Abstract
Aims
5-HT1B-receptor mediated vasoconstriction of cranial arteries is a potential mechanism by which 5-HT1B/1D-receptor agonists such as sumatriptan produce their antimigraine effects. 5-HT1B-receptors exist in other blood vessels which may give rise to unwanted vascular effects. Therefore we examined the distribution of 5-HT1B-receptor immunoreactivity (i.r.) in human blood vessels (including target and nontarget vessels) and confirmed the functionality of this receptor protein, by comparing the vasoconstrictor effects of sumatriptan and 5-HT (the endogenous ligand) in isolated vessels.
Methods
Blood vessels (middle meningeal, pial, temporal and uterine arteries and saphenous veins) were obtained from surgical patients (with consent). Sections of the vessels were prepared for routine immunohistochemical studies using specific 5-HT1B- and 5-HT1D-receptor antibodies. For functional studies, ring segments of the vessels were mounted in organ baths for isometric tension recording.
Results
5-HT1B-receptor i.r. was detected on the smooth muscle layer in middle meningeal, pial and uterine arteries and in saphenous vein and sumatriptan produced contractions in these vessels with potency values (mean pEC50) of 7.00, 7.08, 6.44 and 6.61, respectively, the magnitude of contraction was greatest in the cranial arteries with Emax values of 100.7, 60.3, 23.0 and 35.9%, respectively (expressed as a percentage of the reference agonist 45 mm KCl). 5-HT1B-receptor i.r. was not detected in temporal artery and sumatriptan had no effect in this artery. 5-HT1D-receptor i.r. was not detected in any of the vessels studied.
Conclusions
Sumatriptan can evoke vasoconstriction in antimigraine target vessels and also in nontarget vessels through an action at 5-HT1B-rcceptors. Sumatriptan acts preferentially to cause contraction in human cranial arteries compared with the other blood vessels we examined and this effect is likely to be shared by other drugs of this class.
Keywords: 5-HT1B-receptors, human blood vessels, vasoconstriction
Introduction
5-HT receptors can be classified in 14 distinct subtypes and 5-HT1-, 5-HT2- and 5-HT7-receptors have been demonstrated to mediate changes in vascular tone [1]. 5-HT7-receptor activation causes vasorelaxation [2, 3], whereas 5-HT1B/1D-receptors and/or 5-HT2A-receptors, which are negatively linked to adenylate cyclase, mediate vasoconstriction [4–6]. 5-HT1F-receptors are also negatively coupled to adenylate cyclase [1] and mRNA coding for this receptor subtype is expressed in vascular smooth muscle cells, however, activation of this receptor does not directly influence vascular tone [7, 8].
Sumatriptan, a 5-HT1B/1D-receptor agonist, is a clinically effective antimigraine agent. Its therapeutic action has been attributed, at least in part, to vasoconstriction of cranial blood vessels which become excessively swollen during the migraine attack [9, 10]. It is generally accepted that this vasoconstrictor response in human cranial arteries is 5-HT1B-receptor mediated [7, 8, 11, 12]. RT-PCR studies have shown the selective expression of 5-HT1B-receptor mRNA (and not 5-HT1D-receptor mRNA) in other human blood vessels [13, 14] and this mRNA, if translated into functional 5-HT1B-receptor protein, may give rise to potential unwanted vasoconstrictor effects. Indeed 5-HT1B-receptor mediated vasoconstriction in coronary artery is of potential importance [11, 15].
In the present study we used antibodies which have previously been shown to be specific for either 5-HT1B- or 5-HT1D-receptors [16], to test for the expression of these receptor proteins in human blood vessels, including cranial arteries (middle meningeal, pial and temporal arteries) and peripheral blood vessels (uterine artery and saphenous vein). Secondly, using isolated blood vessels, we examined the functionality of the receptor protein and determined whether there were any differences in the vasoconstrictor effects of sumatriptan and 5-HT (the endogenous ligand) in cranial arteries compared with the peripheral blood vessels.
Methods
Patients
Blood vessels were obtained, with consent, from surgical patients. Middle meningeal, pial and temporal arteries were from patients undergoing neurosurgical procedures for aneurysm, subarachnoid haemorrhage or temporal lobe epilepsy (Addenbrooke's Hospital, UK). These cranial arteries were transported to the Terlings Park laboratory for investigation. Saphenous veins were obtained as discarded vessel segments from patients undergoing coronary bypass (Addenbrooke's Hospital, UK) and uterine arteries from the uterus removed as hysterectomy (Baylor Medical College, USA) and functional studies were performed at these respective sites. All arteries were of macroscopically normal appearance. All vessels were collected in physiological salt solution and transported to the laboratory and prepared for functional studies within 1–2 h of collection, or placed in fixative for immunohistochemistry.
The collection of human material for these studies was approved by the University of Cambridge and Addenbrooke's Hospital Ethics Committees and by the Ethics Committee at Baylor Medical College, Texas, USA. The human material collected in the UK (i.e. cranial arteries and saphenous vein) was excess material remaining following completion of all pathology or surgical procedures and the patients gave written, informed consent for this material to be used for research purposes.
Preparation of vascular sections for immunohistochemistry
The immunohistochemical studies were carried out as previously described by Longmore et al. [17]. Briefly, blood vessel segments were fixed in 10% formal saline (4–6 days), processed to paraffin wax and sectioned (7 µm). Following de-waxing and rehydration in graded alcohols, endogenous peroxidases were inhibited by immersion in 0.3% H2O2 in methanol (1 h, room temperature). Following washing in 0.1 m phosphate buffered saline (PBS, Sigma) the sections were subjected to antigen retrieval techniques by washing in Citra solution (pH 6.0, Biomen UK) followed by microwaving (full power, 5–20min, Panasonic 800 W).
Immuno-staining procedure for tissue sections
Briefly, the sections were exposed (overnight, 4 °C, saturated humidity) to the primary antibodies, i.e. 5-HT1D- and 5-HT1B-receptor antisera (MSD/Affiniti, 1 : 300 and 1 : 100 dilutions, respectively), α-actin (smooth muscle cell marker, Biogenesis, 1 : 1000) and biotinylated Ulex europaeus agglutinin 1 (endothelial cell marker, 1 : 200–1 : 1000). Following washing in PBS the sections were exposed to the secondary antibody (biotinylated goat anti rabbit IgG, Vectastain Elite Kit, 1 h, room temperature), washed and immersed in Avidin-Biotin Complex (Vectastain Elite Kit, 1 h, room temperature). This was followed by further washing and immersion in di-amino benzidine solution (0.0025% DAB in PBS with 0.03% H2O2). Light microscopy was performed on a Leica (DMRB) microscope using normal Kohler illumination. (For detailed methodology see [12, 17]).
Measurement of vasoconstrictor responses
The arteries were cleaned of connective tissue and in the case of the saphenous veins the endothelium was mechanically removed by gently rubbing with a cotton bud. Each vessel was cut into ring segments (each approximately 2–6 mm in length) and 3–6 segments were obtained from each vessel. Segments were mounted for isometric tension recording in tissue baths containing physiological salt solution aerated with 95% O2/5% CO2, pH 7.4, 37 °C. A resting load was applied and the tissues were allowed to equilibrate to their own basal tension level. Tissue viability was assessed by determining the response to the reference agonist 45 mm KCl. This concentration of KCl was selected since it causes membrane depolarization and the opening of voltage-operated calcium channels, giving a substantial, submaximal contraction that is readily reversible on wash-out. For detailed methodology of studies with cranial arteries see [8], for saphenous vein see [18] and for uterine arteries see [19].
Cumulative concentration-effect curves (1 nm–10 µm) to 5-HT and/or sumatriptan were obtained. For middle meningeal, pial and temporal arteries some segments were exposed to both 5-HT and sumatriptan in randomised order since previously we have shown that using this experimental protocol there is no desensitization on exposure to multiple 5-HT receptor agonists [8]. For uterine arteries and saphenous veins, preliminary experiments showed substantial desensitization and therefore each vessel segment was exposed to either 5-HT or sumatriptan.
Analysis of data
Concentration-effect curves for the mean responses for 5-HT and sumatriptan were fitted to a model using weighted least squares nonlinear regression analysis and the equation:
where Emax is the maximum contraction evoked by each agonist (relative to 45 mm KCl = 100%), EC50 is the half maximally effective concentration and nH is the Hill coefficient (GraphPad Prism 2.0b).
Results
Immunohistochemical studies
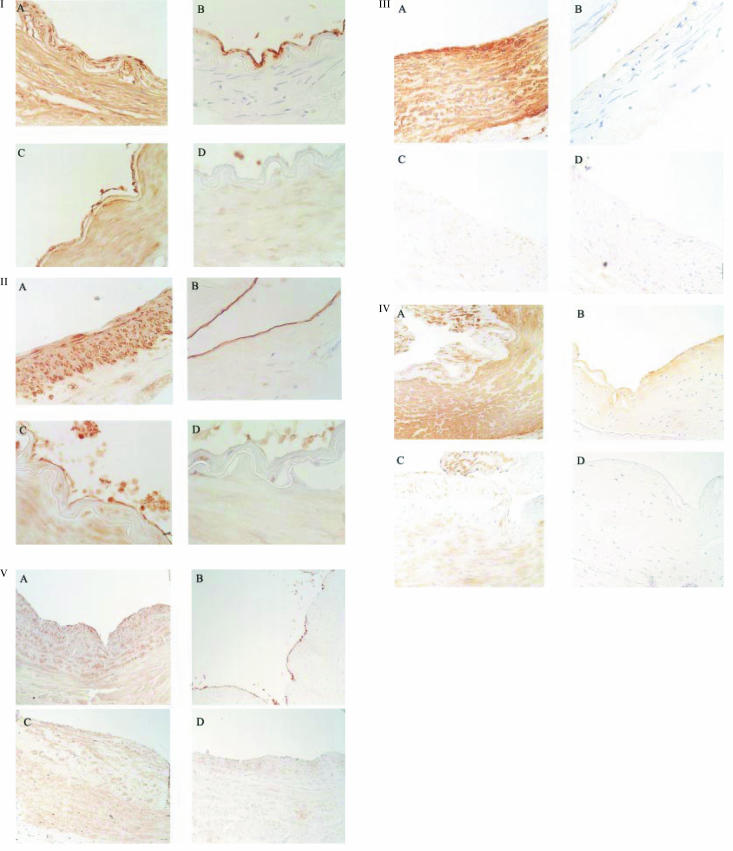
Immunohistochemical studies were performed on vessel segments previously used in organ baths for functional studies (‘used vessels’) and/or on segments which had not been used for functional studies (‘naive vessels’). Figure 1 shows representative immunohistochemical findings from each blood vessel (n = 2–4 patients per group). Between 15 and 20 sections from each individual patient were examined and a consistent picture of immunostaining was obtained in all sections examined. The middle meningeal arteries had a well developed intimal layer and specific 5-HT1B-receptor like immunostaining (5-HT1B-ir), but not 5-HT1D-receptor like immunostaining (5-HT1D-ir), was seen throughout the smooth muscle layer (as defined by smooth muscle actin immunostaining) of the tunica media. Similarly for pial and uterine arteries and saphenous vein 5-HT1B-ir, but not 5-HT1D-ir, was also detected within the smooth muscle layer. Neither 5-HT1B-ir, or 5-HT1D-ir was detected in temporal artery (see Figure 1).
Figure 1.
Representative immunohistochemical findings in: (I) middle meningeal artery; (II) pial artery; (III) temporal artery; (IV) uterine artery and (V) saphenous vein. Immunoreactivity (ir) was detected using di-amine benzidine as the chromagen which gives orange/brown staining and haematoxylin was used as a counterstain to detect cell nuclei (blue/purple stain). Thin (7 µm) sections of the artery were obtained and high power micrographs of parts of these sections are shown (for middle meningeal, pial and temporals arteries magnification is × 400 and for uterine artery and saphenous vein magnification is × 200). Panels A and B show immunostaining to α-actin (smooth muscle cells) and Ulex europeaus (endothelial cells) and Panels C and D show 5-HT1B-receptor and 5-HT1D-receptor immunoreactivity, respectively. Dense 5-HT1B-immunoreactivity was detected on the smooth muscle layer in middle meningeal, pial and uterine arteries and also in saphenous vein but not in temporal artery. Dense 5-HT1B-immunoreactivity was also detected on endothelial cells in middle meningeal and pial arteries and weak 5-HT1B-immunoreactivity was also detected on endothelial cells in saphenous vein. 5-HT1D-immunoreactivity was not detected in any vessel.
In vessel segments used for functional studies there was no immunostaining to Ulex europaeus indicating that the segments were endothelium-denuded (data not shown). In naive vessels, dense Ulex staining was seen in the cranial arteries (see Figure 1) and weak staining was seen in uterine and saphenous vein. 5-HT1B-receptor i.r. was detected on the endothelial layer in middle meningeal and pial and arteries and also on saphenous vein (see Figure 1). This endothelial receptor protein was not expected to influence the functional responses since these experiments were performed using endothelium-denuded segments.
Vasoconstrictor effects of 5-HT-receptor agonists
Functional studies on each of the vessels were carried out in several laboratories using methodology shown to produce reliable and reproducible responses to 5-HT receptor agonists. Any differences in the experimental conditions were considered to be minor and not expected to exert a substantial influence on the comparison of 5-HT and sumatriptan vasoconstrictor responses.
The response to KCl ranged between 0.04 and 4.95 g for middle meningeal artery, 0.04–1.86 g for pial arteries, 0.46–4.55 g for temporal arteries, 5.8–29.0 g for uterine artery and 0.175–3.30 g for saphenous vein. The mean concentration curves for 5-HT and sumatriptan in each of the vessels are shown in Figure 2. The Emax (relative to 45 mm KCl = 100%) and EC50 values for 5-HT and sumatriptan (expressed relative to the KCl reference responses) are shown in Table 1. Sumatriptan produced larger contractile responses (relative to KCl) in middle meningeal artery compared with the other blood vessels studied. The Emax value for sumatriptan in middle meningeal artery was significantly greater that the Emax obtained in the other vessels studies (one-way anovaF = 1170, d.f. = 4, 71, P < 0.001; mean difference (with 95% confidence limits of the differences) for middle meningeal artery vs pial artery was 39.5% (35.8–43.3); vs temporal artery was 93.4% (87.2–99.6); vs uterine artery was 76.8% (72.7–80.9) and vs saphenous vein was 63.9% (61.1–66.8), for all comparisons P < 0.01, Dunnett's test.
Figure 2.
Concentration-effect curves to 5-HT (▪) and sumatriptan (▴) obtained in endothelium-denuded segments of human isolated middle meningeal artery (a), pial artery (b), temporal artery (c), uterine artery (d) and saphenous vein (e). Points represent mean data expressed relative to the contraction evoked by 45 mm KCl. Vertical bars signify ±s.e. mean. Several segments obtained from the same donor were used, the details regarding the total number of segments (s) which were exposed to either 5-HT and/or to sumatriptan (and the number of individual donors from which these were obtained, n) are listed in Table 1. Curves were fitted to the data using nonlinear regression analysis.
Table 1.
Vasoconstrictor effects in human isolated blood vessels. pEC50 values (i.e. agonist potency, the negative logarithm of the concentration required to produce half maximal response) and Emax values (i.e. the maximum contractile response relative to 45 mm KCl = 100%) were derived from the curve fitting procedure. Values shown are symptotic mean (95% confidence limits). For each agonist the total number of arterial segments tested (s)/the number of individual donors (n) from whom tissues were obtained is also shown (s/n).
| Blood vessel | pEC50 | 5-HT Emax | s/n | pEC50 | Sumatriptan Emax | s/n |
|---|---|---|---|---|---|---|
| Middle meningeal artery | 7.22 (7.32–7.12) | 143.0 (136.9–153.7) | 19/13 | 7.00 (7.22–6.72) | 100.7 (88.8–112.7) | 29/15 |
| Pial artery | 7.87 (8.06–7.70) | 86.9 (78.4–95.4) | 8/5 | 7.08 (7.21–6.96) | 60.3 (55.7–64.9) | 10/7 |
| Temporal artery | 7.31 (7.89–6.74) | 74.4 (64.1–84.7) | 3/3 | ND | 6.4* | 3/3 |
| Uterine artery | 6.42 (6.52–6.31) | 48.7 (45.2–52.3) | 9/5 | 6.44 (6.52–6.35) | 23.0 (21.6–24.5) | 8/4 |
| Saphenous vein | 6.73 (6.79–6.67) | 97.9 (94.3–101.4) | 23/5 | 6.61 (6.67–6.55) | 35.9 (34.5–37.4) | 22/5 |
ND = not calculated as no clear maximum achieved
maximum contraction measured at 10 µm.
Discussion
Previously we have shown that the expression of 5-HT1B-receptor, but not 5-HT1D-receptor immunoreactivity, can be detected in human middle meningeal and coronary arteries [11, 17]. RT-PCR studies have also shown the selective expression of 5-HT1B-receptor mRNA transcripts in human blood vessels [13, 14]. The present study has now extended these observations and allowed the cellular localization of 5-HT receptor proteins which is not always possible with RT-PCR studies. Thus we have shown that in human vascular smooth muscle the ‘sumatriptan-receptor’, where present, is of the 5-HT1B-receptor subtype. This contrasts to findings in blood vessels from other species such as dog where expression of both 5-HT1B- and 5-HT1D-receptor mRNAs have been detected [20] and with rabbit where 5-HT1D-receptors can mediate vasoconstriction [21, 22]. The immunohistochemical techniques used in the present study allow only the detection (i.e. absence or presence) of immunoreactivity and not quantification of the levels of expression of 5-HT1B-receptor i.r. across the different blood vessels. Previously we used a radiolabelled secondary antibody to show that 5-HT1B-receptor expression was higher in human middle meningeal artery compared with coronary artery [11]. This kind of experiment is difficult to carry out in an adequately controlled manner, requiring substantial validation of the nonsaturable binding of the primary and secondary antibodies and identical collection/preparation of the blood vessels. Therefore it was considered not feasible to repeat quantitative assesment in the present study.
The present study also showed that 5-HT1B-receptor immunoreactivity, but not 5-HT1D-receptor immunoreactivity, could be detected on the endothelial cell layer in middle meningeal and pial arteries and saphenous vein. This agrees with previous reports where endothelial 5-HT1B-receptor immunoreactivity was detected in human cortical microvessels [23] and in human cerebral arteries [12, 24]. The role of the endothelial 5-HT1B-receptors and the relationship to migraine pathogenesis and/or the therapeutic action of the triptans has not yet been clearly defined. Previous studies have shown that sumatriptan can produce vasorelaxation through an endothelium dependent mechanism [25–27]. On the other hand, responses of human isolated coronary arteries to sumatriptan are similar in both endothelium-intact and endothelium-denuded segments [11, 24, 28]. In dog saphenous vein 5-HT1B/1D-receptor immunoreactivity is present on both smooth muscle and endothelial cells and sumatriptan evoked a contraction attenuated by removal of the endothelium, suggesting a pro-constrictor role for the endothelial receptors [29]. In the present study it was not possible to reliably preserve an intact endothelium in vessels used for functional experiments and therefore not possible to compare responses in endothelium-intact and endothelium-denuded tissues.
We measured vascular reactivity in isolated blood vessels to determine whether the 5-HT1B-receptor immunoreactivity represented functional 5-HT1B-receptor protein. Since both 5-HT2A- and 5-HT1B-receptors mediate vasoconstriction we estimated the 5-HT1B-receptor component by comparing the response evoked by sumatriptan (a selective 5-HT1B/1D-receptor agonist with no affinity for 5-HT2A-receptors [30]), with the 5-HT response. Previously, we have shown that in human middle meningeal artery responses to sumatriptan (over a similar concentration range as used in the present study), are markedly inhibited by the selective 5-HT1B/1D-receptor antagonist GR127935, confirming that over this concentration range sumatriptan retains selectivity as a 5-HT1B-receptor agonist [18]. In both middle meningeal and pial arteries the contractile response to sumatriptan was approximately 70% relative to the response evoked by 5-HT indicating a substantial 5-HT1B-receptor contribution. For uterine artery and saphenous vein the sumatriptan response was smaller (< 50% relative to 5-HT) indicating the involvement of both 5-HT1B-receptors and a larger 5-HT2-receptor component [5]. In contrast in temporal artery, sumatriptan was a weak vasoconstrictor consistent with the absence of 5-HT1B-receptor immunoreactivity [4].
Previously we reported that sumatriptan and rizatriptan act selectively to cause greater vasoconstriction and are approximately 10-fold more potent in middle meningeal artery compared to coronary vessels and that this may result from a higher level of 5-HT1B-receptor expression in cranial over coronary arteries [11]. A similar observation has also been reported for eletriptan [32]. The present experiments in isolated blood vessels support the involvement of multiple factors in the promotion of a selective action of sumatriptan on the craniovasculature: firstly the design of sumatriptan as a selective 5-HT1B/1D-receptor agonist avoids 5-HT2-receptor mediated vasoconstriction in nontarget blood vessels. This is consistent with the relatively small difference in the size of the response to sumatriptan compared to the response to 5-HT in cranial arteries (indicating a minor 5-HT2A-receptor component) compared to the substantial difference seen in nontarget blood vessels (consistent with a greater 5-HT2-receptor contribution); secondly sumatriptan had significantly higher potency (2–3 fold) in middle meningeal and pial arteries over uterine artery and saphenous vein. Finally the magnitude of contractions to both sumatriptan and 5-HT (relative to the reference response) was greater in cranial arteries (most notably middle meningeal artery) compared with the other blood vessels studied, and this also points to a selective craniovascular action of sumatriptan. However, it should be noted that the magnitude of the KCl reference response could vary depending on the relative contributions of l-type calcium channels to contraction across vessel types. If selective vasoconstriction of cranial arteries is important for antimigraine activity, then all the currently available triptans will share this mechanism. The selectivity of triptans for the craniovasculature over other vascular beds appears to be corroborated by the low incidence of serious cardiovascular adverse events associated with triptans in clinical use. 5-HT1B/1D receptor agonists have been associated with rare reports of serious cardiac adverse events and this class of drugs is currently contraindicated in patients with coronary artery disease, those with signs and symptoms of ischaemic heart disease, Prinzmetal's angina or uncontrolled hypertension.
Acknowledgments
Thanks to Reyhana Kajee for technical assistance, to the neurosurgeons and Lynn Maskell at Addenbrooke's for collecting the cranial arteries, the surgeons at Baylor for collecting uterine arteries and Denize Denton for collecting saphenous veins. The generosity of the patients who donated these tissues is very much appreciated and we hope that this work will lead to a better understanding of the mechanism of action of antimigraine drugs.
References
- 1.Hoyer D, Clarke DE, Fozard JR, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1996;46:157–203. [PubMed] [Google Scholar]
- 2.Schoeffter P, Ullmer C, Bobirnac I, Gabbiani G, Lubbert H. Functional endogenously expressed 5-hydroxytryptamine 5-ht7 receptors in human vascular smooth muscle cells. Br J Pharmacol. 1996;117:993–994. doi: 10.1111/j.1476-5381.1996.tb16687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung E, Walsh LKM, Pulido-Rios MT, Eglen RM. Characterisation of putative 5-HT7 receptors mediating direct relaxation in Cynomolgus monkey isolated jugular vein. Br J Pharmacol. 1996;117:926–930. doi: 10.1111/j.1476-5381.1996.tb15282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verheggen R, Freudenthaler S, Meyer-Dulheuer F, Kaumann AJ. Participation of 5-HT1-like and 5-HT2A receptors in the contraction of human temporal artery by 5-hydroxytryptamine and related drugs. Br J Pharmacol. 1996;117:283–289. doi: 10.1111/j.1476-5381.1996.tb15188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bax WA, van Heuven-Nolsen D, Bos E, Somoons ML, Saxena PR. 5-Hydroxytryptamine-induced contractions of the human isolated saphenous vein: involvement of 5-HT2 and 5-HT1D-like receptors, and a comparison with grafted veins. Naunyn-Schmiedeberg's Arch Pharmacol. 1992;345:500–508. doi: 10.1007/BF00168940. [DOI] [PubMed] [Google Scholar]
- 6.Kaumann AJ, Parsons AA, Brown AM. Human arterial constrictor serotonin receptors. Cardiovasc Res. 1993;27:2094–2103. doi: 10.1093/cvr/27.12.2094. [DOI] [PubMed] [Google Scholar]
- 7.Bouchelet I, Cohen Z, Case B, Sequela P, Hamel E. Differential expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in human trigeminal ganglia and cerebral blood vessels. Mol Pharmacol. 1996;50:219–223. [PubMed] [Google Scholar]
- 8.Razzaque Z, Heald MA, Pickard JD, et al. Vasoconstriction in human isolated middle meningeal arteries determining the contribution of 5-HT1B- and 5-HT1F-receptor activation. Br J Clin Pharmacol. 1998;47:75–82. doi: 10.1046/j.1365-2125.1999.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey PPA, Feniuk W. Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol Sci. 1991;12:444–446. doi: 10.1016/0165-6147(91)90630-b. [DOI] [PubMed] [Google Scholar]
- 10.Friberg L, Olesen J, Iversen HK, Sperling B. Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet. 1991;338:13–17. doi: 10.1016/0140-6736(91)90005-a. [DOI] [PubMed] [Google Scholar]
- 11.Longmore J, Razzaque Z, Shaw D, et al. Comparison of the meningeal versus coronary artery selectivity of the 5-HT1B/1D-receptor agonists rizatriptan and sumatriptan using human isolated blood vessels. Br J Clin Pharmacol. 1998;46:577–582. doi: 10.1046/j.1365-2125.1998.00821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson T, Longmore J, Jansen Olesen I, Edvinsson L. Contractile 5-HT1B receptors in human cerebral arteries. Pharmacological characterization and localisation with immunocytochemistry. Br J Pharmacol. 1999;128:1133–1140. doi: 10.1038/sj.bjp.0702773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamel E, Fan E, Linville D, et al. Expression of mRNA for the serotonin 5-hydroxytryptamine1Dβ receptor subtype in human and bovine cerebral arteries. Mol Pharmacol. 1993;44:242–246. [PubMed] [Google Scholar]
- 14.Ullmer C, Schmuck K, Kalkman HO, Lubbert H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Letts. 1995;370:215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor P, Gladstone P. Oral sumatriptan-associated myocardial infarction. Neurol. 1995;45:2274–2276. doi: 10.1212/wnl.45.12.2274. [DOI] [PubMed] [Google Scholar]
- 16.Smith D, Shaw D, Hopkins R, et al. Characterisation of human 5-HT1B- or 5-HT1D-receptor specific antibodies. J Neurosci Meth. 1998;80:155–161. doi: 10.1016/s0165-0270(97)00209-4. [DOI] [PubMed] [Google Scholar]
- 17.Longmore J, Shaw D, Smith D, et al. Differential distribution of 5-HT1B- and 5-HT1D-immunoreactivity within the human trigemino-cerebrovascular system: implications for the discovery of new anti-migraine drugs. Cephalalgia. 1998;17:833–842. doi: 10.1046/j.1468-2982.1997.1708833.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferro A, Longmore J, Hill RG, Brown MJ. A comparison of the contractile effects of 5-hydroxytryptamine, sumatriptan and MK-462 on human coronary artery in vitro. Br J Clin Pharmacol. 1995;40:245–252. doi: 10.1111/j.1365-2125.1995.tb05780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longmore J, Boulanger CM, Desta B, Schofield WN, Hill RG, Taylor AA. 5-HT1D-receptor agonists and human coronary artery reactivity in vitro: crossover comparisons of 5-HT and sumatriptan with MK-462 and L-741,519. Br J Clin Pharmacol. 1996;42:431–441. doi: 10.1046/j.1365-2125.1996.04217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branchek TA, Bard JA, Kucharewicz SA, et al. Frontiers in Headache ResearchExperimental Headache Models. New York: Lippincott-Raven Press; 1995. Migraine. Relationship to cloned canine receptors and human 5-HT1D receptors; pp. 125–134. [Google Scholar]
- 21.Razzaque Z, Longmore J, Hill RG. Differences in the effects of ketanserin on 5-HT receptor mediated responses in rabbit saphenous vein and guinea-pig jugular vein. Eur J Pharmacol. 1995;283:199–206. doi: 10.1016/0014-2999(95)00349-p. [DOI] [PubMed] [Google Scholar]
- 22.Bard JA, Kucharewicz SA, Zgombick JM, et al. Differences in ligand binding profiles between cloned rabbit and human 5-HT1Dα and 5-HT1Dβ–receptors: ketanserin and methiothepin distinguish rabbit 5-HT1D receptor subtypes. Naunyn-Schmiedeberg's Arch Pharmacol. 1996;354:237–244. doi: 10.1007/BF00171053. [DOI] [PubMed] [Google Scholar]
- 23.Riad M, Tong XK, El-Mestikawy S, Hamon M, Hamel E, Descarries L. Endothelial expression of the 5-hydroxytryptamine (1B) antimigraine drug receptor in rat and human brain microvessels. Neurosci. 1998;86:1031–1035. doi: 10.1016/s0306-4522(98)00196-1. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson T, Longmore J, Shaw D, et al. Characterisation of 5-HT receptors in human coronary arteries by molecular and pharmacological techniques. Eur J Pharmacol. 1999;372:49–56. doi: 10.1016/s0014-2999(99)00114-4. [DOI] [PubMed] [Google Scholar]
- 25.Schoeffter P, Hoyer D. Is the sumatriptan (GR 43175) -induced endothelium-dependent relaxation of pig coronary arteries mediated by 5-HT1D-receptors. Eur J Pharmacol. 1989;166:117–119. doi: 10.1016/0014-2999(89)90692-4. [DOI] [PubMed] [Google Scholar]
- 26.Schoeffter P, Hoyer D. 5-hydroxytryptamine (5-HT)-induced endothelium-dependent relaxation of pig coronary arteries is mediated by 5-HT receptors similar to the 5-HT1D-receptor subtype. J Pharmacol Exp Ther. 1990;252:387–395. [PubMed] [Google Scholar]
- 27.Elhusseiny A, Hamel E. Sumatriptan elicits both constriction and dilation in human and bovine brain intracortical arterioles. Br J Pharmacol. 2001;132:55–62. doi: 10.1038/sj.bjp.0703763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maassen VanDenBrink A, Reekers M, Bax WA, et al. Coronary side-effect potential of current and prospective antimigraine drugs. Circulation. 1998;98:25–30. doi: 10.1161/01.cir.98.1.25. [DOI] [PubMed] [Google Scholar]
- 29.Razzaque Z, Taylor P, Smith D, Wang T, Longmore J. J Physiol. 2001. The role of the endothelium on 5-HT1B/1D evoked contraction of dog isolated saphenous vein. (Abstract) [Google Scholar]
- 30.Humphrey PPA, Feniuk W, Perren MJ, Connor HE, Oxford A. The pharmacology of the novel 5-HT1-like receptor agonist, GR43175. Cephalalgia. 1989;9(Suppl 9):21–23. doi: 10.1111/J.1468-2982.1989.TB00069.X. [DOI] [PubMed] [Google Scholar]
- 31.Kaumann AJ, Frenken M, Posival H, Brown AM. Variable participation of 5-HT1-like receptors and 5-HT2 receptors in serotonin-induced contraction of human isolated coronary artery. Circulation. 1994;90:1141–1153. doi: 10.1161/01.cir.90.3.1141. [DOI] [PubMed] [Google Scholar]
- 32.Maassen VanDenBrink A, van den Broek RWM, de Vries R, et al. Craniovascular selectivity of eletriptan and sumatriptan in human isolated blood vessels. Neurol. 2000;55:1524–1530. doi: 10.1212/wnl.55.10.1524. [DOI] [PubMed] [Google Scholar]