg−1 min−1.
Erythromycin caused a statistically significant reduction in midazolam clearance from the original value of 3.8 to 2.5 ml kg−1 min−1 (95% CI for the difference −2.27, −0.35 ml kg−1 min−1). However, no statistically significant change in the clearance of lignocaine was observed (95% CI for the difference −2,74, 1.51 ml kg−1 min−1). Figure 3 shows the correlation between midazolam and lignocaine clearance. In the absence of erythromycin, a statistically significant correlation was observed which could be described by the equation CLmidazolam=0.41 × CLlignocaine + 1.2 (r2 = 0.857; P < 0.001). However cotreatment with erythromycin resulted in a loss of this correlation (r2 = 0.39; P = 0.1).
Figure 3.
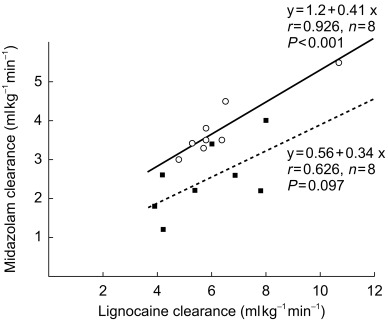
Lignocaine and midazolam clearances given without (solid line, open circles) and with (dotted line, solid squares) erythromycin administration on days 5 and 10, respectively.
The individual values of the MEGX-test (i.e. serum MEGX concentrations at 15 and 30 min after an intravenous dose of 1 mg kg−1 of lignocaine) are shown in Table 3. No statistically significant effect of midazolam treatment on MEGX concentrations was observed on day 5 of the study nor of erythromycin treatment on days 9 and 10 (Figure 4). When considering the data on day 1, 3 and 5 the intra-and inter-individual variability in lignocaine clearance were 18.3% and 21%, respectively. For the MEGX test at 15 min these values were 45.3% and 57.7%, respectively, and at 30 min 23.5% and 31.6%. No statistically significant correlation was observed between lignocaine clearance and the MEGX-test at 15 min and 30 min (Figure 5). The plasma 1-hydroxymidazolam/midazolam concentration ratio 30 min after administration of midazolam could not be calculated because most 1-hydroxymidazolam concentrations were below the detection limit of 10 µg l−1.
Table 3.
MEGX-concentrations (µg l−1) 15 and 30 min after lignocaine administration on subsequent days.
| MEGX t=15 min | MEGX t=30 min | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Day 1 | Day 3 | Day 5 | Day 9 | Day 10 | Day 1 | Day 3 | Day 5 | Day 9 | Day 10 |
| 1 | 22.3 | 29.9 | 40.2 | 72.7 | 25.5 | 36.4 | 45.4 | 41.7 | 42.6 | 23.2 |
| 2 | 25.3 | 163.9 | 83.9 | 96.1 | 122 | 52.2 | 103.1 | 66.3 | 86.2 | 89.2 |
| 3 | 76.9 | 37.8 | 132.2 | 108.2 | 113.3 | 86 | 50.7 | 103 | 91.9 | 106.8 |
| 4 | 48.5 | 31.2 | 57.4 | 35.1 | 62.6 | 65.1 | 45.4 | 61.9 | 53.1 | 67.7 |
| 5 | 26.6 | 84 | 115.3 | 93.5 | 86.5 | 47.8 | 81.9 | 93.8 | 83.4 | 82.3 |
| 6 | 58.4 | 63 | 88.2 | 74.5 | 46.3 | 63.3 | 64.8 | 76.5 | 73.5 | 52.8 |
| 7 | 69.1 | 135.2 | 85.4 | 82.5 | 56 | 72.4 | 108.8 | 81.7 | 91.6 | 58.2 |
| 8 | 19 | 62.6 | 58.5 | 28.1 | 51.1 | 37.8 | 62 | 59.5 | 47.2 | 59.2 |
| Average | 43.3 | 76 | 82.6 | 73.8 | 70.9 | 57 | 71.5 | 73.1 | 71.2 | 67.4 |
| s.d. | 22.9 | 49.6 | 30.7 | 28.6 | 34.1 | 17.8 | 23.9 | 19.8 | 20.5 | 25.5 |
Figure 4.
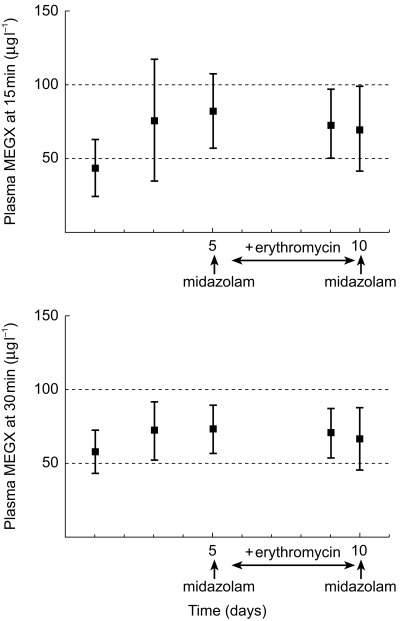
Plasma MEGX concentration at t=15 and t=30 min after lignocaine administration (mean data and 95% confidence intervals).
Figure 5.
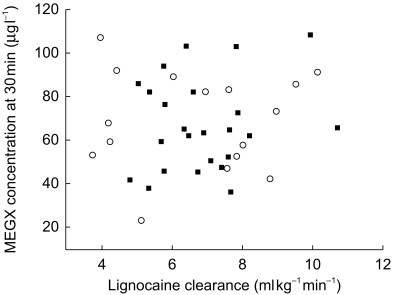
Lignocaine clearance and MEGX concentrations at 30 min after dosing alone (solid squares) and during erythromycin cotreatment (open circles).
Discussion
The correlation between lignocaine and midazolam clearances suggests the possibility that the two drugs may share a common elimination pathway, and that the metabolism of one drug might be a marker for the other. However, midazolam clearance, but not lignocaine clearance, is reduced by erythromycin and the correlation is lost. This suggests either that differences in the pharmacokinetic properties of the two drugs result in a differential effect on clearance as a result of enzyme inhibition or that the drugs do not in fact share a common elimination pathway.
Between lignocaine and midazolam there is a difference in hepatic extraction ratio. As a result there may be differences in the contribution of variability in hepatic blood flow to the clearance. Because lignocaine has an intermediate-high hepatic extraction ratio, its clearance rate and MEGX formation depend on both metabolic capacity and hepatic blood flow. Stimulation of hepatic blood flow by food intake did not influence the MEGX test in a study by Pritchard-Davies et al. [22], whereas Elvin et al.[23] demonstrated that a high protein meal increases hepatic clearance of intravenously administered lignocaine by 21%. Our study design resembles that of Pritchard-Davies et al.[22], because the volunteers were allowed to have a light breakfast. Therefore, only a small part of the variability might be explained by the influence of differences in food intake between study days. Furthermore, since midazolam has a lower extraction ratio of 0.3 to 0.4 [10, 24] as compared with lignocaine's value 0.75 [25], it is unlikely that variation in hepatic blood flow explains an important part of the observed variability in healthy volunteers. However, because of lignocaine's higher extraction ratio, enzyme inhibition may not result in as much a change in clearance as it does for midazolam.
Isohanni et al.[17] showed that concomitant administration of erythromycin does not affect lignocaine pharmacokinetics. Interactions between erythromycin and midazolam have been investigated by several authors. Olkkola et al.[19] administered erythromycin 500 mg three times daily for 7 days and detected an increase of the elimination half-life of midazolam 15 mg orally from 2.4 to 5.7 h. If midazolam 0.05 mg kg−1 was administered intravenously after the same dose of erythromycin the elimination half-life increased from 3.5 to 6.2 h. Aranko et al.[20] and Zimmermann et al.[21] showed an increase in the half-life of midazolam 15 mg orally from 1.5 to 2.6 and from 2.2 to 4.1 h after 6 and 5 days respectively of erythromycin 500 mg three times daily. We also found a significant prolongation of the midazolam half-life and decrease of its clearance.
The interaction between CYP3A4 and its substrates and inhibitors is complex, which is probably attributable to multiple binding sites within the active site of the enzyme. As a consequence, the interactions observed with one CYP3A4 probe may not be representative for those observed with other CYP3A4 substrates [26]. Kenworthy et al.[26] identified two distinct groups of CYP3A4 substrates based on their response to chemical modifiers of CYP3A4 activity. Erythromycin and cyclosporin belong to one group, midazolam to the other. Unfortunately lignocaine was not included in this study. Our results suggest that lignocaine may belong to a third, not yet identified, group. Furthermore the difference between lignocaine and midazolam might be explained by the fact that there is an alternative metabolic route for lignocaine. Hydroxylation mediated by CYP1A2 might contribute more to MEGX formation than CYP3A4 at low lignocaine concentrations [16, 27]. For midazolam there are no known alternative metabolic routes for hydroxylation other than mediated by CYP3A4 [9].
With regard to the variability of the MEGX-test, Oellerich et al.[13] and Huang et al.[15] also showed in their studies large interindividual variation in both their control patients and patients with impaired liver function. There was a significant overlap of MEGX levels between the control group and diseased groups. This did not restrain them from the conclusion that the MEGX formation test is valuable both in the quantitative evaluation of liver function and in the prognostic prediction of adults with liver disease. In our study the presumed cut-off value of 50 μg l−1 as an indicator for hepatic dysfunction [13, 14] falls within the 95% confidence interval of the first 2 study days as shown in Figure 4 (95% CI day 1, 24.1, 62.4, day 3, 34.5, 117.4). The mean value of MEGX formation after administration of erythromycin on day 9 did not fall within the 95% confidence interval of day 1, but did in that of day 3. Inhibition of CYP3A4 by erythromycin therefore cannot be predicted by the MEGX-test. These results and the large variability indicate that MEGX formation is not a valuable indicator for oxidative metabolic liver function or even for changes in metabolic liver function.
Interpretation is also complicated by the fact that some MEGX can be produced elsewhere in the body, probably in the intestine and kidney, since there is evidence for its extrahepatic production in an anhepatic woman, showing a serum MEGX concentration at 15 min of approximately 15 μg l−1[28]. Furthermore there are sex related differences in MEGX formation and the influence of contraceptives has to be taken into account when the test results are evaluated. Premenopausal women show significantly lower MEGX concentrations 30 min after lignocaine administration than men, the lowest values being observed in women taking contraceptives [29]. Gender does not influence midazolam pharmacokinetics, but its clearance tends to be diminished in women taking contraceptives [30]. This discrepancy also indicates lignocaine to be a member of a third group of CYP3A4 substrates other than erythromycin and midazolam.
The main advantage of the MEGX-test was that it can be performed rapidly. However, our results show that the predictive value of MEGX concentrations for the estimation of clearance of drugs metabolized by CYP3A4 is low. Although it is possible to predict individual drug pharmacokinetics with only a few data points of its plasma concentration, this will inevitably take much more time. Further investigations are necessary to find a suitable test which can be performed quickly and has good predictive value for a priori drug dosing.
In conclusion, there is a correlation between midazolam and lignocaine clearances, but the decrease of midazolam clearance induced by erythromycin administration is not observed with lignocaine. The values of plasma MEGX concentrations 15 and 30 min after administration of a bolus of 1 mg kg−1 of lignocaine vary considerably. The effects of administration of midazolam or erythromycin on the test results could not be determined due to these large variations. No correlation was found between MEGX concentrations 15 and 30 min after administration and lignocaine or midazolam clearance. This makes the MEGX test inappropriate to predict optimal drug dosing for midazolam and perhaps also other drugs whose pharmacokinetics depend on CYP3A4 activity. Individual measurement of plasma concentrations remains necessary to adjust drug dosing to liver oxidative function.
We thank Mr J. Sewnath and Mr R. Mooren for their skilful determination of the drug concentrations. The study was supported by Abbott Laboratories, Amstelveen, the Netherlands.
References
- 1.Malacrida R, Fritz ME, Suter PM, Crevoisier C. Pharmacokinetics of midazolam administered by continuous intravenous infusion to intensive care patients. Crit Care Med. 1991;20:1123–1126. doi: 10.1097/00003246-199208000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Shelly MP, Sultan MA, Bodenham A, Park GR. Midazolam infusions in critically ill patients. Eur J Anaesth. 1991;8:21–27. [PubMed] [Google Scholar]
- 3.Pohlman AS, Simpson KP, Hall JB. Continuous intravenous infusions of lorazepam versus midazolam for sedation during mechanical ventilatory support: a prospective, randomized study. Crit Care Med. 1994;22:1241–1247. doi: 10.1097/00003246-199408000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Shafer A. Complications of sedation with midazolam in the intensive care unit and a comparison with other sedative regimens. Crit Care Med. 1998;26:947–956. doi: 10.1097/00003246-199805000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Swart EL, Strack van Schijndel RJM, van Loenen AC, Thijs LG. Continuous infusion of lorazepam vs midazolam in patients in the intensive care unit: sedation with lorazepam is easier to manage and is more cost-effective. Crit Care Med. 1999;27:1461–1465. doi: 10.1097/00003246-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Oldenhof H, de Jong M, Steenhoek A, Janknegt R. Clinical pharmacokinetics of midazolam in intensive care patients, a wide interpatient variability? Clin Pharmacol Ther. 1988;43:263–269. doi: 10.1038/clpt.1988.31. [DOI] [PubMed] [Google Scholar]
- 7.MacGilchrist AJ, Birnie GG, Cook A, et al. Pharmacokinetics and pharmacodynamics of intravenous midazolam in patients with severe alcoholic cirrhosis. Gut. 1986;27:190–195. doi: 10.1136/gut.27.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park GR, Miller E. What changes drug metabolism in critically ill patients-III? Effect of pre-existing disease on the metabolism of midazolam. Anaesthesia. 1996;51:431–434. doi: 10.1111/j.1365-2044.1996.tb07785.x. [DOI] [PubMed] [Google Scholar]
- 9.Pentikäinen PJ, Välisalmi L, Himberg J-J, Crevoisier C. Pharmacokinetics of midazolam following intravenous and oral administration in patients with chronic liver disease and in healthy subjects. J Clin Pharmacol. 1989;29:272–277. doi: 10.1002/j.1552-4604.1989.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 10.Thummel KE, Shen DD, Podoll TD, et al. vitro–in vivo correlations in liver transplant patients. J Pharmacol Exp Ther. 1994;271:549–556. Use of midazolam as a human cytochrome P 450, 3A probe. I. [PubMed] [Google Scholar]
- 11.Ha HR, Rentsch KM, Kneer J, Vonderschmitt DJ. Determination of midazolam and its 1-hydroxymetabolite in human plasma and urine by high-performance liquid chromatography. Ther Drug Monit. 1993;15:338–343. doi: 10.1097/00007691-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Vletter AA, Burm AGL, Breimer LTM, Spierdijk J. High performance liquid chromatographic assay of midazolam and flumazenil simultaneously in human plasma. J Chromatogr, Biomed Appl. 1990;530:177–185. doi: 10.1016/s0378-4347(00)82319-1. [DOI] [PubMed] [Google Scholar]
- 13.Oellerich M, Raude E, Burdelski M, et al. Monoethylglycinexylidide formation kinetics. A novel approach to assessment of liver function. J Clin Chem Clin Biochem. 1987;25:845–853. doi: 10.1515/cclm.1987.25.12.845. [DOI] [PubMed] [Google Scholar]
- 14.Oellerich M, Burdelski M, Lautz HU, Schulz M, Schmidt FW, Herrmann H. Lidocaine metabolite formation as a measure of liver function in patients with cirrhosis. Ther Drug Monit. 1990;12:219–226. doi: 10.1097/00007691-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Huang YS, Lee SD, Deng JF, et al. Measuring lidocaine metabolite – monoethylglycinexylidide as a quantitative index of hepatic function in adults with chronic hepatitis and cirrhosis. J Hepatol. 1993;18:140–147. doi: 10.1016/s0168-8278(05)80187-4. [DOI] [PubMed] [Google Scholar]
- 16.Chandel B, Shapiro MJ, Kurtz M, et al. MEGX (monoethylglycinexylidide): a novel in vivo test to measure early hepatic dysfunction after hypovolemic shock. Shock. 1995;3:51–53. doi: 10.1097/00024382-199501000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Isohanni MH, Neuvonen PJ, Olkkola KT. Effect of erythromycin and itraconazole on the pharmacokinetics of intravenous lignocaine. Eur J Clin Pharmacol. 1998;54:561–565. doi: 10.1007/s002280050513. [DOI] [PubMed] [Google Scholar]
- 18.Beaird SL. HMG-CoA reductase inhibitors. Assessing differences in drug interactions and safety profiles. J Am Pharm Assoc. 2000;40:637–644. doi: 10.1016/s1086-5802(16)31104-4. [DOI] [PubMed] [Google Scholar]
- 19.Olkkola KT, Aranko K, Luurila H, et al. A potentially hazardous interaction between erythromycin and midazolam. Clin Pharmacol Ther. 1993;53:298–305. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]
- 20.Aranko K, Olkkola KT, Hiller A, Saarnivaara L. Clinically important interaction between erythromycin and midazolam. Br J Clin Pharmacol. 1992;33:217–218P. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann T, Yeates RA, Laufen H, Scharpf F, Leitold M, Wildfeuer A. Influence of the antibiotics erythromycin and azitromycin on the pharmacokinetics and pharmacodynamics of midazolam. Arzneimittel-Forschung. 1996;46:213–217. [PubMed] [Google Scholar]
- 22.Pritchard-Davies R, Gross AS, Shenfield GM. The effect of liver disease and food on plasma MEGX concentrations. Br J Clin Pharmacol. 1994;37:298–301. doi: 10.1111/j.1365-2125.1994.tb04279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elvin AT, Cole AFD, Pieper JA, Rolbin SH, Lalka D. Effect of food on lidocaine kinetics: mechanism of food-related alteration in high intrinsic clearance drug elimination. Clin Pharmacol Ther. 1981;30:455–460. doi: 10.1038/clpt.1981.188. [DOI] [PubMed] [Google Scholar]
- 24.Klotz U, Ziegler G. Physiologic and temporal variation in hepatic elimination of midazolam. Clin Pharmacol Ther. 1982;32:107–112. doi: 10.1038/clpt.1982.133. [DOI] [PubMed] [Google Scholar]
- 25.Molino G, Avagnina P, Belforte G, Bircher J. Assessment of the hepatic circulation in humans: new concepts based on evidence derived from a d-sorbitol clearance method. J Laboratory Clin Med. 1998;131:393–405. doi: 10.1016/s0022-2143(98)90139-1. [DOI] [PubMed] [Google Scholar]
- 26.Kenworthy KE, Bloomer JC, Clarke SE, Houston JB. CYP3A4 drug interactions. correlation of 10 in vitro probe substrates. Br J Clin Pharmacol. 1999;48:716–727. doi: 10.1046/j.1365-2125.1999.00073.x. DOI: 10.1046/j.1365-2125.1999.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JS, Backman JT, Taavitsainen P, Neuvonen PJ, Kivistö KT. Involvement of CYP1A2 and CYP3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos. 2000;28:959–965. [PubMed] [Google Scholar]
- 28.Sallie RW, Tredger JM, Williams R. Extrahepatic production of the lignocaine metabolite monoethylglycinexylidide (MEGX) Biopharmaceut Drug Disp. 1992;13:555–558. doi: 10.1002/bdd.2510130709. [DOI] [PubMed] [Google Scholar]
- 29.Oellerich M, Schütz E, Polzien F, et al. Influence of gender on the monoethylglycinexylidide test in normal subjets and liver donors. Ther Drug Monit. 1994;16:225–231. doi: 10.1097/00007691-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Holazo AA, Winkler MB, Patel IH. Effect of age, gender and oral contraceptives on intramuscular midazolam pharmacokinetics. J Clin Pharmacol. 1988;28:1040–1045. doi: 10.1002/j.1552-4604.1988.tb03127.x. [DOI] [PubMed] [Google Scholar]


