Abstract
Aims
It has been widely recognized that classical antihistamines induce sedation as an adverse effect, while second-generation antihistamines have few if any sedative effects. In order to evaluate the sedative properties of ebastine, a second-generation antihistamine, its effect on cognitive performance in healthy subjects was compared with placebo and (+)-chlorpheniramine.
Methods
Twelve healthy male subjects were instructed to perform six types of attention-demanding cognitive tasks, and objective measurements of reaction times and accuracy was made before and after drug administration. Their sleepiness levels were also monitored. Test drugs were ebastine 10 mg, placebo and two doses of (+)-chlorpheniramine 2 mg and 6 mg, as positive controls. Plasma drug concentrations at the end of the study were analysed.
Results
After treatments with (+)-chlorpheniramine, the reaction times of the tasks were significantly prolonged (e.g. ratios of after/before dosing: placebo (0.998±0.113) vs (+)-chlorpheniramine 2 mg (1.103±0.083; P < 0.05) or (+)-chlorpheniramine 6 mg (1.170±0.139; P < 0.001) in a 7 ms visual discrimination time task) and the accuracy was significantly decreased (e.g. ratios: placebo (1.038±0.158) vs (+)-chlorpheniramine 2 mg (0.792±0.202; P < 0.01) or (+)-chlorpheniramine 6 mg (0.837±0.222; P < 0.05) in a 7 ms task). On the other hand, performance was not affected by ebastine or placebo treatment (e.g. ebastine 10 mg (reaction time ratio; 1.014±0.067 and accuracy ratio; 0.990±0.146) in a 7 ms task). Subjective sleepiness was also not affected by ebastine but (+)-chlorpheniramine significantly increased sedation. With respect to the relationship between plasma drug concentrations and task performance, the latter deteriorated with an increase in plasma (+)-chlorpheniramine concentration (e.g. r = 0.439 (P = 0.007) in a 5 ms and r = 0.352 (P = 0.039) in a 7 ms task), but it did not correlate with the plasma concentration of carebastine, an active metabolite of ebastine.
Conclusions
Ebastine 10 mg did not cause any cognitive impairment or subjective sleepiness. On the other hand, (+)-chlorpheniramine impaired cognitive function and induced sleepiness even at 2 mg, the recommended dose in over-the-counter medication. In addition, impaired CNS performance was significantly correlated with plasma (+)-chlorpheniramine concentration.
Keywords: (+)-chlorpheniramine, cognition, ebastine, plasma concentration, tachistoscope
Introduction
Antihistamines have been widely used for many years in the treatment of peripheral allergic diseases such as urticaria and rhinitis [1]. However, sedation is a serious problem. The sedative properties of classical antihistamines have been reported to cause accidents while driving and working, and to contribute to a decline in productivity and learning efficiency [2–5]. These effects are due to penetration through the blood–brain-barrier and occupation of brain histamine H1-receptors [6]. Using positron emission tomography, we have demonstrated that impaired cognitive performance induced by (+)-chlorpheniramine was parallel to its occupation of human brain H1-receptors [7, 8].
The consequent development of second-generation antihistamines resulted in drugs that cause much less sedation and anticholinergic effects. However, each drug should be thoroughly evaluated for its potential sedative properties.
Ebastine, a second-generation antihistamine [9], is efficacious in seasonal/perennial allergic rhinitis [10–14], and chronic idiopathic urticaria [15, 16]. It is well absorbed from the digestive tract and extensively metabolized on first-pass to the antihistaminergic active metabolite carebastine [17–20]. Phase III studies conducted in our country revealed that the incidence of sleepiness caused by ebastine was lower than that of ketotifen [14, 21]. In addition, several studies reported the low potential of ebastine for sedation [22]. However, few studies focusing on the relationship between cognitive performance and plasma concentration have been reported.
In this study, we investigated the effect of ebastine on cognitive performance in healthy subjects and compared it with those of placebo and (+)-chlorpheniramine using attention-demanding cognitive tasks. We also studied the relationship between task performance and plasma drug concentration.
Methods
The study was approved by the Committee on Clinical Investigation, Tohoku University School of Medicine (the appropriate ethics committee) and was performed in accordance with the policy of the Declaration of Helsinki.
Subjects
Twelve healthy male Japanese volunteers, aged between 21 and 24 years old (average 22.5±1.0 years), were enrolled in this study. All subjects had no visual and cognitive impairment and had no history of alcohol- or other drug-dependency, or drug allergy. They were requested not to take medication containing antihistamines a week before the study, and were asked to abstain from any drugs and alcohol the night before the study and from tobacco, alcohol, caffeine, grapefruit, grapefruit-containing beverages and any other drugs during the test. Written informed consent was obtained from all subjects.
Subjects were instructed to train for all tasks to achieve their own plateau level of task performance prior to testing. They also underwent clinical examination and laboratory tests for haematology (haemoglobin, red blood cell count, haematocrit, leucocyte count, differential leucocyte count ratios and platelet count) as well as biochemical tests (alkaline phosphatase, γ-glutamyl transpeptidase, aspartate aminotransferase, alanine aminotransferase, lactic acid dehydrogenase, blood urea nitrogen, creatinine, total protein and albumin).
Drug administration
Drugs used in the study were ebastine (10 mg) (E), (+)-chlorpheniramine (2 mg (C2) and 6 mg (Repetabs) (C6)) and a placebo tablet (P). Drugs were taken orally with approximately 150 ml of water.
Subjective sleepiness and alertness
Subjective sleepiness and alertness were evaluated with the Stanford Sleepiness Scale [23], composed of a 7 level self-report measure. Subjects were instructed to select the statement best reflecting their current level of sleepiness and alertness.
Attention-demanding cognitive tasks
(1) Visual discrimination time task (VDT) [8]
To maintain a highly attention-demanding condition, visual stimuli were shown with near-visual threshold presentation time. Subjects viewed a single visual stimulus displayed on an AV tachistoscope (IS702, Iwatsu, Japan), subtending a 2°×2° visual angle. The task was to distinguish target stimuli from nontarget stimuli and to push a button promptly with the right index finger when the target stimulus was presented. The latter were selected from 10 kinds of digits and nontarget stimuli were selected from 46 kinds of Japanese phonograms called ‘hiragana’. Target stimuli were presented with a probability of 20% of the total stimuli. The presenting times of visual stimuli were fixed at 3, 5, 7, and 20 ms length during each session. In total, one-hundred and twenty single digits or letters were serially presented during each 3 min session. Task performance was evaluated by measuring two kinds of parameter, namely reaction time and accuracy. These tasks clearly revealed in our previous study that cognition was impaired by intravenous injection of (+)-chlorpheniramine 2 mg [8].
(2) Choice reaction time task (CRT) and simple reaction time task (SRT)
To maintain a highly attention-demanding condition, the time between one stimulus and the next was random. Subjects viewed a single visual stimulus displayed on an AV tachistoscope. The CRT involved choosing a left or right stimulus against the centre of the display, and to push a button promptly with the left or right index finger when the corresponding-side stimulus was presented. The SRT required the subject to push a button promptly with the right index finger when the stimulus was presented. The stimulus was a closed circle symbol, which was randomly presented on the left or right against the centre of the display in the CRT and only on the left against the centre of the display in the SRT. A total of 30 stimuli were serially presented during about 3 min of a session. The reaction time and accuracy (CRT only) were also used as task performance parameters.
Study design
A single-blind, randomized, crossover study with 12 healthy subjects was performed. Tasks were given each day before and after drug treatment in order to correct possible interday differences in performance. Tasks after each treatment were performed at the time when the plasma drug concentration was expected to be at its maximum value.
Each drug was randomly given to subjects on different days with intervals of at least 6 days. Prior to the pre-dose or post-dose evaluations, task exercises consisting of CRT, SRT, 5 ms and 7 ms VDTs were given to the subjects for 15–20 min at around 10.00 h or 16.00 h for adaptation purposes. At the end of these task exercises, the pre- and post-dose tests were performed immediately. Ebastine and (+)-chlorpheniramine were given to subjects 5 h and 2 h before the postdose test, respectively, since it was reported that the times to reach their respective peak plasma concentration were approximately 5 and 2 h after dosing [18, 24]. In order to maintain close attention during the tasks, subjects took a 2 min rest after each one. Before the first task and after each task, levels of subjective sleepiness and alertness were evaluated. Blood was taken from subjects at the end of all tasks (approximately 6 h after the ebastine administration and 3 h after the (+)-chlorpheniramine administration) for analysis of plasma ebastine, carebastine and (+)-chlorpheniramine.
Analysis of plasma drug concentration
Plasma concentrations of ebastine and carebastine were measured by high performance liquid chromatography (h.p.l.c.) as described previously [25]. Plasma concentration of (+)-chlorpheniramine was measured by liquid chromatography-mass spectrometry (LC-MS) [26]. Briefly, plasma was pretreated with a solid-phase or liquid–liquid extraction and the extract was applied to a h.p.l.c. system with ultraviolet detection at 254 nm or a LC-MS system using an electrospray ionization probe in the positive ion mode, in which the ion mass of (+)-chlorpheniramine was detected as 275 (m/z). Coefficients of variation were in the ranges of 0.6–11.0% for ebastine, 1.1–12.2% for carebastine, and 3.9–13.5% for (+)-chlorpheniramine, respectively. The lower limits of quantification were 3 ng ml−1 for ebastine and carebastine using 1 ml of plasma, and 0.5 ng ml−1 for (+)-chlorpheniramine using 0.5 ml of plasma, respectively.
Data analysis
Results are expressed as means±s.d. anova followed by Dunnett's test was conducted for multiple comparison of the task performance among the drug groups. The relationship between the plasma drug concentration and the task performance was evaluated using Pearson's correlation. A probability less than 0.05 was considered to be statistically significant.
Results
Subjective sleepiness and alertness
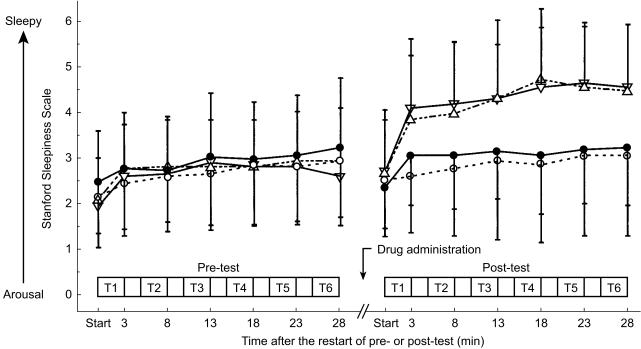
Time courses of the subjective evaluation of sleepiness and alertness are shown in Figure 1. During the pre-dose test, the results were virtually the same among all drug groups. In the post-dose test period, levels of sleepiness and alertness in the ebastine group as well as the placebo group were not different from those during the pre-dose test. However, feelings of sleepiness in the two (+)-chlorpheniramine groups significantly increased compared with the placebo groups (P vs C2 or C6: P < 0.05).
Figure 1.
Subjective sleepiness and alertness before and after dosing (• placebo; ^ ebastine 10 mg; ▵ (+)-chlorpheniramine 2 mg; ▿ (+)-chlorpheniramine 6 mg. T1-6 represent the periods when tasks were performed, T1: CRT, T2: SRT, and T3-6: VDTs (4 types randomly assigned). Each task took about 3 min with a 2 min resting interval.
Plasma concentration of drugs
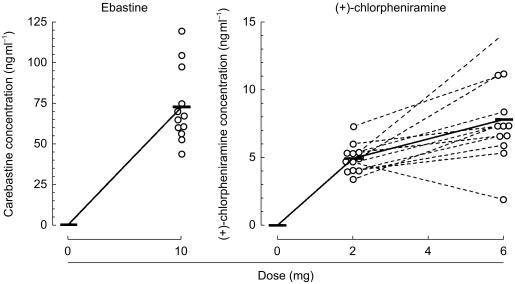
Mean and individual plasma concentrations of carebastine and (+)-chlorpheniramine are plotted against their respective doses in Figure 2. Unchanged ebastine was not detected in any plasma samples 6 h after treatment with ebastine, and the mean (±s.d.) plasma concentration of carebastine was 73±23 ng ml−1 (range 44–120 ng ml−1). Three hours after oral (+)-chlorpheniramine at doses of 2 and 6 mg, mean (±s.d.) plasma concentrations were 5.0±1.1 ng ml−1 (3.4–7.4 ng ml−1) and 7.9±3.2 ng ml−1 (2.0–14.3 ng ml−1), respectively.
Figure 2.
Plasma concentrations of carebastine and (+)-chlorpheniramine 6 h and 3 h after drug administration, respectively. Ebastine, the unchanged compound, was not detected in any plasma samples. The concentrations were plotted as a function of each dose; mean (—) and individual (^) data.
Attention-demanding cognitive tasks
(1) Primary task performance
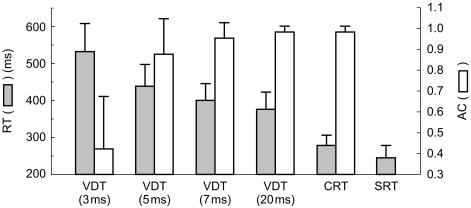
Before drug administration, mean (±s.d.) values of reaction times after 3, 5, 7 and 20 ms tasks were 530±76 ms, 440±58 ms, 400±46 ms and 380±45 ms, respectively, and mean (±s.d.) values of accuracy were 0.42±0.25, 0.88±0.17, 0.95±0.08 and 0.99±0.02. The mean (±s.d.) reaction times from the CRT and SRT were 280±29 ms and 240±32 ms, respectively, and the accuracy from the CRT was 0.99±0.02 (Figure 3). These data indicate that the longer the stimulus-presenting time, the shorter the reaction time, and that the accuracy tended to increase to a plateau level.
Figure 3.
Mean (±s.d.) results of the VDTs, CRT and SRT before drug treatment. Stimulus presenting times are in parenthesis. Data were compiled from all pre-dose tests (n = 48). RT reaction time, AC accuracy.
(2) Visual discrimination time tasks (VDTs)
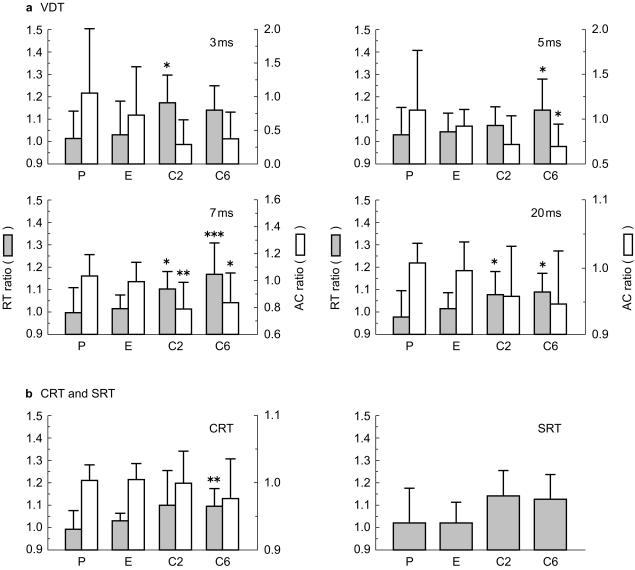
Figure 4a shows the ratios of reaction time and accuracy in the post-dose test phase to those in the pre-dose test phase. All task performances of the ebastine group were similar to those of the placebo group. However, the reaction time ratios in the two (+)-chlorpheniramine groups were significantly higher than those in the placebo group for a 3 ms (C2: P < 0.05, 95% confidence interval for difference (CI): −0.309, −0.017), a 5 ms (C6: P < 0.05, 95% CI: −0.223, −0.004), a 7 ms (C2: P < 0.05, 95% CI: −0.208, −0.001 and C6: P < 0.001, 95% CI: −0.276, −0.069) and a 20 ms visual stimulus (C2: P < 0.05, 95% CI: −0.197, −0.005 and C6: P < 0.05, 95% CI: −0.209, −0.017). The accuracy ratios in the (+)-chlorpheniramine groups were also significantly lower than the placebo group for a 5 ms (C6: P < 0.05, 95% CI: 0.001, 0.809) and a 7 ms visual stimulus (C2: P < 0.01, 95% CI: 0.063, 0.429 and C6: P < 0.05, 95% CI: 0.018, 0.384).
Figure 4.
Effects of drug treatment on reaction time and accuracy for the (a) VDTs, and (b) CRT and SRT. Each figure represents the results at each stimulus-presenting time. The x-axis shows P (placebo), E (ebastine), C2 ((+)-chlorpheniramine 2 mg) and C6 ((+)-chlorpheniramine 6 mg), and the y-axes show the ratios of reaction time or accuracy of post-dose to those of pre-dose. *P < 0.05, **P < 0.01 and ***P < 0.001 vs placebo (Dunnett's multiple comparison test).
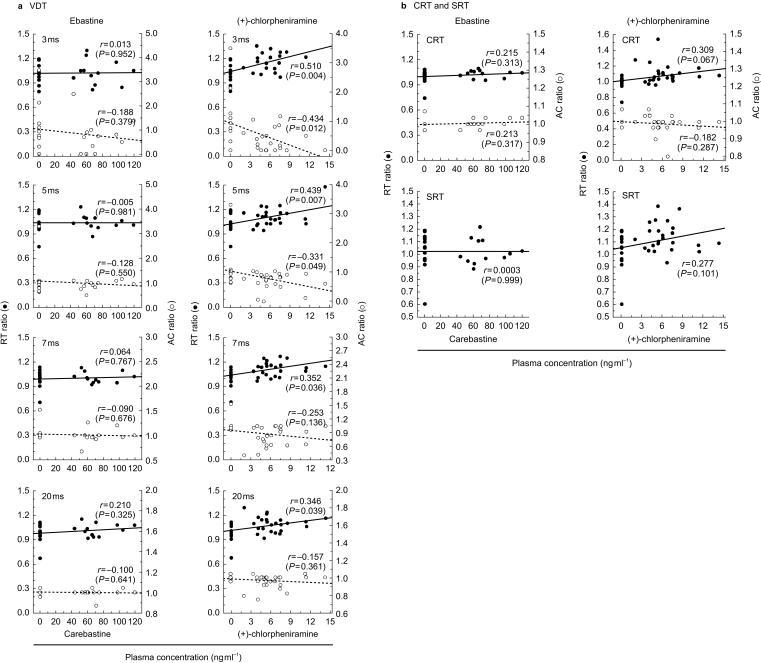
The relationships between the plasma concentration of carebastine or (+)-chlorpheniramine and task performance of individuals are illustrated in Figure 5a. The ratios of reaction time or accuracy of all four kinds of the VDTs were not significantly correlated with the plasma concentration of carebastine. However, the ratios of reaction time had a significant positive correlation in all tasks with the plasma concentration of (+)-chlorpheniramine (3 ms visual stimulus: r = 0.510 (P = 0.004), 5 ms: r = 0.439 (P = 0.007), 7 ms: r = 0.352 (P = 0.036), 20 ms: r = 0.346 (P = 0.039)). The accuracy ratios also had a significant negative correlation for the 3 ms (r = −0.434, P = 0.012) and a 5 ms visual stimulus (r = −0.331, P = 0.049).
Figure 5.
Relationships between plasma concentrations and ratios of reaction time or accuracy for the (a) VDTs, and (b) CRT and SRT. The x-axis for ebastine shows carebastine concentration, and the y-axes show the ratios of reaction time (RT) or accuracy (AC) of post-dose to those of pre-dose.
(3) CRT and SRT
The ratios of reaction time or accuracy in the CRT and SRT are shown in Figure 4b. The results obtained were similar to those from the VDT studies, in that the ratios of reaction time or accuracy in the ebastine group were not significantly different from those in the placebo group. On the other hand, in the (+)-chlorpheniramine groups, the ratios of reaction time were higher than those in the placebo group for the CRT (C6: P < 0.01, 95% CI: −0.206, −0.006), and the accuracy ratio for the CRT was less than that of the placebo group.
The relationships between the plasma concentration and ratios of reaction time or accuracy are shown in Figure 5b, but no significant correlations were found. On the other hand, the CRT and SRT tended to be performed less well with increasing the plasma concentration of (+)-chlorpheniramine when evaluated with reaction time.
Discussion
We investigated the effects of ebastine on human cognitive function in comparison with placebo and (+)-chlorpheniramine. Ebastine was found not to affect task performance and subjective sleepiness. These results agree with data obtained in phase III studies [14, 21], in which the incidence of sleepiness, as one of self-reported adverse effects, by ebastine was only 5–6% out of a total of 212 patients with allergic diseases, although that of ketotifen (the reference drug) was 23–28% from 206 patients. In addition, ebastine did not affect subjective drowsiness, cognitive performance and vigilance tests at normal doses [27, 28]. Furthermore, subjects showed no significant impairment in car driving performance during a 5 day repeated treatment [29]. These together with our present findings clearly indicate that ebastine causes little sedation.
In contrast, we found that (+)-chlorpheniramine at doses of 2 and 6 mg caused impairments of task performance and subjective sleepiness. Similar effects to these have been previously reviewed [30, 31], and the drug has been shown to impair visuomotor coordination performance [32], car driving performance [33], and slowed P3 latency [34], as well as increased subjective sedation [35], at dose higher than 4 mg. In our previous study, 2 mg of intravenous (+)-chlorpheniramine was shown to impair task performance at a human brain H1-receptor occupancy of approximately 60% [8].
In the present study, we also examined the relationship between task performance and plasma drug concentrations. The latter showed considerable interindividual fluctuations for both ebastine and (+)-chlorpheniramine. Task performance deteriorated with an increase in plasma (+)-chlorpheniramine concentration, but was not related to carebastine concentration. These results suggest that impaired cognitive performance and sleepiness were caused by (+)-chlorpheniramine exposure, and penetration of the agent into the CNS as shown by our PET studies [7, 8]. In contrast, the data suggest a lack of penetration of carebastine into the brain despite its presence in the systemic circulation.
In conclusion, ebastine at a dose of 10 mg did not cause detectable sedation nor CNS impairment while (+)-chlorpheniramine even at the low dose of 2 mg, available over the counter, did show sedative effects. In addition, the level of cognitive performance deteriorated along as the plasma concentration of (+)-chlorpheniramine increased, but such a relationship did not exist for carebastine. This study also revealed that VDT was more sensitive and useful for detecting drug-induced cognitive impairment than either the SRT or the CRT.
Acknowledgments
This work was supported by grants-in-aid from the Ministry of Education, Science and Culture, the Ministry of Health and the Shimadzu Science Foundation. We thank Professor R. Kato (Keio University School of Medicine) for his encouragement during this study and Dr I. Sato (Miyagi Cancer Center) for his suggestions for the statistical evaluation.
References
- 1.Simons FE, Simons KJ. The pharmacology and use of H1-receptor-antagonist drugs. N Engl J Med. 1994;330:1663–1670. doi: 10.1056/NEJM199406093302307. [DOI] [PubMed] [Google Scholar]
- 2.Cimbura G, Lucas DM, Bennett RC, Warren RA, Simpson HM. Incidence and toxicological aspects of drugs detected in 484 fatally injured drivers and pedestrians in Ontario. J Forensic Sci. 1982;27:855–867. [PubMed] [Google Scholar]
- 3.Fireman P. Treatment of allergic rhinitis: effect on occupation productivity and work force costs. Allergy Asthma Proc. 1997;18:63–67. doi: 10.2500/108854197778605482. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore TM, Alexander BH, Mueller BA, Rivara FP. Occupational injuries and medication use. Am J Ind Med. 1996;30:234–239. doi: 10.1002/(SICI)1097-0274(199608)30:2<234::AID-AJIM16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 5.Vuurman EF, van Veggel LLM, Uiterwijk MM, Leutner D, O'Hanlon JF. Seasonal allergic rhinitis and antihistamine effects on children's learning. Ann Allergy. 1993;71:121–126. [PubMed] [Google Scholar]
- 6.Goldberg MJ, Spector R, Chiang CK. Transport of diphenhydramine in the central nervous system. J Pharmacol Exp Ther. 1987;240:717–722. [PubMed] [Google Scholar]
- 7.Yanai K, Okamura N, Tagawa M, Itoh M, Watanabe T. New findings in pharmacological effects induced by antihistamines: from PET studies to knock-out mice. Clin Exp Allergy. 1999;29(Suppl 3):29–36. [PubMed] [Google Scholar]
- 8.Okamura N, Yanai K, Higuchi M, et al. Functional neuroimaging of cognition impaired by a classical antihistamine, d-chlorpheniramine. Br J Pharmacol. 2000;129:115–123. doi: 10.1038/sj.bjp.0702994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yakuo I, Ishii K, Seto Y, et al. Pharmacological study of ebastine, a novel histamine H1-receptor antagonist. Folia Pharmacol Jpn. 1994;103:121–135. doi: 10.1254/fpj.103.121. [DOI] [PubMed] [Google Scholar]
- 10.Luria X. Comparative clinical studies with ebastine: efficacy and tolerability. Drug Saf. 1999;21(Suppl 1):63–67. doi: 10.2165/00002018-199921001-00008. [DOI] [PubMed] [Google Scholar]
- 11.de Molina M, Cadahia L, Cano L, et al. Efficacy and tolerability of ebastine at two dose levels in the treatment of seasonal allergic rhinitis. Drug Invest. 1989;1:40–46. [Google Scholar]
- 12.Gehannol P, Brémard-Oury C, Zeisser P. A double-blind multicenter randomized study comparing once daily oral administration of ebastine 20 mg, ebastine 10 mg, and cetirizine 10 mg for the treatment of seasonal allergic rhinitis in adults. Rhône-Poulenc Rorer (Collegeville) (Data on file) [Google Scholar]
- 13.Picado VC, Cadahia GA, Cistero BA, Cano CL, Sanz AA, Zayas SJM. Ebastine in perennial allergic rhinitis. Ann Allergy. 1991;67:615–618. [PubMed] [Google Scholar]
- 14.Baba S, Mamiya S, Sakakura Y, et al. Clinical trial of LAS-90 on perennial allergic rhinitis: a double blind study in comparison with ketotifen fumarate [in Japanese] Rinsho Iyaku. 1994;10:1143–1162. [Google Scholar]
- 15.Kalis B, Brémard-Oury C. A 3-month double-blind comparative study of ebastine (10 mg o.d.), terfenadine (60 mg b.i.d.) and placebo in the treatment of chronic urticaria. Allergy. 1995;50(Suppl):380. [Google Scholar]
- 16.Peyri J, Vidal J, Marrón J, et al. Ebastine in chronic urticaria: a double-blind placebo-controlled study. J Dermatol Treat. 1991;2:51–53. [Google Scholar]
- 17.Martinez-Tobed A, Tarrus E, Segura J, et al. Pharmacokinetic studies in rats, dogs and man. Drugs Today. 1992;28(Suppl B):57–67. [Google Scholar]
- 18.Yamaguchi T, Hashizume T, Matsuda M, et al. Pharmacokinetics of the H1-receptor antagonist ebastine and its active metabolite carebastine in healthy subjects. Arzneimittelforschung Drug Res. 1994;44:59–64. [PubMed] [Google Scholar]
- 19.Vincent J, Liminana R, Meredith PA, Reid JL. The pharmacokinetics, antihistamine and concentration-effect relationship of ebastine in healthy subjects. Br J Clin Pharmacol. 1988;26:497–502. doi: 10.1111/j.1365-2125.1988.tb05288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashizume T, Mise M, Terauchi Y, et al. N-Dealkylation and hydroxylation of ebastine by human liver cytochrome P450. Drug Metab Dispos. 1998;26:566–571. [PubMed] [Google Scholar]
- 21.Kukita A, Harada S, Yoshida H, et al. Phase III study of LAS-90 on chronic urticaria: double blind comparative study with ketotifen fumarate [in Japanese] Rinsho Iyaku. 1994;10:895–912. [Google Scholar]
- 22.Roberts DJ, Gispert J. The non-cardiac systemic side-effects of antihistamines: ebastine. Clin Exp Allergy. 1999;29(Suppl 3):151–155. doi: 10.1046/j.1365-2222.1999.0290s3151.x. [DOI] [PubMed] [Google Scholar]
- 23.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 24.Peets EA, Jackson M, Symchowicz S. Metabolism of chlorpheniramine maleate in man. J Pharmacol Exp Ther. 1972;180:464–474. [PubMed] [Google Scholar]
- 25.Matsuda M, Mizuki Y, Terauchi Y. Simultaneous determination of the histamine H1-receptor antagonist ebastine and its two metabolites, carebastine and hydroxyebastine, in human plasma using high-performance liquid chromatography. J Chromatogr B. 757:173–179. doi: 10.1016/s0378-4347(00)00494-1. [DOI] [PubMed] [Google Scholar]
- 26.Takagaki T, Matsuda M, Mizuki Y, Terauchi Y. A simple and sensitive method of chlorpheniramine maleate in human plasma using LC/MS. J Chromatogr B. doi: 10.1016/s1570-0232(02)00315-x. in press. [DOI] [PubMed] [Google Scholar]
- 27.Vincent J, Sumner DJ, Reid JL. Ebastine: the effect of a new antihistamine on psychomotor performance and autonomic responses in healthy subjects. Br J Clin Pharmacol. 1988;26:503–508. doi: 10.1111/j.1365-2125.1988.tb05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hopes H, Meuret GH, Ungethum W, Leopold G, Wiemann H. Placebo controlled comparison of acute effects of ebastine and clemastine on performance and EEG. Eur J Clin Pharmacol. 1992;42:55–59. doi: 10.1007/BF00314920. [DOI] [PubMed] [Google Scholar]
- 29.Brookhuis KA, de Vries G, de Waard D. Acute and subchronic effects of the H1-histamine receptor antagonist ebastine in 10, 20 and 30 mg dose, and triprolidine 10 mg on car driving performance. Br J Clin Pharmacol. 1993;36:67–70. doi: 10.1111/j.1365-2125.1993.tb05894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simons FE. H1-receptor antagonists. Comparative tolerability and safety. Drug Saf. 1994;10:350–380. doi: 10.2165/00002018-199410050-00002. [DOI] [PubMed] [Google Scholar]
- 31.Nolen TM. Sedative effects of antihistamine: safety, performance, learning, and quality of life. Clin Ther. 1997;19:39–55. doi: 10.1016/s0149-2918(97)80071-9. [DOI] [PubMed] [Google Scholar]
- 32.Clarke CH, Nicholson AN. Performance studies with antihistamines. Br J Clin Pharmacol. 1978;6:31–35. doi: 10.1111/j.1365-2125.1978.tb01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aso T, Sakai Y. Effects of terfenadine on actual driving performance. Jpn J Pharmacol Ther. 1988;19:681–688. [Google Scholar]
- 34.Meador KJ, Loring DW, Thompson EE, Thompson WO. Differential cognitive effects of terfenadine and chlorpheniramine. J Allergy Clin Immunol. 1989;84:322–325. doi: 10.1016/0091-6749(89)90415-6. [DOI] [PubMed] [Google Scholar]
- 35.Kulshrestha VK, Gupta PP, Turner P, Wadsworth J. Some clinical pharmacological studies with terfenadine, a new antihistamine drug. Br J Clin Pharmacol. 1978;6:25–29. doi: 10.1111/j.1365-2125.1978.tb01677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]