Abstract
Aims
Helicobacter pylori (H. pylori) eradication rate varies according to the treatment regimen used and other factors, e.g. antimicrobial resistance and patient compliance. The aim of the present study was to evaluate the influence of patient counselling and follow-up on H. pylori eradication rates and to document the effectiveness of a 1 week eradication regimen consisting of lansoprazole (30 mg once daily), amoxicillin (1 g twice daily) and clarithromycin (500 mg twice daily).
Methods
Seventy-six dyspeptic patients, who at endoscopy were found to have gastritis, duodenitis or ulceration, and a positive H. pylori urease test, were recruited. Patients were randomly assigned to an intervention group (n = 38) or a control group (n = 38). Intervention patients received their medicines via the hospital pharmacy and were counselled (and followed up) by a hospital pharmacist. Control patients were given a standard advice sheet and referred to their GP who prescribed the same therapy.
Results
Intervention patients exhibited a statistically significant improvement in the H. pylori eradication rate (94.7% vs 73.7%; P = 0.02) and compliance (92.1% vs 23.7; P < 0.001). Of the 64 H. pylori eradicated patients, 62 were able to eliminate their antisecretory medication compared with only 12 of the H. pylori persistent patients (P < 0.001). A pharmacoeconomic evaluation indicated that counselling and follow-up reduced the direct costs of eradication by approximately £30 per patient.
Conclusions
Structured patient counselling and follow-up can have a significant effect on H. pylori eradication rates and should be a routine part of therapy.
Keywords: H. pylori, patient counselling, peptic ulcer disease
Introduction
Helicobacter pylori (H. pylori) infection of the mucosa of stomach and duodenum has been positively associated with gastritis, duodenal ulcer, gastric ulcer and gastric carcinoma [1–3].
Although antisecretory drugs have been shown to heal ulcers, once treatment is withdrawn almost all ulcers can be expected to recur within 2 years. Eradication of H. pylori significantly decreases ulcer recurrence rates and the complications associated with ulcer disease [4]. Several consensus groups therefore recommend that all patients with peptic ulcers associated with the presence of H. pylori should be treated with antimicrobial agents to eradicate the bacteria [5]. Several combinations of antimicrobial agents and antisecretory drugs have been used in clinical trials to date. The best results have been achieved with triple therapies, however, the results have been variable, possibly due to differences in patient compliance and bacterial resistance to the antimicrobial agents used [6–10].
Patient compliance is an important factor in the successful outcome of any therapeutic programme [11,12] and the critical effect of compliance on the efficacy of triple therapy for H. pylori has been demonstrated [13]. One major factor predisposing to poor patient compliance is lack of proper counselling on the part of medical and paramedical staff [14–16].
The main aim of the present study was to evaluate, in a randomized controlled trial in one centre, the influence of patient counselling and follow-up on H. pylori eradication rate and patient outcomes. A second goal of the study was to evaluate the effectiveness and tolerability of an eradication regimen consisting of lansoprazole, amoxicillin and clarithromycin.
Methods
Study site
The study was conducted at the endoscopy unit of the Antrim Area Hospital (a 378-bed district hospital).
Study design
This was a prospective, randomized, controlled study.
Patients
All adult patients who were referred by their GP for investigation of dyspepsia, by outpatient referral to the GI clinic or open access referral to the Endoscopy Unit, Antrim Area Hospital, and who endoscopically were found to have macroscopic changes of gastritis, duodenitis or ulceration, with the presence of H. pylori in the stomach, using a rapid urease test (Clotest−, Delta West Pty Ltd, Australia), were invited to participate in the study. Those patients who gave their informed consent to participate in the study were enrolled. Consent was verbal due to the sensitivity of patient compliance to the Hawthorn effect [17]. Patients were excluded if found to be hypersensitive to any component of the combination therapy or clinically judged unsuitable for eradication therapy. Ethical approval for the study was obtained from the Ethics Committee, The Queen's University of Belfast, Northern Ireland.
Methodology
Following diagnosis and enrolment into the study, patients were prescribed a 1 week regimen of lansoprazole 30 mg once daily, amoxicillin 1 g twice daily, and clarithromycin 500 mg twice daily. Lansoprazole was the selected proton pump inhibitor as it was considered to be the most cost-effective agent at the time of the study. Using a sealed envelope technique [18] patients were randomly assigned to either the control group or the intervention group.
Patient groups
Patients in the intervention group received their medicines via the Hospital Pharmacy and on receipt of their medicines each patient was counselled by the same hospital pharmacist on: their disease and the importance of eradication of the organism; the medicines to be taken, including possible side-effects; the importance of compliance with the prescribed dosage regimen. In addition the intervention patients were given a patient information leaflet (Figure 1) about their medicines and the need for H. pylori eradication. They were also given a compliance diary chart (Figure 2). Intervention group patients were also telephoned 3 days after the initiation of therapy to provide further counselling on the importance of medication compliance.
Figure 1.
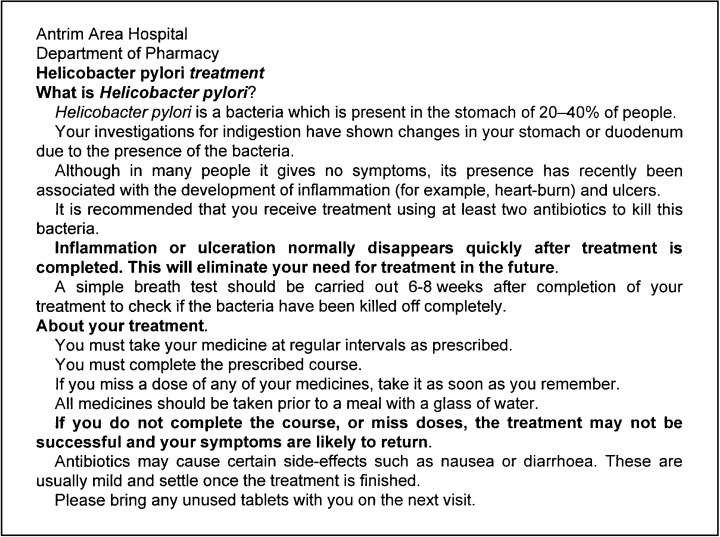
Information sheet given to intervention patients.
Figure 2.
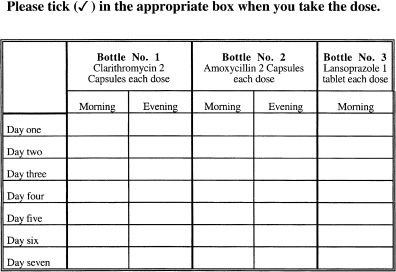
Patient's diary chart given to patients in the intervention group.
Control group patients were treated according to normal hospital procedures. They were given a letter to be taken to their GP, with the recommendation that the triple therapy regimen detailed above be prescribed, together with a standard letter then in use in the department which explained the nature of the infection, the need for treatment and importance of compliance.
Assessment of H. pylori status
All patients, as was the usual practice, were asked to return to the hospital for a urea breath test, 4–6 weeks after the treatment regimen had finished, to confirm whether eradication had been successful. Eradication was defined as absence of H. pylori as judged by the urea breath test [19,20].
Assessment of adverse drug reactions
All patients, in both intervention and control groups, were contacted by telephone by the same hospital pharmacist (structured questionnaire) approximately 10 days post endoscopy and asked about any adverse effects experienced during the eradication therapy, such as nausea, vomiting, diarrhoea, taste disturbance, abdominal pain, headache, itching or rash.
Assessment of compliance
Compliance was measured by two indirect techniques in both groups, i.e. patient interview by telephone (structured questionnaire) after completion of the H. pylori eradication therapy and pill counts (patients were asked to bring unused tablets and capsules when they returned to the hospital to have a urea breath test performed). Using these techniques the number of doses missed was recorded.
Assessment of clinical outcome measures
Patient response to the therapy was assessed using the following parameters: severity of dyspeptic symptoms and use of antisecretory medication after eradication therapy. A modified version of the Gastrointestinal Symptom Rating Scale [21] was used to assess the presence and severity of dyspeptic symptoms, including epigastric pain or discomfort, heartburn, nausea, vomiting and wind. The presence and severity of the dyspeptic symptoms were judged by the patient. The following scoring system was used: no symptoms, score one, mild symptoms, score two, moderate symptoms, score three and severe symptoms, score four. Patients were interviewed on the day of endoscopy and 1 month and 6 months after completion of H. pylori eradication therapy by the same interviewer (hospital pharmacist) using a standard questionnaire. They were also asked at the 6 month interview if they had consulted their GP for dyspeptic symptoms during the past 6 months.
Statistical analysis
Data were coded and entered into a desktop computer and analysed using SPSS (Version 7). The chi-squared test and the Fisher's exact test were used to compare noncontinuous variables as appropriate. Continuous variables were analysed using the independent Student's t-test. In addition to comparing the control and intervention groups, symptoms were compared in the patients who had H. pylori eradicated vs those in patients in which H. pylori persisted. The Sign test was used to compare the severity of the dyspeptic symptoms 1 month and 6 months after completion of H. pylori eradication therapy. P values <0.05 were considered significant.
Results
Patient characteristics
Eighty patients fulfilled the entrance criteria and were randomly assigned to the intervention and control groups. Two patients in the intervention group were excluded, one because of an adverse drug reaction causing discontinuation of therapy and a second for failing to attend for the urea breath test after three appointments. Two patients were also excluded from the control group one because of allergy to penicillin and a second because the GP prescribed a longer eradication treatment regimen (2 weeks). Data from 38 patients in each group were therefore suitable for evaluation.
Table 1 summarizes the demography of patients participating in the study. The mean (± s.d.) age of the intervention group was 49.3 (± 16.3) ranging from 22 to 85 years compared with 50.7 (± 15.7) ranging from 20 to 82 years in the control group (P = 0.71). The majority of patients in both groups were male (73.7% in the intervention compared to 68.4% in the control group; P = 0.80). Patients in both groups were similar in terms of smoking (P = 0.29) and although there was an increased reported alcohol consumption in the intervention group, this did not reach statistical significance (P = 0.06).
Table 1.
Demographic details of the study patients.
| Control | Intervention | P value | |
|---|---|---|---|
| Number of patients | 38 | 38 | |
| Age (years) | |||
| mean (± s.d.) | 50.7 (± 15.7) | 49.3 (± 16.3) | 0.705§ |
| range | (25–81) | (21–83) | |
| Sex (%) | 0.800† | ||
| Female | 12 (31.6%) | 10 (26.3%) | |
| Male | 26 (68.4%) | 28 (73.7%) | |
| Social habits | |||
| Smoking | 0.287† | ||
| current | 20 (52.6%) | 21 (55.3%) | |
| former | 8 (13.2%) | 12 (31.6%) | |
| never | 10 (34.2%) | 5 (13.2%) | |
| Alcohol intake | 0.060† | ||
| weekly | 15 (39.5%) | 23 (60.5%) | |
| occasionally | 5 (13.2%) | 7 (18.4%) | |
| none | 18 (47.4%) | 8 (21.1%) | |
chi-squared test;
Student's t-test.
The median duration of dyspeptic symptoms prior to eradication therapy was similar in both groups (48 months in the control group and 46 in the intervention group; P = 0.41; Table 2).
Table 2.
Diagnoses, antisecretory medications and duration of dyspeptic symptoms prior to eradication therapy.
| Control (n = 38) | Intervention (n = 38) | P value | |
|---|---|---|---|
| Diagnosis | |||
| Duodenal ulcer (DU) | 15 (39.5%) | 13 (34.2%) | |
| Gastric ulcer (GU) | 5 (13.1%) | 2 (5.3%) | |
| DU and GU | 2 (5.3%) | 5 (13.2%) | |
| Duodenitis | 8 (21.0%) | 10 (26.3%) | |
| Gastritis | 2 (5.3%) | 1 (2.6%) | |
| Duodenitis and gastritis | 6 (15.8%) | 7 (18.4%) | |
| Antisecretory medications used | 38 | 38 | |
| Cimetidine | 8 | 13 | |
| Lansoprazole | 2 | 1 | |
| Nizatidine | 5 | 6 | |
| Omeprazole | 5 | 7 | |
| Ranitidine | 18 | 11 | |
| Duration of antisecretory medication usage (months) | |||
| mean (± s.d.) | 40.4 (± 5.6) | 38.9 (± 3.9) | 0.199§ |
| range | 18–72 | 12–96 | |
| Duration of dyspeptic symptoms (months) | |||
| median | 48 | 46 | 0.412* |
| interquartile range | 44.0–50.5 | 42.7–49.0 | |
Student's t-test;
Mann–Whitney U-test.
H. pylori status and eradication rate
Of the 38 patients in the intervention group, 36 were urea breath test negative while of the 38 patients in the control group, only 28 patients were urea breath test negative, i.e. the eradication rate was 94.7% in the intervention compared with 73.7% in the control group (95% CIs of difference: 5.3%, 36.7%; P = 0.027). The overall (intervention and control, n = 76) eradication rate achieved using this combination therapy containing amoxicillin, clarithromycin and lansoprazole for 1 week was 84.2% (i.e. 64 eradicated; 12 persistent).
Adverse drug reactions
A number of patients in both groups reported that they experienced adverse effects during H. pylori eradication therapy, 17 patients (44.7%) in the control group compared with 19 (50%) in the intervention (Table 3, P = 0.81). The most frequent adverse drug reactions were diarrhoea and taste disturbance.
Table 3.
Adverse drug reactions (ADRs) reported by the patients.
| Control | Intervention | P value | |
|---|---|---|---|
| Number of patients who experienced an ADR | 17 (44.7%) | 19 (50.0%) | 0.810† |
| ADRs reported | |||
| Abdominal pain | 1 (2.6%) | 3 (7.9%) | |
| Diarrhoea | 4 (10.5%) | 8 (21.0%) | |
| Headache | 2 (5.3%) | 4 (10.5%) | |
| Loose stool | 6 (15.8%) | 2 (5.3%) | |
| Itching | 0 | 1 (2.6%) | |
| Metallic taste | 6 (15.8%) | 7 (18.4%) | |
| Nausea | 1 (2.6%) | 4 (10.5%) | |
| Rash | 1 (2.6%) | 2 (5.3%) | |
| Sore mouth | 3 (7.9%) | 3 (7.9%) | |
| Tired | 4 (10.5%) | 2 (5.3%) | |
| Vomiting | 1 (2.6%) | 3 (7.9%) | |
Chi-squared test.
Counselling and compliance rate
The average time (± s.d.) spent counselling each patient by a hospital pharmacist was 9.5 ± 2.3 min. This resulted in a 100% compliance rate (Table 4) being recorded in 92.1% of the patients in the intervention group. The equivalent number of patients with 100% compliance in the control was much lower (23.7%; 95% CIs of the difference: 52.3, 84.5%; P < 0.001). All H. pylori persistent patients took less than 60% of their prescribed regimen, while all eradicated patients took more than 65% of their prescribed medication.
Table 4.
Doses missed during the eradication therapy.
| Control | Intervention | P value | |
|---|---|---|---|
| Number of patients who took all prescribed doses | 9 (23.7%) | 35 (92.1%) | < 0.001† |
| Number of patients who missed the following number of doses | |||
| One dose | 6 (15.8%) | 1 (2.6%) | |
| Two doses | 4 (10.5%) | 0 | |
| Three doses | 5 (13.2%) | 0 | |
| Four doses | 3 (21.4%) | 0 | |
| Five doses | 1 (2.6%) | 0 | |
| Six doses | 1 (2.6%) | 0 | |
| Seven doses | 2 (5.3%) | 0 | |
| Eight doses | 5 (13.2%) | 1 (2.6%) | |
| Nine doses | 1 (2.6%) | 1 (2.6%) | |
| Ten doses | 1 (2.6%) | 0 | |
Chi-squared test.
Clinical outcome measures
Dyspeptic symptoms
At the 1 month follow-up the severity scores for individual dyspeptic symptoms were, as expected, much lower for the patients in whom H. pylori had been successfully eradicated (Table 5). The differences in the severity scores of epigastric discomfort, heartburn, nausea, vomiting and wind were statistically significant (P < 0.001). In the H. pylori persistent patients the severity scores for nausea and vomiting were, however, statistically lower (P < 0.001) after H. pylori eradication therapy than before, while the severity scores for epigastric discomfort, heartburn and wind were not statistically different (P > 0.05). The same results were found at the 6 month follow-up in the H. pylori eradicated patients (P < 0.001) with little more reduction in the severity scores for epigastric discomfort, heartburn, nausea, vomiting and wind. In H. pylori persistent patients, at the 6 month follow-up the severity scores for epigastric discomfort heartburn, nausea, vomiting, and wind had almost returned to baseline (P > 0.05).
Table 5.
Dyspeptic symptoms assessment.
| H. pylori eradicated | H. pylori persistent | ||||||
|---|---|---|---|---|---|---|---|
| Dyspeptic | Follow-up pre and post treatment (n = 64) | Follow-up pre and post treatment (n = 12) | |||||
| symptoms | Time* | 0 | 1 | 6 | 0 | 1 | 6 |
| Epigastric pain | |||||||
| nil | 4 | 61 | 63 | 2 | 2 | 2 | |
| mild | 1 | 3 | 1 | 0 | 0 | 0 | |
| moderate | 9 | 0 | 0 | 1 | 2 | 2 | |
| severe | 50 | 0 | 0 | 9 | 8 | 8 | |
| % with symptom | 93.8% | 4.7% | 1.6% | 83.3% | 83.3% | 83.3% | |
| Heartburn | |||||||
| nil | 16 | 50 | 61 | 3 | 3 | 3 | |
| mild | 1 | 14 | 3 | 0 | 0 | 0 | |
| moderate | 7 | 0 | 0 | 1 | 2 | 0 | |
| severe | 40 | 0 | 0 | 8 | 7 | 9 | |
| % with symptom | 75% | 21.9% | 3.2% | 75% | 75% | 75% | |
| Nausea | |||||||
| nil | 29 | 60 | 64 | 5 | 7 | 3 | |
| mild | 15 | 3 | 0 | 3 | 2 | 3 | |
| moderate | 10 | 1 | 0 | 3 | 2 | 2 | |
| severe | 10 | 0 | 0 | 2 | 1 | 4 | |
| % with symptom | 55% | 4.7% | 0% | 66.7% | 41.7% | 75% | |
| Vomiting | |||||||
| nil | 33 | 63 | 64 | 6 | 12 | 6 | |
| mild | 22 | 1 | 0 | 5 | 0 | 5 | |
| moderate | 7 | 0 | 0 | 1 | 0 | 1 | |
| severe | 2 | 0 | 0 | 0 | 0 | 0 | |
| % with symptom | 48.4% | 1.6% | 0% | 50% | 0% | 50% | |
| Wind | |||||||
| nil | 30 | 52 | 64 | 4 | 4 | 4 | |
| mild | 3 | 12 | 0 | 0 | 0 | 0 | |
| moderate | 9 | 0 | 0 | 3 | 2 | 2 | |
| severe | 22 | 0 | 0 | 5 | 6 | 6 | |
| % with symptom | 46.8% | 18.8% | 0% | 66.7% | 66.7% | 66.7% | |
0: symptoms severity before eradication therapy; 1, 6: symptoms severity 1 and 6 months after completion of eradication therapy.
In addition, at the 6 month follow-up, the incidence of epigastric discomfort had decreased from 93.8% before treatment to 1.6%; heartburn from 75% to 3.2%; nausea from 55% to 0%; vomiting from 48.4% to 0%; and wind from 46.8% to 0% in H. pylori eradicated patients. In H. pylori persistent patients, the number of patients remained similar (P > 0.05) for all symptoms at the 6 month follow-up. However, at the 1 month follow-up, the number of patients with nausea and vomiting had decreased significantly (P < 0.001).
Antisecretory medications usage
The mean duration of prescribed antisecretory medications which were most commonly H2-receptor antagonists prior to entry into the study was similar for both groups (40.4 months in the control group vs 38.9 in the intervention group; Tables 2, P = 0.199). At the 1 month follow-up, the usage of antisecretory medications was significantly reduced in all H. pylori eradicated patients compared with H. pylori persistent patients (P < 0.001). At the 6 month follow-up usage of antisecretory medications was eliminated in all H. pylori eradicated patients except two, whilst all H. pylori persistent patients continued to receive antisecretory medication. Again the difference was statistically significant (P < 0.001).
Visit to GPs
At the 6 month follow-up, H. pylori persistent patients were much more likely to have consulted their GPs for dyspeptic symptoms (91.7% of patients) compared with the H. pylori eradicated patients (4.7%; P < 0.001). In the H. pylori persistent group, seven patients had consulted their GPs once during the 6 month follow-up, two patients twice and another two patients three times, while the three that consulted their GPs in the H. pylori eradicated group had done so only once.
Treatment costs
The direct costs of a 1 week course of the triple therapy regimen, other expenses involving the urea breath test and patient counselling together with the financial implications of a second eradication programme are summarized in Table 6. The community-based costs for 1 year of treatment with the antisecretory medications that were being used by patients before eradication therapy, with the exception of cimetidine therapy, far exceeded the eradication costs. In this study, of the 64 H. pylori eradicated patients 62 were able to completely stop antisecretory medications.
Table 6.
Cost of H. pylori eradication.
| Cost element | Cost (£)* |
|---|---|
| Expenses per successful eradicated case | |
| Eradication therapy | 42.3 |
| Urea breath test (13C test plus labour) | 32 |
| Patient counselling | 3 |
| (average time spent+phone call+diary card) | |
| Total | 77.3 |
| Expenses per one failure case | |
| Eradication therapy | 42.3 |
| Urea breath test (13C test plus labour) | 32 |
| GP consultations (average) | 52.3 |
| Another course of eradication therapy | 42.3 |
| Another urea breath test | 32 |
| Total | 200.9 |
Costs obtained from Monthly Index of Medical Specialties (MIMS), Hospital Trust and Health Board sources.
Overall costs
In economic terms the present data indicate that £8402 would be needed to eradicate H. pylori in 100 patients using the study treatment regimen plus counselling, while an additional £3026 would be needed to eradicate H. pylori in 100 patients using the same eradication therapy without counselling. Patient counselling and follow-up therefore appears to be a cost-effective option.
Discussion
Guidelines identifying which patients, infected with Helicobacter, should be given eradication treatment have been published [22]. Of the many treatment regimes reported, proton pump inhibitor-based triple therapies for a period of 1 week appear, at present, to represent the optimal therapeutic option for eradication of H. pylori[23]. Inevitably most regimes show variation in reported eradication rates, and this has been particularly marked for dual therapy regimes with a proton pump inhibitor and amoxicillin [24]. It is likely that variations in reported success rates are related to differences in patient populations, including their compliance, variability in the details of trial design and the prevalence of antimicrobial resistance, particularly to metronidazole. The view has been expressed that clinical practice may be at variance with results of clinical trials of Helicobacter eradication [10] though others have disagreed [25]. The experience in this hospital has been that it is difficult to reproduce the effectiveness of clinical trial regimes for eradication of Helicobacter. In the present population metronidazole resistance was present in 24% of Helicobacter cultures (unpublished data), but it was felt that other factors may have contributed to the difficulties in replicating clinical trial results.
The aim of the present study was to determine if the effectiveness of a triple therapy regimen, which did not include a nitro-imidazole, could be enhanced by paying particular attention to improving patient compliance. This has been recognized as an important issue, which impacts on the eradication rate achieved by different types of H. pylori eradication therapy [26,27]. In this study patients in the intervention group received detailed counselling from a pharmacist about the rationale for treatment, the possible side-effects and the importance of good compliance. These patients were also provided with the medication by the hospital pharmacist, with the required instructions of dosing at the time of the initial counselling session. The control population received a standard letter explaining the nature of the infection and the need for treatment. They were asked to contact their GP to obtain the necessary prescription. The study demonstrated the value of patient counselling since the intervention group were significantly more compliant that those in the control group (92.1% vs 23.7%, who took all prescribed doses; P < 0.001). All patients who showed a failure of eradication had taken less than 60% of their prescribed dosage regime. As a result, eradication rates were significantly higher in the intervention group (94.7% compared with 73.7% in the control group; P = 0.027). Before the study started, the eradication rate in our hospital, using the same regime, was 72% in 86 patients (unpublished data). The higher eradication rate achieved in the intervention group would appear to be due to the influence of patient counselling about their disease, drug treatment and the resultant good compliance with the prescribed regime. There may also have been an effect of the further checks undertaken in the intervention group during follow-up telephone calls. As a result the eradication rate was significantly improved to as good as, or better than, appropriate clinical trials [28] indicating that in clinical practice it is important to try to duplicate the instruction, education and counselling which takes place in a clinical trial.
As expected, symptoms in patients in whom H. pylori was successfully eradicated were significantly improved. The number of patients with dyspeptic symptoms was significantly reduced, and more importantly the severity of each of the measured dyspeptic symptoms was significantly reduced, both 1 month and 6 months after treatment. H. pylori persistent patients showed slight improvements in nausea and vomiting at 1 month, but by 6 months symptoms, frequency and severity were unchanged from the pre-treatment levels. Similar results have been reported by other investigators [29], and indeed it has been suggested that symptom control is a useful surrogate for confirming that eradication has taken place [30].
In this study H. pylori persistent patients continued to require antisecretory medications, while those who were eradicated did not, and as other studies have shown had much reduced contact with their GPs following eradication [31]. This has significant cost implications since most of the difference in costs of different regimes is related to the cost of failure of eradication. The appropriateness of using lansoprazole 30 mg daily in our chosen regime may be questioned since using a twice daily dose, as with the other proton pump inhibitors, may be more effective. Others have, however, found good eradication rates with once daily proton pump regimens [32,33].
The possible suboptimal nature of the regime may have permitted the benefit of detailed patient counselling to become apparent, and indicates that a highly acceptable eradication rate can be achieved with such a regime if careful attention is paid to patient counselling and compliance. In the present study, adverse drug reactions were generally mild in nature and well tolerated.
The present study suggests that variations in Helicobacter eradication rates may be due to factors other than the drug regime chosen, and that patient counselling can have a significant impact on improving success rates. It would seem important that details of the instructions and information given to patients together with subsequent monitoring should be included in clinical trial reports. The present study reinforces the view that a central part of any eradication regime must be careful explanation of the rationale of treatment, the need for full compliance and discussion of possible side-effects with the patient [33,34].
In conclusion, the results of this study document the significant difference between the routine clinical practice (control group) and counselling enhanced treatment (intervention group) and highlight the urgent need for structured patient counselling to be introduced into routine clinical practice. Combined with well structured patient counselling, a lansoprazole, amoxicillin and clarithromycin combination can result in excellent compliance and highly efficient, cost-effective eradication of H. pylori.
References
- 1.Bourke B, Sherman P, Drumm B. Peptic ulcer disease: what is the role for Helicobacter pylori. Semin Gastrointest Dis. 1994;5:24–31. [PubMed] [Google Scholar]
- 2.Sipporen P. Gastric cancer – A long term consequence of Helicobacter pylori infection? Scand J Gastroenterol. 1994;29(Suppl 201):24–27. [PubMed] [Google Scholar]
- 3.Sipporen P, Hyvarinen H. Role of Helicobacter pylori in the pathogensis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol. 1993;28(Suppl 196):3–6. doi: 10.3109/00365529309098333. [DOI] [PubMed] [Google Scholar]
- 4.Bell GD, Powell KU, Burridge SM, et al. Rapid eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 1995;9:41–46. doi: 10.1111/j.1365-2036.1995.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 5.The National Institute of Health Consensus Development Panel. Helicobacter pylori in peptic ulcer disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 6.Chiba N, Rao BV, Rademaker JW, Hunt RH. Meta-analysis of the efficacy of antibiotic therapy in eradicating Helicobacter pylori. Am J Gastroenterol. 1992;87:1716–1727. [PubMed] [Google Scholar]
- 7.Toracchio S, Cellini L, Campli E, et al. Role of antimicrobial sensitivity testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment Pharmacvol Ther. 2000;14:1639–1643. doi: 10.1046/j.1365-2036.2000.00870.x. [DOI] [PubMed] [Google Scholar]
- 8.Rauws EA, Tytgat GNJ. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 9.Borody TJ, Cole P, Noonan S, et al. Recurrence of duodenal ulcer and Campylobacter pylori infection after eradication. Med J Aust. 1989;151:431–435. doi: 10.5694/j.1326-5377.1989.tb101251.x. [DOI] [PubMed] [Google Scholar]
- 10.Labeij RJF, Van Russum LGM, Jansen JBMJ, Staatman H, Verbeek ALM. Evaluation of treatment regimens to cure Helicobacter pylori infection – a meta analysis. Aliment Pharmacol Ther. 1999;13:857–864. doi: 10.1046/j.1365-2036.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 11.Penston JG. Helicobacter pylori eradication – understandable caution but no excuse for inertia. Aliment Pharmacol Ther. 1994;8:369–389. doi: 10.1111/j.1365-2036.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 12.Stewart RB. Noncompliance in the elderly. Drugs Aging. 1991;1:163–167. doi: 10.2165/00002512-199101030-00001. [DOI] [PubMed] [Google Scholar]
- 13.Graham DY, Lew GM, Malaty HM, et al. Factors influencing the eradication of Helicobacter therapy with triple therapy. Gastroenterology. 1992;102:493–496. doi: 10.1016/0016-5085(92)90095-g. [DOI] [PubMed] [Google Scholar]
- 14.Cramer JA, Mattson RH, Prevey L, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? JAMA. 1989;261:3273–3277. [PubMed] [Google Scholar]
- 15.Gul V, Mackenzie M. Medical counselling and patient compliance in Turkish and Greek speaking communities in Haringey and Enfield. J R Soc Health. 1993;113:286–287. doi: 10.1177/146642409311300601. [DOI] [PubMed] [Google Scholar]
- 16.Goodyer LI, Miskelly F, Milligan P. Does encouraging good compliance improve patients clinical condition in heart failure? Br J Clin Pract. 1995;49:173–176. [PubMed] [Google Scholar]
- 17.Roethlisberger FJ, Dickson WJ. Management and the Worker – an Account of a Research Program Conducted by the Western Electric Company, Hawthorne Works. Chicago. Cambridge Massachusetts: Harvard University Press; 1939. [Google Scholar]
- 18.Altman DG, Gore SM. 1982. Statistics in practice Articles from the BMJ. BMA, London,
- 19.Logan RPH, Dill S, Bauer EF, et al. The European 13C-urea breath test for the detection of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1991;3:915–921. [Google Scholar]
- 20.Cutler AF, Havstad S, Chen K, et al. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136–141. doi: 10.1016/0016-5085(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 21.Svedlund J, Sjodin I, Dotevall G. GSRS – a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 22.The European Helicobacter pylori Study Group. Current European concepts in management of Helicobacter pylori infection. Gut. 1997;41:8–13. doi: 10.1136/gut.41.1.8. The Maastricht Consensus Report. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lind T, Veldhuyzen van Zanten SJD, Unge P, et al. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials. Helicobacter. 1996;1:138–144. doi: 10.1111/j.1523-5378.1996.tb00027.x. The MACH 1 Study. [DOI] [PubMed] [Google Scholar]
- 24.Axon ATR, Moayyedi P. Eradication of Helicobacter pylori: Omeprazole in combination with antibiotics. Scand J Gastroenterol. 1996;31(Suppl 215):82–87. [PubMed] [Google Scholar]
- 25.Johnston BJ, Levi S. What is the relevance of the randomised controlled clinical trial in H. pylori eradication to routine clinical practice. Gut. 1997;41(Suppl 1):A91. [Google Scholar]
- 26.Graham DY, Lew GM, Klein PD, et al. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric and duodenal ulcer. A randomised controlled study. Ann Int Med. 1992;116:705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 27.Cutler AF, Schubert TT. Patient factors affecting Helicobacter pylori eradication with triple therapy. Am J Gastroenterol. 1993;88:505–509. [PubMed] [Google Scholar]
- 28.Unge P. Review of Helicobacter pylori: Eradication regimes. Scand J Gastroenterol. 1996;31(Suppl 215):74–81. [PubMed] [Google Scholar]
- 29.Phull PS, Ryder SD, Halliday D, Price AB, Levi AJ, Jacyna MR. The economic and quality-of-life benefits of Helicobacter pylori eradication in chronic duodenal ulcer disease – a community-based study. Postgrad Med J. 1995;71:413–418. doi: 10.1136/pgmj.71.837.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labenz J, Tillenburg B, Peitz U, Borsch G. Long term consequences of Helicobacter pylori eradication – clinical aspects. Scand J Gastroenterol. 1996;31(Suppl 215):111–115. [PubMed] [Google Scholar]
- 31.Reilly TG, Ayres RCS, Poxon V, Walt RP. Helicobacter pylori eradication in a clinical setting. Success rates and the effect on the quality of life in peptic ulcer. Aliment Pharmacol Ther. 1995;9:438–490. doi: 10.1111/j.1365-2036.1995.tb00410.x. [DOI] [PubMed] [Google Scholar]
- 32.Bazzoli F, Zagori RM, Fossi S, et al. Short-term low dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol. 1996;6:773–777. [Google Scholar]
- 33.Pryce DI, Harris AW, Gabe SM, et al. One week of lansoprazole, clarithromycin and metronidazole eradication of Helicobacter pylori. Gastroenterology. 1996;110:A235. [PubMed] [Google Scholar]
- 34.Lee M, Kemp JA, Canning A, Egan C, Tataronis G, Farraye FA. A randomised controlled trial of an enhanced patient compliance program for Helicobacter pylori therapy. Arch Intern Med. 1999;159:2312–2316. doi: 10.1001/archinte.159.19.2312. [DOI] [PubMed] [Google Scholar]


