There is a growing interest in therapeutic drug monitoring (TDM) of protease inhibitors (PIs) as a potential tool to optimize the treatment of HIV-infection, as recently discussed in this journal [1]. A prerequisite for the introduction of TDM is the definition of minimal effective concentrations. To date, the target concentrations of the PIs are largely unknown. Proposed minimal effective concentrations for the PI saquinavir range from 100 to 200 ng ml−1[1]. Results from the CHEESE study, however, suggest that the threshold concentration may be even lower for antiretroviral naive HIV-1-infected patients concomitantly treated with zidovudine (AZT) and lamivudine (3TC).
In the CHEESE study, antiretroviral naive patients were treated with saquinavir soft-gelatin capsules 1200 mg three times daily (or indinavir 800 mg three times daily), in combination with AZT 200 mg three times daily plus 3TC 150 mg twice daily [2]. To explore pharmacokinetic–pharmacodynamic (PK–PD) relationships, blood samples for the quantification of saquinavir were obtained at regular intervals. Plasma concentrations of saquinavir were determined with a sensitive and validated assay [3]. The accuracy of this assay was confirmed in a cross-validation with a commercial contract laboratory using a radioimmunoassay.
Three patients experienced virological treatment failure prior to week 48 (one had two plasma saquinavir concentrations below 100 ng ml−1, one had no concentrations below 200 ng ml−1, and no saquinavir concentrations were available from the remaining patient). Twenty-two patients (median baseline plasma HIV-1 RNA concentration (viral load) 4.99 log10 copies/ml), completed 48 weeks follow-up. After 48 weeks, 19/22 patients (86%) had a plasma viral load below 50 copies/ml. A total of 151 plasma saquinavir concentrations were available ranging from undetectable (four samples from three patients) to 3792 ng ml−1 (median 145 ng ml−1) (Figure 1).
Figure 1.
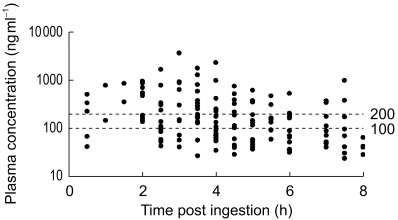
Scatterplot of the saquinavir plasma concentrations (n=151) obtained from 22 HIV-1 infected patients at regular intervals during a 48 week period. The patients used saquinavir soft gelatin capsules 1200 mg three times daily plus zidovudine and lamivudine. The dotted lines represent threshold concentrations of 100 and 200 ng ml−1.
No PK–PD relationships were observed, possibly due to a lack of statistical power. Surprisingly, however, a potent and durable suppression of viral replication (< 50 copies/ml) was observed in 86% of the patients, despite low plasma saquinavir concentrations throughout the study period. When applying the proposed threshold concentrations of 100 and 200 ng ml−1, the proportion of patients with at least one ‘suboptimal’ saquinavir concentration was 77 and 91%, respectively. Fifteen patients continued the study drugs beyond 48 weeks. After a median follow-up of 93 weeks (range 62–106 weeks), 13/15 patients (87%) maintained a plasma HIV-1 RNA concentration below 50 copies/ml, despite continuing low plasma saquinavir concentrations (median concentration 52 ng ml−1).
The satisfactory antiviral response may be explained by the intracellular pharmacokinetics of saquinavir. Compared with other protease inhibitors, saquinavir showed the most pronounced accumulation in peripheral blood mononuclear cells, resulting in a 5.45-fold higher exposure intracellularly as compared with plasma [4]. The intracellularly saquinavir concentration may be a better predictor of the virological response to therapy than the plasma concentration, since viral replication takes place within cells.
Despite low plasma concentrations, saquinavir contributed to the virological response. A decline in plasma viral load of 2.4 log10 copies/ml was observed after 24 weeks [2], whereas previous studies of AZT plus 3TC in naive patients showed only a drop of about 1.1 log10 copies/ml after 24 weeks [5].
Recent results from a randomized, prospective study on TDM (the ATHENA trial), suggested that antiretroviral naive HIV-1-infected patients may benefit from TDM of indinavir and nelfinavir [6]. TDM of PIs seems thus an attractive option to optimize antiretroviral therapy, and is increasingly advocated as the next tool in the management of HIV-1-infected patients. We argue, however, that more knowledge on the PK–PD relationships of saquinavir is required, before the introduction of TDM in daily clinical practice can be justified. Definition of target concentrations for TDM is crucial to prevent a waste of health-care resources.
The authors would like to thank the following for their contribution to the CHEESE Study: L. van Belle, B. Bravenboer, K. Brinkman, C. A. B. Boucher, W. Dorama, T. de Groot, D. Hamann, H. J. M. ter Hofstede, J. R. Jutmann, F. P. Kroon, P. P. Koopmans, N. Langebeek, R. van Leusen, P. L. Meenhorst, P. van der Meulen, C. Richter, M. Roos, R. Schuurman, R. Snijder, H. G. Sprenger, B. van der Ven, E. Waalberg-Tammeling, A. Wicherink, and B. Zomer. This research was supported by the Ministry of Public Health, Welfare, and Sports as part of the Stimulation Program on AIDS Research of the Dutch Program Committee for AIDS Research (project 1307), and by Roche Nederland BV, Mijdrecht, the Netherlands.
References
- 1.Back DJ, Khoo SH, Gibbons SE, Merry C. The role of therapeutic drug monitoring in treatment of HIV infection. Br J Clin Pharmacol. 2001;51:301–308. doi: 10.1046/j.1365-2125.2001.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen Stuart JWT, Schuurman R, Burger DM, et al. Randomized trial comparing saquinavir soft-gelatin capsules versus indinavir as part of triple therapy (CHEESE Study) AIDS. 1999;13:F53–F58. doi: 10.1097/00002030-199905070-00001. DOI: 10.1097/00002030-199905070-00001. [DOI] [PubMed] [Google Scholar]
- 3.Heeswijk van RPG, Hoetelmans RMW, Harms R, et al. Simulatenous quantitative determination of the HIV protease inhibitors amprenavir, indinavir, nelfinavir, ritonavir, and saquinavir in human plasma by ion-pair high-performance liquid chromatography with ultraviolet detection. J Chromatogr B. 1998;719:159–168. doi: 10.1016/s0378-4347(98)00392-2. [DOI] [PubMed] [Google Scholar]
- 4.Khoo S, Hennesy M, Mulcahy F, et al. 2001. Differences in intracellular drug accumulation between the protease inhibitors8th Conference on Retroviruses and Opportunistic Infections, Chicago, IL, USA, February 4–8, [Abstract 258]
- 5.Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200–500 CD4+ cells per cubic millimeter. N Engl J Med. 1995;333:1662–1669. doi: 10.1056/NEJM199512213332502. [DOI] [PubMed] [Google Scholar]
- 6.Burger DM, Hugen PWH, Droste J, et al. 2001. Therapeutic drug monitoring (TDM) of nelfinavir (NFV) and indinavir (IDV) in treatment naive patients improves therapeutic outcome after 1 year: Results from ATHENAThe 1st IAS Conference on HIV Pathogenesis and Treatment, Buenos Aires, Argentina, July 8–11, [Abstract 30]


